Validation of Osteoporosis Scores with Urinary N-telopeptide Bone Marker in Assessment Bone Mineral Density in Autoimmune Rheumatic Diseases Patients
Article Information
Marwa A Besar1*, Youssef Abulatta2, Mohamed Hussein2, Mahmoud Abdelhadi2
1Lecturer of Internal medicine, Rheumatology& Immunology unit, Faculty of medicine, Mansoura University, Mansoura, Egypt
2House officer at Mansoura University Hospital MMMP (Mansoura Manchester Medical program), Faculty of medicine, Mansoura University, Mansoura, Egypt
*Corresponding Author: Marwa A Besar, Lecturer of Internal medicine, Rheumatology& Immunology unit, Faculty of medicine, Mansoura University, Mansoura, Egypt.
Received: 21 December 2023; Accepted: 06 January 2024; Published: 30 January 2024.
Citation: Marwa A Besar, Youssef Abulatta, Mohamed Hussein, Mahmoud Abdelhadi. Validation of Osteoporosis Scores with Urinary N-telopeptide Bone Marker in Assessment Bone Mineral Density in Autoimmune Rheumatic Diseases Patients. Fortune Journal of Rheumatology 6 (2024): 01-07.
Share at FacebookAbstract
Introduction: Osteoporosis is a condition characterised by decreased bone strength. Many tools are used to assess osteoporosis e.g Dual-energy x-ray absorptiometry (DXA) and Fracture Risk Assessment Tool (FRAX). Also, different bone resorption marker can predict osteoporosis e.g Urinary N-telopeptide is a sensitive and specific marker of bone resorption. Objective: assess the validity DXA and FRAX scores in early detection of osteoporosis in autoimmune diseases. Also, serum vitamin D and urinary N-telopeptide as marker for bone resorption in them.
Method: A cross sectional observational study where a (180) autoimmune rheumatic patients were assessed by DXA, FRAX scores, serum vitamin D, serum uric acid and Urinary N-telopeptide.
Result: The mean age of studied patients (180) was 46±12.6 years old, BMI 33.7 (29.4-37.8), S Uric acid 6 (5-7), S vitamin D 26 (13.3-32), urinary Telopeptide 105 (89-175), Lt femur neck T score -0.9 (-1.5…-0.3), Lt forearm T score -0.8 (-1.7….-0.1), osteoporosis 2 (1-2), FRAX score osteoporosis % 3.7 (2.6-7.5), FRAX score hip fracture % 0.2 (0.1-0.6) and risk hip fracture 1 (1-1). Left femur neck T score and Lumbar T score were significantly correlated with age, S uric acid, percentage of FRAX score osteoporosis and FRAX score hip fracture.
Conclusion: If we suspect osteoporosis, it is better to go for urinary N-telopeptide and those who test positive can go for current gold standard DXA scan. Combination of two diagnostic tools; urinary N-telopeptide with osteoporosis scores could help early identification of high risk for fracture in autoimmune diseases.
Keywords
Osteoporosis, Autoimmune Diseases, Fracture Risk Assessment (Frax), Bone mineral density (Bmd), Dual energy X-ray absorptiometry (Dexa).
Osteoporosis articles; Autoimmune Diseases articles; Fracture Risk Assessment (Frax) articles; Bone mineral density (Bmd) articles; Dual energy X-ray absorptiometry (Dexa) articles.
Osteoporosis articles Osteoporosis Research articles Osteoporosis review articles Osteoporosis PubMed articles Osteoporosis PubMed Central articles Osteoporosis 2023 articles Osteoporosis 2024 articles Osteoporosis Scopus articles Osteoporosis impact factor journals Osteoporosis Scopus journals Osteoporosis PubMed journals Osteoporosis medical journals Osteoporosis free journals Osteoporosis best journals Osteoporosis top journals Osteoporosis free medical journals Osteoporosis famous journals Osteoporosis Google Scholar indexed journals Autoimmune Diseases articles Autoimmune Diseases Research articles Autoimmune Diseases review articles Autoimmune Diseases PubMed articles Autoimmune Diseases PubMed Central articles Autoimmune Diseases 2023 articles Autoimmune Diseases 2024 articles Autoimmune Diseases Scopus articles Autoimmune Diseases impact factor journals Autoimmune Diseases Scopus journals Autoimmune Diseases PubMed journals Autoimmune Diseases medical journals Autoimmune Diseases free journals Autoimmune Diseases best journals Autoimmune Diseases top journals Autoimmune Diseases free medical journals Autoimmune Diseases famous journals Autoimmune Diseases Google Scholar indexed journals Fracture Risk Assessment (Frax) articles Fracture Risk Assessment (Frax) Research articles Fracture Risk Assessment (Frax) review articles Fracture Risk Assessment (Frax) PubMed articles Fracture Risk Assessment (Frax) PubMed Central articles Fracture Risk Assessment (Frax) 2023 articles Fracture Risk Assessment (Frax) 2024 articles Fracture Risk Assessment (Frax) Scopus articles Fracture Risk Assessment (Frax) impact factor journals Fracture Risk Assessment (Frax) Scopus journals Fracture Risk Assessment (Frax) PubMed journals Fracture Risk Assessment (Frax) medical journals Fracture Risk Assessment (Frax) free journals Fracture Risk Assessment (Frax) best journals Fracture Risk Assessment (Frax) top journals Fracture Risk Assessment (Frax) free medical journals Fracture Risk Assessment (Frax) famous journals Fracture Risk Assessment (Frax) Google Scholar indexed journals Bone mineral density (Bmd) articles Bone mineral density (Bmd) Research articles Bone mineral density (Bmd) review articles Bone mineral density (Bmd) PubMed articles Bone mineral density (Bmd) PubMed Central articles Bone mineral density (Bmd) 2023 articles Bone mineral density (Bmd) 2024 articles Bone mineral density (Bmd) Scopus articles Bone mineral density (Bmd) impact factor journals Bone mineral density (Bmd) Scopus journals Bone mineral density (Bmd) PubMed journals Bone mineral density (Bmd) medical journals Bone mineral density (Bmd) free journals Bone mineral density (Bmd) best journals Bone mineral density (Bmd) top journals Bone mineral density (Bmd) free medical journals Bone mineral density (Bmd) famous journals Bone mineral density (Bmd) Google Scholar indexed journals Dual energy X-ray absorptiometry (Dexa) articles Dual energy X-ray absorptiometry (Dexa) Research articles Dual energy X-ray absorptiometry (Dexa) review articles Dual energy X-ray absorptiometry (Dexa) PubMed articles Dual energy X-ray absorptiometry (Dexa) PubMed Central articles Dual energy X-ray absorptiometry (Dexa) 2023 articles Dual energy X-ray absorptiometry (Dexa) 2024 articles Dual energy X-ray absorptiometry (Dexa) Scopus articles Dual energy X-ray absorptiometry (Dexa) impact factor journals Dual energy X-ray absorptiometry (Dexa) Scopus journals Dual energy X-ray absorptiometry (Dexa) PubMed journals Dual energy X-ray absorptiometry (Dexa) medical journals Dual energy X-ray absorptiometry (Dexa) free journals Dual energy X-ray absorptiometry (Dexa) best journals Dual energy X-ray absorptiometry (Dexa) top journals Dual energy X-ray absorptiometry (Dexa) free medical journals Dual energy X-ray absorptiometry (Dexa) famous journals Dual energy X-ray absorptiometry (Dexa) Google Scholar indexed journals Rheumatology& Immunology unit articles Rheumatology& Immunology unit Research articles Rheumatology& Immunology unit review articles Rheumatology& Immunology unit PubMed articles Rheumatology& Immunology unit PubMed Central articles Rheumatology& Immunology unit 2023 articles Rheumatology& Immunology unit 2024 articles Rheumatology& Immunology unit Scopus articles Rheumatology& Immunology unit impact factor journals Rheumatology& Immunology unit Scopus journals Rheumatology& Immunology unit PubMed journals Rheumatology& Immunology unit medical journals Rheumatology& Immunology unit free journals Rheumatology& Immunology unit best journals Rheumatology& Immunology unit top journals Rheumatology& Immunology unit free medical journals Rheumatology& Immunology unit famous journals Rheumatology& Immunology unit Google Scholar indexed journals rheumatic patients articles rheumatic patients Research articles rheumatic patients review articles rheumatic patients PubMed articles rheumatic patients PubMed Central articles rheumatic patients 2023 articles rheumatic patients 2024 articles rheumatic patients Scopus articles rheumatic patients impact factor journals rheumatic patients Scopus journals rheumatic patients PubMed journals rheumatic patients medical journals rheumatic patients free journals rheumatic patients best journals rheumatic patients top journals rheumatic patients free medical journals rheumatic patients famous journals rheumatic patients Google Scholar indexed journals serum uric acid articles serum uric acid Research articles serum uric acid review articles serum uric acid PubMed articles serum uric acid PubMed Central articles serum uric acid 2023 articles serum uric acid 2024 articles serum uric acid Scopus articles serum uric acid impact factor journals serum uric acid Scopus journals serum uric acid PubMed journals serum uric acid medical journals serum uric acid free journals serum uric acid best journals serum uric acid top journals serum uric acid free medical journals serum uric acid famous journals serum uric acid Google Scholar indexed journals hip fracture articles hip fracture Research articles hip fracture review articles hip fracture PubMed articles hip fracture PubMed Central articles hip fracture 2023 articles hip fracture 2024 articles hip fracture Scopus articles hip fracture impact factor journals hip fracture Scopus journals hip fracture PubMed journals hip fracture medical journals hip fracture free journals hip fracture best journals hip fracture top journals hip fracture free medical journals hip fracture famous journals hip fracture Google Scholar indexed journals corticosteroid articles corticosteroid Research articles corticosteroid review articles corticosteroid PubMed articles corticosteroid PubMed Central articles corticosteroid 2023 articles corticosteroid 2024 articles corticosteroid Scopus articles corticosteroid impact factor journals corticosteroid Scopus journals corticosteroid PubMed journals corticosteroid medical journals corticosteroid free journals corticosteroid best journals corticosteroid top journals corticosteroid free medical journals corticosteroid famous journals corticosteroid Google Scholar indexed journals
Article Details
1. Introduction
Osteoporosis is a condition characterised by decreased bone strength that culminates in an increased risk of fractures in response to minimal or low velocity force in autoimmune rheumatic diseases due to many factors; the disease itself, the age and side effect of medication used in treatment especially corticosteroid [16]. Many tools used to assess the osteoporosis in them e.g Dual-energy x-ray absorptiometry (DXA) san, is the gold standard test for diagnosing osteoporosis and monitoring its treatment [14]. It depends on the amount of x-ray energy passing through bone and correlates it with the amount of mineral present, so DXA is a valuable tool in assessment of quantity in bone, but it is poor assessment of quality of bone. Another important point; DXA is unquestionably a useful tool to detect early bone loss that fracture risk which can occur at any T-score [2].So; incorporation anew tool in evolution the risk of fracture in these vulnerable group based on clinical history to assess the risk factor e.g the Fracture Risk Assessment Tool (FRAX) [9]. FRAX has worldwide applicability and validation in different countries. Its calculations may be part of a DXA report, or clinicians may use web-based tools to run the calculations. It provides an intervention threshold for decision analysis. [8] it allows the use of trabecular bone score data to help calculate intervention thresholds for major and hip osteoporotic fractures [12] but it isn’t validated to assess patients under the age of 40 years, may be restricted to specific geographic populations and they do not quantify factors in the calculations such as duration and amount of glucocorticoid use and the severity of secondary diseases [6]. Bone resorption markers are important indicators of disease activity in patients with osteoporosis. Urinary N-telopeptide has been used for monitoring treatment for osteoporosis for a long time [7], but now, clinicians are using it to predict the onset of osteoporosis. Urinary N-telopeptide is a sensitive and specific marker of bone resorption.
Aim of the work:
This study was conducted to validity of WHO fracture risk assessment tool (FRAX) and Dual-energy x-ray absorptiometry (DXA) scan in assessment of osteoporosis risk in autoimmune diseases. Beside assessment the role of serum Vitamin D and urinary telopeptide in predication of osteoporosis in them.
2. Patients and Methods
A cross sectional observational study where a total of (180) autoimmune rheumatic patients were assessed for osteoporosis during one-year duration in the rheumatology and immunology outpatient clinic, Mansoura University Hospital (MUH).
2.1 Methods
Thorough autoimmune and rheumatic history (which one, duration, line of management and disease activity at that time).Osteoporosis assessment (DXA, FRAX score assessment) and Line of management (whether anabolic or antiresorptive therapy, duration and response to prescribed medication).Serum vitamin D and uric acid and Twenty-four hours urine was collected in a sterile plastic container and sent for analysis of urinary telopeptide.
2.2 Inclusion criteria
All patients diagnosed as autoimmune rheumatic diseases patients who fulfil the criteria of definition of osteoporosis attending Rheumatology &Immunology unit in internal medicine department and outpatient’s clinic during the duration of the study.
2.3 Statistical analysis
Data were entered and statistically analyzed using the Statistical Package for Social Sciences (SPSS) version 23. Qualitative data were described as numbers and percentages. Quantitative data were described as means (SD) or medians, after testing for normality by Kolmogorov-Smirnov test.
3. Results
Our work target about 180 Rheumatic patients with the mean age of them was 46±12.6 years old, 92.8% (167/180) were female, more than fifty 57.2 (103/180) of studied patients were RA, 33 % (60/180) were SLE and less than 10% of them were Behcet 4.4%, Scleroderma 0.6%, Sjogren syndrome 1.7%, Vascuilitis 0.6% and 2.2% PSA. Most of them 86.1% (155/180) were controlled and 67.2% (121/180) of them had no complication. Majority of studied group 77.8% (140/180) were treated with DMARDs, 89.4% (161/180) were treated with corticosteroid Table (2). The median of height of studied patients was 160 (154-165) cm, weight 85 (75-95)kg, BMI 33.7 (29.4-37.8)cm/kg2, S Uric acid 6 (5-7)ng/dl, S vitamin D 26 (13.3-32)ng/dl, urinary Telopeptide 105 (89-175) nm BCE. It was noticed that more than Fifty of studied Rheumatic patients had normal bone density, 34% had osteopenia and about 14% of them had osteoporosis Figure (1), where Lt femur neck T score -0.9 (-1.5…-0.3), Lt femur neck z score -0.6 (-1.2….0), Lt forearm T score -0.8 (-1.7….-0.1), Lt forearm Z score -0.5 (-1.3…….0.3), osteoporosis 2 (1-2), FRAX score osteoporosis % 3.7 (2.6-7.5), FRAX score hip fracture % 0.2 (0.1-0.6) and risk hip fracture 1 (1-1). The mean of BMI (10-year probability of fracture) was 33.5±6.6, lumbar spine T score -1.1±1.3 and lumbar spine Z score -1.1±1.3 Table (1). Type of Osteoporosis treatment received by our studied patients varied where Bisphosphonate was used in 47.2%, Densoumab 5%, Tripeptide 2.2% while Calcium and vitamin D supplement was used alone in 37.2% and one year duration was used in 57.8% of treated patients, two year duration in 31.1% while three year duration was only in 7.8% of them. Majority of treated patients 91.1% had stationary course, fortunately no major osteoporotic fracture in majority of them 96.1% with mild side effect in the form FHMA and hypocalcaemia in about 35.6% Table (2). With correlation of osteoporosis score with other parameter, there was highly significant negatively correlated with age, percentage of FRAX score osteoporosis and BMI (10year probability of fractur), but positively significant correlation with risk hip fracture. Weight and BMI were positively significant correlated with age, BMI (10year probability of fractur) and risk hip fracture, but significant negatively correlated with percentage of FRAX score hip fracture. S Uric acid was positively significant correlated with age, percentage of FRAX score osteoporosis and FRAX score hip fracture, but significant negatively correlated with osteoporosis and S. vitamin D. S. Vitamin D was significant negatively correlated with percentage of FRAX score osteoporosis and FRAX score hip fracture and S. uric acid but significant positively correlated osteoporosis. Left femur neck T score was significant negatively correlated with age, S uric acid, S vitamin D, percentage of FRAX score osteoporosis and FRAX score hip fracture, but positively significant correlated with osteoporosis and risk hip fracture. Left femur neck Z score was significant negatively correlated with S uric acid and S vitamin D, but positively significant correlated with osteoporosis. Left forearm T score was significant negatively correlated with age, S uric acid, percentage of FRAX score osteoporosis and FRAX score hip fracture, but positively significant correlated with S vitamin D, osteoporosis, risk hip fracture and BMI (10year probability of fractur). Left forearm Z score was significant negatively correlated with S uric acid, percentage of FRAX score osteoporosis and FRAX score hip fracture, but positively significant correlated with osteoporosis, S vitamin D and BMI (10year probability of fractur). Lumbar T score was significant negatively correlated with age, S uric acid, percentage of FRAX score osteoporosis and FRAX score hip fracture, but positively significant correlated with S vitamin D and osteoporosis, risk hip fracture. Lumbar Z score was significant positively correlated with age, S vitamin D, percentage of FRAX score osteoporosis and FRAX score hip fracture, but negatively significant correlated with S uric acid and risk hip fracture. Table (3)
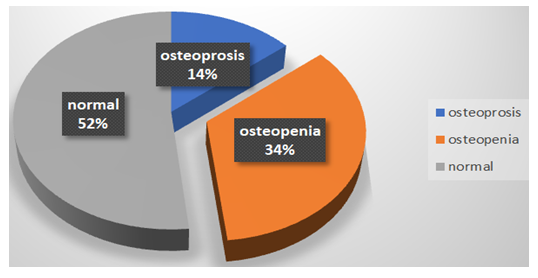
Figure 1: Percentage of osteoporosis among studied patients (n=180)
Table 1: Characteristics of studied patients (n=180):
|
Age |
Mean±SD |
180 |
46.0 ± 12.6 |
||
|
Hight |
Median (Q1-Q3) |
180 |
160 |
154 |
165 |
|
weight |
Median (Q1-Q3) |
180 |
85 |
75 |
95 |
|
BMI |
Median (Q1-Q3) |
175 |
33.7 |
29.4 |
37.8 |
|
duration/years |
Median (Q1-Q3) |
180 |
5 |
3 |
8 |
|
S Uric acid |
Median (Q1-Q3) |
180 |
6 |
5 |
7 |
|
S Vit D |
Median (Q1-Q3) |
180 |
26 |
13.3 |
32 |
|
Urinary Telopeptide |
Median (Q1-Q3) |
180 |
105 |
89 |
175 |
|
LT femur neck T score |
Median (Q1-Q3) |
180 |
-0.9 |
-1.5 |
-0.3 |
|
LT femur neck Z score |
Median (Q1-Q3) |
170 |
-0.6 |
-1.2 |
0 |
|
Lt forearm T score |
Median (Q1-Q3) |
174 |
-0.8 |
-1.7 |
-0.1 |
|
Lt forearm Z score |
Median (Q1-Q3) |
162 |
-0.5 |
-1.3 |
0.3 |
|
lumbar spine T score |
Mean±SD |
177 |
-1.1 ± 1.3 |
||
|
lumbar spine Z score |
Mean±SD |
175 |
-1.1 ± 1.3 |
||
|
Osteoporosis |
Median (Q1-Q3) |
180 |
2 |
1 |
2 |
|
FRAX score osteoporosis % |
Median (Q1-Q3) |
180 |
3.7 |
2.6 |
7.5 |
|
FRAX score hip fracture % |
Median (Q1-Q3) |
180 |
0.2 |
0.1 |
0.6 |
|
Risk hip fracture |
Median (Q1-Q3) |
180 |
1 |
1 |
1 |
|
BMI (10year probability of fracture) |
Mean±SD |
180 |
33.5 ± 6.6 |
||
Table 2: demographic data of studied patients (n=180):
|
n |
% |
||
|
Gender |
Female |
167 |
92.8 |
|
Male |
13 |
7.2 |
|
|
Rhematic disease |
RA |
103 |
57.2 |
|
SLE |
60 |
33.3 |
|
|
Behcet |
8 |
4.4 |
|
|
Scleroderma |
1 |
0.6 |
|
|
Sjogran syndrome |
3 |
1.7 |
|
|
Vascuilitis |
1 |
0.6 |
|
|
PSA |
4 |
2.2 |
|
|
Controlled |
Yes |
155 |
86.1 |
|
No |
25 |
13.9 |
|
|
Complications |
Yes |
59 |
32.8 |
|
NO |
121 |
67.2 |
|
|
Medications DMARDs |
Yes |
140 |
77.8 |
|
No |
40 |
22.2 |
|
|
Corticosteroid |
Yes |
161 |
89.4 |
|
No |
19 |
10.6 |
|
|
Type of osteoporosis treatment |
NO treatment |
15 |
8.3 |
|
Ca+Vit D |
67 |
37.2 |
|
|
Bisphosphonate |
85 |
47.2 |
|
|
Densumab |
9 |
5 |
|
|
Teripeptide |
4 |
2.2 |
|
|
Duration of osteoporosis treatment |
3 years |
14 |
7.8 |
|
2 years |
56 |
31.1 |
|
|
1 year |
104 |
57.8 |
|
|
Response to osteoporosis treatment |
Improved |
16 |
8.9 |
|
Stationary |
164 |
91.1 |
|
|
Worsen |
0 |
0 |
|
|
Side effects osteoporosis treatment |
FHMA |
6 |
3.3 |
|
pain site injection |
3 |
1.7 |
|
|
hypocalcaemia |
14 |
7.8 |
|
|
FHMA + hypocalcaemia |
64 |
35.6 |
|
|
hypocalcaemia + pain site injection |
9 |
5 |
|
|
No |
84 |
46.7 |
|
|
Major osteoporotic fracture |
Yes |
7 |
3.9 |
|
No |
173 |
96.1 |
|
Table 3: Correlation of osteoporosis scores: -
Spearman correlation
4. Discussion
Due to impact of autoimmune diseases and medication used in management especially corticosteroid, most of them had low bone density with different degree of fracture, early predication of low bone mineral density is the main preventive tool to prevent fracture in these vulnerable group [19]. Our study revealed that half of studied autoimmune diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Bechet, psoriatic arthritis (PSA), Scleroderma and Sjogren syndrome had low bone mass that in line with(Weitzmann, et al) [18] stated that rapid bone loss and increased fracture risk are implicated in a range of autoimmune diseases. The older age of studied autoimmune patients with average age of them was 46±12.6 years old that put them in high risk for osteoporosis and fracture as in agreement with (Marques A et al) who concluded that Age is a variable in all clinical risk calculators and with aging, fracture rates rise exponentially as bone density decreases. Added that fractures are less likely to occur in younger people than in older people, even with similar bone density measurements. Clinical data show that this paradox reflects age-dependent microarchitecture degradation [11]. It was noticed that S. vitamin D is low, median 6 (5-7)ng/ml that may a risk factor for low bone density in studied patients as mentioned by (Pietschmann, P et al 2016) that Osteoporotic hormones such as 1,25 dihydroxy vitamin D3 (1,25(OH)2 D3), parathyroid hormone (PTH) and prostaglandin E2 (PGE2) are responsible for upregulating expression of RANKL which represents the central process through which bone loss is regulated [13]. It was observed that usefulness of osteoporosis treatment in autoimmune studied patients with stationary course without risk of atypical fracture that in line to A 2019 meta-analysis [1] that Osteoporosis drugs produce a spectrum of changes in vertebral bone density, added that a strong correlation between improvements in BMD and greater reductions in rates of vertebral and hip fracture, reassuring practitioners of the usefulness of DXA to monitor treatment.
Assessment of bone density can be done by different tools; DEXA scan, FRAX score for fracture risk assessment each one has advantage and disadvantage [10]. DEXA scan in autoimmune studied patients detected that osteopenia in 34%, osteoporosis 14% while the median of FRAX score osteoporosis % was 3.7 (2.6-7.5) with high risk for osteoporosis that was in consistent with (Humes, D.H et al) stated that risk of osteoporosis increases significantly in autoimmune patients with each standard deviation below peak bone mass (or 1 unit decrease in T-score), it is reported a woman's risk of fracture approximately doubles [4]. Our studied autoimmune patients had high risk of fracture according to 10-year probability of major osteoporotic fracture and 10-year probability of hip fracture based on thresholds in the USA as described in the National Osteoporosis Foundation (NOF) Clinician’s Guide (Tosteson AN et al) [17] For clinical use, the International Osteoporosis Foundation proposed different markers that reflect bone metabolism or turnover, but these are not diagnostic tools for osteoporosis and are not a substitute for DXA analysis. ( Eastell R et al) [8]. The N-telopeptide is specific to bone due to its unique amino acid sequence. Bone density as measured by DXA provides a static snapshot of bones and does not distinguish if bone loss is ongoing or not (Jayaram N et al). But urinary N-telopeptide is a dynamic measurement of what is happening in bone at any given time [5] The median urinary Telopeptide was high in studied autoimmune patients 105 (89-175) which is important tool in the evaluation of quality of bone and a predicator for fracture as in agreement with (Singer FR et al) [15] stated that urinary N-telopeptide can be considered as a new diagnostic tool for diagnosing osteoporosis.
Conclusion
Although urinary N-telopeptide is a valuable marker for bone metabolism and predication of risk of fracture, it is not a substitute for DXA and FRAX score. So, If we suspect osteoporosis, it is better to go for urinary N-telopeptide and those who test positive can go for current gold standard DXA scan. Thus, combination of these two diagnostic tests could be useful to improve the identification of high risk for fracture.
Limitations of our analysis: include the cross-sectional nature of the data collected, to some extant small sample size, single Centre experience and lack of long-term follow-up to evaluate the incidence of fractures.
Declarations:
DOI (Declaration of conflict of interest):
Marwa A Besar, Youssef Abulatta, Mohamed Hussein and Mahmoud Abdelhadi have no conflict of interest.
Ethics approval: Ethical approval was taken by Mansoura Medical Ethics Research committee (MMERC) of faculty of Medicine, IRB Mansoura
Code number: R.22.10.1908 Date: 5/11/2022.
Human Ethics and Consent to participate: Ethical approval from the patients were taken to share their data and for publication.
Availability of data and materials: All data are confidential.
Competing interests:
Marwa A Besar, Youssef Abulatta, Mohamed Hussein and Mahmoud Abdelhadi have not competing interest
Funding: Marwa A Besar, Youssef Abulatta, Mohamed Hussein and Mahmoud Abdelhadi have no external funds.
Authors' contributions:
Main author; Corresponding author; (Marwa A Besar) Marwa Abo Elmaaty Besar; Lecturer of Internal medicine, Rheumatology& Immunology unit. Collected main data wrote, main paper and added supplementary data. besarmarwa@mans.edu.eg
Co-authors:
(Youssef Abulatta) Youssef Mohammed Metwally Abulatta: House officer at Mansoura University Hospital MMMP (Mansoura Manchester Medical program), help collected the data and approved the submitted final version. Yabuelatta@gmail.com
(Mohamed Hussein) Mohamed IssamHafez Hussein, House officer at Mansoura University Hospital MMMP (Mansoura Manchester Medical program), help collected the data and approved the submitted final version. m.essam.hafez977@gmail.com
(Mahmoud Abdelhadi) Mahmoud Ali Ali Abdelhadi: House officer at Mansoura University Hospital MMMP (Mansoura Manchester Medical program), help collected the data and approved the submitted final version. Mahmoud.abdulhady1998@gmail.com.
References
- Bouxsein ML, Eastell R, Lui LY, et al. FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta regression of published trials. J Bone Miner Res 34 (2019): 632-642.
- Choksi P, Clines GA, Jepsen KJ. The challenges of diagnosing osteoporosis and the limitations of currently available tools. Clin Diabetes Endocrinol 4 (2018): 12.
- Eastell R, Gossiel F, Naylor KE, et al. Diagnosis of endocrine disease: bone turnover markers: are they clinically useful? Eur J Endocrinol 178 (2018): 19-31.
- Humes DH. Osteoporosis. In: Humes, D.H. (Ed.), Kelley's Essentials of Internal Medicine. 2ed Lippincott Williams & Wilkins, Philadelphia, PA (2011).
- Jayaram N, Bijoor AR, Rajagopalan N, et al. The value of serumand urinary n-telopeptide in the diagnosis of osteoporosis. Indian J Orthop 36 (2002): 9-13.
- Kanis JA, Cooper C, Harvey NC, et al. A systematic review of intervention thresholds based on FRAX: a report prepared for theNational Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos 11 (2016): 25.
- Kleerekoper M, Edelson GW, et al. Biochemical studies in the evaluation and management of osteoporosis: current status and future prospects. Endocr Pract 2 (1996): 13-19.
- Leslie WD, Morin SN, Lix LM, et al. The diagnostic threshold for osteoporosis impedes fracture prevention in women at high risk for fracture: a registry-based cohort study. Bone 114 (2018): 298-303.
- Lindsay R, Cosman F, et al. Osteoporosis 25 (2014): 2359-2381.
- Meshcheryakova A, Ellinger I, Föger-Samwald U, et al. Harrison's Principles of Internal Medicine, 19e New York. McGraw-Hill, NY.
- Marques A, Carmona L, da Silva JA, et al. The accuracy of osteoporotic fracture risk prediction tools: a systematic review and meta-analysis. Ann Rheum Dis 74 (2015): 1958-1967.
- Martineau P, Leslie WD, Johansson H, et al. Clinical utility of using lumbar spine trabecular bone score to adjust fracture probability: the Manitoba BMD cohort. J Bone Miner Res 32 (2017): 1568-1574.
- Pietschmann P, Mechtcheriakova D. Immunology of osteoporosis: a mini-review. Gerontology 62 (2016): 128-137.
- Punda M, Grazio S et al. Bone densitometry–the gold standard for diagnosis of osteoporosis [in Croatian]. Reumatizam 61 (2014): 70-74.
- Singer FR, Eyre DR et al. Using biochemical markers of bone turnover in clinical practice. Cleve Clin J Med 75 (2008): 739-750.
- Siris ES, Barrett-Connor E, Miller PD, et al. Identifi cation and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 286 (2001): 2815-2822.
- Tosteson AN, Dawson-Hughes B, Melton LJ 3rd et al. Costeffective osteoporosis- treatment thresholds: the United States perspective. Osteoporos Int 19 (2008): 437-447.
- Weitzmann MN. T-cells and B-cells in osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes 21 (2014): 461-467.
- Xiao Y, de Groot DC, van Tol MJ, et al. Identification of the common origins of osteoclasts, macrophages, and dendritic cells in human hematopoiesis. Stem Cell Rep 4 (2015): 984-994.
