Virlaza™ Improves Lung Function, Mechanics and Inflammation and Muscle Strength of Post-Covid-19 Patient: A Case Report
Article Information
Maysa Alves Rodrigues Brandao-Rangel1#, Dobroslav Melamed2#, Claudio Ricardo Frison1, Razi Ronen2, Boris Brill2, Rodolfo P Vieira1,3,4,5*
1Laboratory of Pulmonary and Exercise Immunology, Federal University of São Paulo (UNIFESP), São José dos Campos – SP, Brazil
2Research and Development Department, Libipharm, Rechovot, Israel
3Post-graduate Program in Bioenginnering, Universidade Brasil, São Paulo – SP, Brazil
4Brazilian Institute of Teaching and Research in Pulmonary and Exercise Immunology, São José dos Campos – SP, Brazil
5Post-graduate Program in Human Movement and Rehabilitation and in Pharmaceutical Sciences, Evangelical University of Goias (Unievangélica), Anápolis – GO, Brazil
#Both authors have equally contributed to this study.
*Corresponding Author: Rodolfo P Vieira, Prof. Dr. Evangelical University of Goiás (Unievangélica)
Avenida Universitária Km 3, 5, Anápolis – GO, Brazil
Received: 16 July 2023; Accepted: 31 July 2023; Published: 24 August 2023
Citation: Maysa Alves Rodrigues Brandao-Rangel, Dobroslav Melamed, Claudio Ricardo Frison, Razi Ronen, Boris Brill, Rodolfo P Vieira. Virlaza™ Improves Lung Function, Mechanics and Inflammation and Muscle Strength of Post-Covid-19 Patient: A Case Report. Archives of Clinical and Medical Case Reports. 7 (2023): 361-363.
Share at FacebookAbstract
Coronavirus disease 2019 (COVID-19) affects different organs and a severe pro-inflammatory response, denominated “cytokine storm” have been described as a key underlying mechanism. The lungs are the most affected organ, and a fibrotic response after infection by COVID-19 is often found. This case report demonstrates that 12 days of oral administration of Virlaza™, an herbal medicine developed in Israel (based on tinctures and natural antioxidants), was able to accelerate the lung recovery of a moderate/severe post-COVID-19 patient, who presents metabolic syndrome, characterized by grade II obesity, dyslipidemia, and hypertension.
After 12 days of hospitalization needing high flow nasal oxygen, non-invasive ventilation, awake (non-intubated) proning, the patient was discharged still presenting the following characteristics: 75% of ground-glass opacities of the lungs in computerized tomography (CT), severe reduction of the lung function, high levels of fractional exhaled nitric oxide (FeNO), leukopenia and neutrophilia, reduced respiratory muscle strength and peripheral muscle strength. So, a very quick recovery was observed after 12 days of Virlaza™, resulting in an improvement to 25% ground-glass opacities of the lungs in the CT, as well as an improvement for all parameters of lung function, FeNO, normalization of blood leukocytes and improvement of respiratory and peripheral muscle strength. So, Virlaza™ may be considered as an herbal medicine able to improve the recovery of post-COVID-19 patients and reduce COVID-19 comorbidities.
Keywords
COVID-19; Lung Function; Nitric Oxide; Peripheral Muscle Strength; Respiratory Muscle Strength; SARS-Cov-2
COVID-19 articles; Lung Function; Nitric Oxide articles; Peripheral Muscle Strength articles; Respiratory Muscle Strength articles; SARS-Cov-2 articles
Article Details
1. Introduction
The COVID-19 imposed a pandemic in the whole world, affecting more than 619 million cases and 6 million deaths due to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The progression, severity, and mortality of COVID-19 differ from no, mild, or moderate symptoms in most of the patients, while up to 15% develop the severe and critical form of the disease with high mortality rates [2, 3]. Among the factors underlying the development of the severe and critical forms of COVID-19, advanced age [4], comorbidities such as obesity [4], hyperactivation of the immune system [4, 5] are proven to have a central role. In addition, hyperactivation of the immune system results in the cytokine storm, which is characterized by the synthesis and release of high levels of proinflammatory cytokines [4, 5]. In this way, cytokine storm plays a major role in COVID-19 pathogenesis, progression, and severity, impairing several organ functions, such as the lungs, heart, blood vessels, etc., [4, 5].
2. Case Presentation
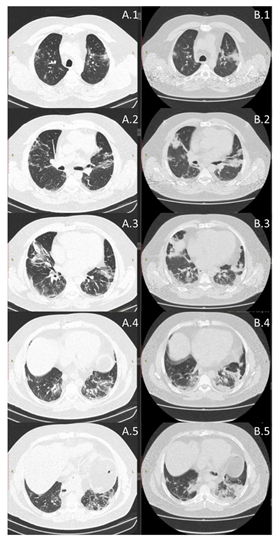
A 50-year-old man, without any history of previous respiratory diseases or allergy, with historic of two cardiovascular surgeries, grade II obese (BMI 35.91), insulin resistance, flu-like symptoms, fatigue and breathlessness, partial oxygen saturation of 80%, chest CT of the lungs (>75% ground-glass opacities) and diagnosed with coronavirus disease 19 (COVID-19) by real time polymerase chain reaction. After twelve days of hospitalization, under treatment with antibiotic (Amoxicillin 500mg/2x/day and Clavulanate Potassium 125mg/2x/day), corticosteroid (Prednisone 20mg/day) and sodium enoxaparin (100mg/day), the patient has been discharged from the hospital, still presenting 75% of ground-glass opacities of the lungs. After hospital discharged these symptoms remained: acute fatigue and breathlessness for minimal effort. The measurement of partial oxygen saturation at rest, lung function by spirometry (Masterscreen, Jaeger, Germany), according to ATS/ERS recommendations was performed by using forced maneuver [6]. Whole blood analysis (red and white cells) (automatic hematology analyzer Sysmex® XS-800i, Japan) [7], the levels of FeNO (NoBreath®, Bedfont Scientific, UK) [7], maximal inspiratory (MIP) and expiratory (MEP) pressure (analogical manovacuometer, Murenas®, Brazil) [7], and hand grip strength (analogical dynamometer, Jamar®, USA) [8] were performed before and after 12 days of use of Virlaza™ (20 drops, 2x/day). Virlaza™ is a tincture-based 10% concentration of: Clove Glycerin, Eucalyptus Glycerin, Basil Glycerin, Sage Glycerin, Maritime Pine Glycerin, Clove Ethyl Alcohol, Eucalyptus Ethyl Alcohol, Maritime Pine Ethyl Alcohol, Basilica Ethyl Alcohol, Sage Ethyl Alcohol. The results demonstrated that 12 days of Virlaza™ improved: lung function, forced vital capacity (FVC; 1.91 L x 2.31 L; 41.2% x 61.7% of predicted), forced expiratory volume in the first second (VEF1; 1.51 L x 1.68 L; 40.4% x 55.9% of predicted), inspiratory vital capacity (VC In; 1.59 L x 1.63 L, 36.3% x 46.7% of predicted), forced expiratory flow 25% (FEF25%; 0.47 L/s x 0.58 L/s, 35.8% x 58.3% of predicted), forced expiratory flow 50% (FEF50%; 1.42 L/s x 1.62 L/s, 37.4% x 41.9% of predicted), forced expiratory flow 75% (FEF75%; 2.91 L/s x 3.58 L/s, 48.3% x 49.7% of predicted), peak expiratory flow (PEF; 2.99 L/s x 3.97 L/s, 36.4% x 38%). The CT revealed a significant reduction in ground-glass opacities, reducing from >75% to 25% after 12 days of Virlaza™ (Figure 1). The levels of FeNO were initially of 28 parts per billion (ppb) to 11 ppb after treatment with Virlaza™. Partial oxygen saturation also presented significant improvement, from 92% to 99% after 12 days of treatment with Virlaza™. The hematological response was improved by Virlaza™ (platelets 250x103/mm3 x 350x103/mm3; total leukocytes 5,37/mm3 x 9,77/mm3; neutrophils 87% x 52.2%; lymphocytes 11% x 38%; eosinophils 0.9% x 0%; monocytes 2% x 0.1%; basophils 0.02% x 0.01%). The strength of respiratory muscles presented improvement after treatment with Virlaza™, where MIP was -100 cmH2O x -120 cmH2O and MEP 40 cmH2O x 60 cmH2O. Virlaza™ improved hand grip strength on right (28.9 Kg x 36.2 Kg) and left (19.8 Kg x 23.1 Kg) hands. Due to the reduction in the levels of FeNO demonstrating anti-inflammatory effects of Virlaza™, we point out the importance of controlling the lung inflammation to inhibit a poor prognosis during the recovery of COVID-19, which has been associated with lung fibrosis [9]. Such result is strengthened by the finding of a significant reduction in the lung ground opacities (>75% to 25%), followed by a very significant improvement in the lung function. As a study limitation, a longer period of follow up of the patient should be done. In conclusion, this case study reveals important beneficial effects of Virlaza™ on clinical and functional parameters of the severe post-COVID-19 patients.
Consent
This study was approved by Ethical Committee of Anhembi Morumbi University, registration number 5.193.830. Written and verbal consent was obtained from the patient for this case report.
Conflict of Interest
MARBR and CRF have no conflict of interest related to this study. DM and BB are the owners and scientific director of Libipharm™. RPV declares he has received consulting fee from Libipharm™.
Data Availability
All data obtained during the current study are not publicly available due to confidential issues but might be available at a reasonable request to the corresponding author.
Authors’ Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgements
We thank Sao Paulo Research Foundation (FAPESP) to provide the funding to perform this study, grant #2012/15165-2. MARBR holds a PhD fellowship from FAPESP #2019/05739-0. We thank the company Libipharm for providing the product Virlaza™ to allow us to perform the present study. FAPESP and Libipharm had no role in the study design and analysis.
References
- WHO Coronavirus (COVID-19) Dashboard (2022).
- Poor HD. Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest (2021): S0012-S3692.
- de Roquetaillade C, Mansouri S, Brumpt C, et al. Comparison of Circulating Immune Cells Profiles and Kinetics Between Coronavirus Disease 2019 and Bacterial Sepsis. Crit Care Med (2021).
- Lange A, Lange J, Jaskula E. Cytokine Overproduction and Immune System Dysregulation in alloHSCT and COVID-19 Patients. Front Immunol 12 (2021): 658896.
- Chua RL, Lukassen S, Trump S, et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol 38 (2020): 970-979.
- Culver BH, Graham BL, Coates AL, et al. ATS Committee on Proficiency Standards for Pulmonary Function Laboratories. Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am J Respir Crit Care Med 196 (2017): 1463-1472.
- Silva-Reis A, Rodrigues Brandao-Rangel MA, Moraes-Ferreira R, et al. Combined resistance and aerobic training improves lung function and mechanics and fibrotic biomarkers in overweight and obese women. Front Physiol 13 (2022): 946402.
- Brandao-Rangel MAR, Moraes-Ferreira R, Oliveira-Junior MC, et al. Pulmonary function changes in older adults with and without metabolic syndrome. Sci Rep 11 (2021): 17337.
- Myall KJ, Mukherjee B, Castanheira AM, et al. Persistent Post-COVID-19 Interstitial Lung Disease. An Observational Study of Corticosteroid Treatment. Ann Am Thorac Soc 18 (2021): 799-806.