Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreen: Potential Impact on Cytokine Expression in Human Skin Pre- and Post-UVB Exposure
Article Information
Shaina Ailawadi╪, Raghav Talreja╪, Nicole Panstingel, Courtney E.W. Sulentic*
Department of Pharmacology and Toxicology, Boonshoft School of Medicine, Wright State University, Dayton, OH, USA
*Corresponding author: Courtney E.W. Sulentic, Department of Pharmacology and Toxicology, Boonshoft School of Medicine, Wright State University, Dayton, OH, USA
╪Equally contributing authors
Received: 15 November 2023 Accepted: 22 November 2023 Published: 14 December 2023
Citation: Shaina Ailawadi, Raghav Talreja, Nicole Panstingel, Courtney E.W. Sulentic. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreen: Potential Impact on Cytokine Expression in Human Skin Pre- and Post-UVB Exposure. Archives of Microbiology and Immunology. 7 (2023): 443-455
Share at FacebookAbstract
Background: Nanoparticles have been widely used in sunscreen products to prevent UVB-mediated skin damage. Research has shown that ZnO and TiO2 nanoparticles effectively scatter, reflect, and absorb light in the UV range. However, little is known regarding the impact of nanoparticle and UVB exposure on cytokine expression. This study investigates the influence of ZnO and TiO2 nanoparticles on the expression of pro- and anti-inflammatory cytokines in human skin exposed to UVB radiation.
Methods: De-identified, discarded skin from three abdominoplasty surgeries were exposed to UVB with or without the application of ZnO or TiO2 nanoparticles. Samples were analyzed using a BioRad Bio-Plex Pro Human Cytokine 27-plex Assay to determine cytokine levels of various pro- and anti-inflammatory cytokines.
Results: UVB exposure or application of ZnO or TiO2 nanoparticles had very little effect on cytokine levels compared to the no treatment control when evaluated 24 hrs after exposure. However, application of TiO2 following UVB exposure resulted in increased cytokine levels for nearly all the cytokines evaluated. This effect was absent when a combination of ZnO and TiO2 nanoparticles were applied. Interestingly, pre-, and post-UVB application of ZnO or a combination of ZnO and TiO2 nanoparticles decreased IL-6 levels or IL-6 and IL-8 levels, respectively.
Discussion: These results suggest a potential for nanoparticle sunscreen to enhance or reduce the inflammatory response in skin depending on conditions of UVB exposure and the nanoparticle composition and how it is applied. Further studies to evaluate the safety and efficacy of using nanoparticle sunscreens are warranted.
Keywords
Titanium dioxide, Zinc oxide, Nanoparticle sunscreen, UVB, Cytokines, Human Skin
Titanium dioxide articles, Zinc oxide articles, Nanoparticle sunscreen articles, UVB articles, Cytokines articles, Human Skin articles
Article Details
1. Introduction
Ultraviolet radiation is an invisible component of the light spectrum and one of the most common environmental exposures to humans. From this spectrum, UVA (320-400nm) and UVB (280-320nm) are primarily responsible for carcinogenic damage of the skin [1]. UV induces skin vasodilation, erythema, epidermal hyperplasia, and inflammation [2]. These pathophysiologic effects have been linked to the release of angiogenic and inflammatory cytokines, neutrophil and macrophage migration, the generation of reactive oxygen and nitrogen species, and the formation of pyrimidine dimers and DNA strand breaks [3, 4]. Sunburn injury can result in acute damage to tissue, which is predominantly mediated by inflammatory cytokine production and p53-mediated keratinocyte apoptosis [5, 6]. Chronic, cyclic exposure to UV radiation causes a cascade of DNA damage leading to dysplasia and eventually metaplasia [7]. Additionally, the production of proinflammatory cytokines from UVB exposure can cause downstream effects on several disease loci, which have also been shown to have an important role for dysregulation of epidermal barrier genes involved in forming tight junctions and desmosomes within the skin [8, 9].
To prevent UV skin damage, organic and inorganic sunscreen products have been developed with mixed results in cancer protection. Squamous cell carcinoma incidence rates declined 40% when patients applied sunscreen daily, but use of sunscreen was not associated with a decreased incidence rate of basal cell carcinoma [10]. In other studies, sunscreen decreased the incidence rate by 22% to 36% of actinic keratoses, which are premalignant lesions that can predispose patients to squamous cell carcinoma or basal cell carcinoma [11, 12]. There remains no consensus on the potential risk reduction by sunscreen usage and the most dangerous skin cancer, melanoma [13, 14]. Primary research on cancer protection was largely done using organic sunscreens, and there remains a lack of randomized controlled trials that assess the efficacy of nanoparticle-based sunscreen products in cancer prevention. Nonetheless, health agencies worldwide recommend regular use of sunscreen [15]. Additionally, guidelines are predominantly based on sunscreen protection factor (SPF) rating, which is a measure of solar energy required to produce sunburn on protected skin compared to energy required to burn unprotected skin. How this translates to protection against skin cancer as discussed above or oxidative stress and inflammation is unclear [16].
Sunscreens protect by reflecting, scattering, and absorbing light, or using antioxidants and osmolytes that limit the cascade of UV-induced cellular damage [17]. The efficacy of inorganic sunscreens depends on reflective properties including the reflective index, size of the particles, application thickness and dispersion index. Older inorganic sunscreen products formed a cosmetically unappealing white film on the skin; thus, newer formulations have been developed in the nanoparticle size range that have addressed this cosmetic shortcoming [18]. Two commonly used nanoparticles for sunscreen include titanium dioxide (TiO2) and zinc oxide (ZnO) [19]. Nanoparticles are defined as materials that range in size from 1 to 100 nm. Nanoparticles possess unique physical and chemical properties due to their small size and high surface area, which allows for a wide range of characteristics suitable for use in commercial, medical, and environmental applications [20, 21].
As mentioned above, the efficacy of nanoparticle sunscreen in providing protection against cancer has not been established through randomized controlled trials. However, nanoparticle sunscreen products have been approved for use by the Food and Drug Administration. The risks and benefits of nanoparticle-containing products remain a concern for consumers [22, 23]. Collectively, studies suggest minimal to no absorption of TiO2 or ZnO across dermal barriers and support their general safety when dermally applied (reviewed by [22-27]). In contrast, inhalation of ZnO has been shown to induce acute lung toxicity in animal models and metal fume fever in occupational settings [28, 29]. Additionally, few studies have examined the effect of TiO2 or ZnO before or after exposure to UV on skin inflammatory markers. Therefore, we conducted a pilot study to directly assess the impact of TiO2 and ZnO on cytokine levels both prior to and following exposure to UVB using a human skin explant model with the overall goals of determining 1) the feasibility of assessing an acute inflammatory response using a human skin explant model and 2) whether further studies were warranted to evaluate different TiO2 and ZnO-containing sunscreen formulations and exposure scenarios on skin inflammation using a larger and more diverse (i.e. skin types) study cohort.
Our study demonstrated a striking increase in cytokine levels for nearly all of the cytokines assessed in a multiplex cytokine panel when TiO2 was applied after UVB exposure. This increase in cytokine level was not observed with combined application of TiO2 and ZnO or when TiO2 was also applied prior to UVB exposure. These results suggest the potential for a direct inflammatory response of the skin to UVB and sunscreen containing TiO2 depending on the sunscreen formulation and timing of application. Since sunscreens typically contain at least two organic active ingredients to offer broad protection [19], further studies are necessary to determine if current formulations of TiO2-containing sunscreens produce a similar effect on cytokine production when applied after UVB exposure and if altering the composition could mitigate this effect and if certain skin types or more or less sensitive.
2. Materials and Methods
2.1 Nanoparticle Characterization and Usage
The TiO2 (40 nm) and ZnO (85 nm) nanoparticles were purchased from nanaComposix (San Diego, CA) and previously characterized [30-32]. Nanoparticles were resuspended in DMEM media (0.25 mg/ml) and 1 ml applied evenly to the surface of an approximately 5 cm X 5 cm section of the skin explant for a final treatment of 0.25 mg or 0.01 mg/cm2 of TiO2 or ZnO.
2.2 Explant Skin Preparation, Treatments, and Culture Conditions
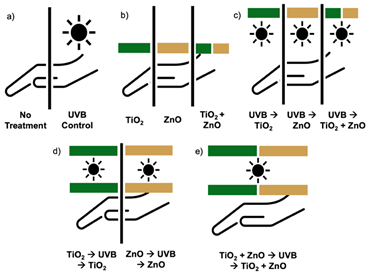
Three full-thickness human skin explants were obtained via medical waste post-surgically (abdominoplasty surgeries in Dayton, OH) and immediately used for experiments. Skin samples were received de-identified with only sex and age recorded. Based on visual assessment using the Fitzpatrick Scale, two samples were from patients with Type II or fair complexion, and one was a patient of color with Type IV or light brown, olive complexion (indicated by the maroon data points in Figs. 2-4). All patients were female, ranging in age from 32 to 60. The subcutaneous adipose was removed and the skin was sectioned into approximately 5 cm X 5 cm pieces and placed into a 100 mm sterile petri dish with 6 mL of DMEM media containing penicillin and streptomycin. Each skin sample (N=3) was treated as follows: 1) untreated, naïve control, 2) UVB irradiated for 3 minutes and 20 seconds with a lighting source that provides 5 J/m2/sec at a distance of 6 cm from the sample for a total UVB dose of 1000 J/m2, 3) 0.25 mg (0.01 mg/cm2) of TiO2 or ZnO nanoparticles applied alone or in combination, 4) UVB exposure then subsequent application of nanoparticles (TiO2 or ZnO or in combination), or 5) application of nanoparticles (TiO2 or ZnO or in combination) pre- and post-UVB (Fig. 1). Once treated, samples were incubated for 1 hr or 24 hrs in a 37ºC water bath, ensuring the water did not contact the skin samples.
2.3 Sample Collection and Storage
After the incubation period, approximately 6 mm-sized samples were cut in triplicate from the exposed 5 cm X 5 cm skin explant and placed into tared 1.5 mL Eppendorf tubes. The samples were flash-frozen using liquid nitrogen and then stored in a freezer at -80ºC. An additional sample was collected for histological processing. The sample was placed in 10% formalin buffered solution for approximately 2 hours after which the FBS was removed, and 70% ethanol was added. The samples remained in 70% ethanol until they were embedded in paraffin. Embedded tissues were sectioned and stained with hematoxylin and eosin by AML Laboratories (St. Augustine, FL).
2.4 Multiplex Cytokine Assay
To prepare skin samples for analysis, surgical scissors and a motorized pestle were used to homogenize the full-thickness sample with 1X PBS containing fresh protease inhibitor (Complete Mini Protease Inhibitor Cocktail, Millipore Sigma, Burlington, MA). Once homogenized, samples were centrifuged for approximately 10 seconds to remove any large debris and were transferred to a new 1.5 mL Eppendorf tube. A Bradford assay (BioRad, Hercules, CA) was utilized to quantify the amount of protein present in each sample. Using a Bio-Plex Pro Human Cytokine 27-plex Assay (BioRad), the following cytokines were assayed simultaneously: IL-1β, IL-1RA, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17, FGF basic, Eotaxin, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α and VEGF.
2.5 Statistical Analysis
Cytokine concentration was normalized to total protein concentration and then calculated as the fold-change (mean ± standard deviation; N=3) relative to either the 24-hr naïve control or the 24-hr UVB control set to 1. High out of range values were extrapolated based on the highest standard that fit the standard curve. As indicated in the figure legends, statistical significance was determined by repeated ANOVA measures after performing a natural log transformation to justify the constant variance and normality assumptions. Treatments were then compared to the natural log of 1 (i.e. 0), which represents the naïve or UVB control to which the samples were normalized. The Bonferroni stepdown multiple comparison procedure was used to adjust the p-values to account for the multiple comparisons.
3. Results
ZnO and TiO2 nanoparticles are used in sunscreens but there are limited studies evaluating the effect of UV and nanoparticles on cytokine levels in the skin under different consumer application scenarios, such as applying sunscreen after sun exposure. This study evaluated the acute effects of UVB and sunscreen-associated nanoparticles on cytokine levels in human skin explants 24 hrs after different exposure scenarios (Fig. 1). We utilized commercially available 40 nm TiO2 and 85 nm ZnO, which was previously characterized as having an average particle diameter of 42.3 nm and 71 nm, respectively [32]. The particle diameter increased to 1307 ± 313.7 nm for TiO2 and 188.9 ± 37.2 nm for ZnO in media [32].
Figure 1: Experimental exposure conditions of human skin to UVB and sunscreen-relevant nanoparticles TiO2 and ZnO. Human skin from three separate donors was either a) left untreated or exposed to 1000 J/m 2 UVB, b) treated with 0.25 mg of 40 nm TiO 2 or 85 nm ZnO or a combination of TiO2 and ZnO, c) exposed to UVB then treated with TiO2 or ZnO or a combination of TiO2 and ZnO, d) treated with TiO2 or ZnO before and after UVB exposure, or e) treated with a combination of TiO2 and ZnO before and after exposure to UVB. Skin samples were processed one hr or 24 hrs following exposure.
3.1 Limited effect of UVB or nanoparticle application on cytokine expression
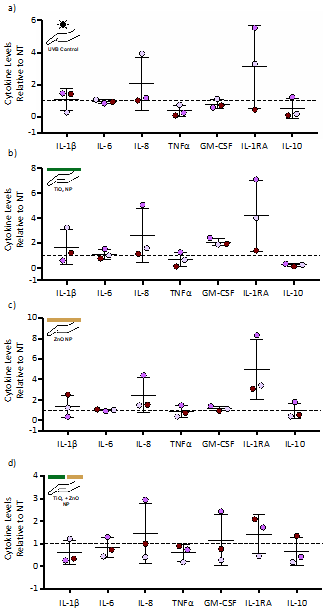
Cytokine levels 24 hours after exposure to UVB alone or nanoparticles alone exhibited very little change for the 27 cytokines analyzed compared to the no treatment control (Table 1 and representative cytokines in Fig. 2). Although not significantly different, the anti-inflammatory cytokine IL-1RA trended towards an increase in two of the three donor skin explants under all treatment scenarios (Fig. 2). TiO2 exposure alone also trended toward an increase in GM-CSF and a decrease in IL-10 (Fig. 2b).
Figure 2: Cytokine levels following exposure to UVB or application of TiO2 or ZnO nanoparticles. Human skin from three separate donors was exposed to a total of 1000 J/m2 of UVB light (a) or 0.25 mg of TiO2 (b) or ZnO (c) or a combination of TiO2 and ZnO (d). Skin samples were processed 24 hrs following exposure and analyzed for cytokine levels via a multiplex cytokine assay. Seven of the 27 cytokines are shown (see Table 1 for all cytokine values). Cytokine levels are expressed as fold change (mean ± SD) relative to the no treatment (NT) control, which was set to 1 and represented by the dashed line. Statistical differences compared to the NT control was determined by a repeated measures ANOVA with a Bonferroni stepdown multiple comparison procedure. There was no statistical significance.
Table 1: Cytokine levels in human skin under different UVB and nanoparticle exposure scenarios
Cytokines were evaluated in skin explants from three patients using a BioRad Bio-Plex Pro Human Cytokine 27-plex Assay at 1 hr (T1) or 24 hrs (T24) following treatment with 0.25 mg of TiO2 or ZnO pre- or post-UVB exposure at 1000 J/m2. T1 NT represents the no treatment or background cytokine levels (mean pg/ml ± standard deviation) at ~1 hr after the initiation of experiments. T24 NT relative to T1 NT represents the fold change of cytokine levels for the 24 hr no treatment group compared to the T1 NT. UVB relative to T24 NT represents the fold change in cytokine levels at 24 hr following UVB exposure compared to the T24 NT. Cytokine levels at 24 hrs following treatment with TiO2 or ZnO nanoparticles alone or in different combinations as described in figure 1 is represented as fold change relative to T24 UVB.
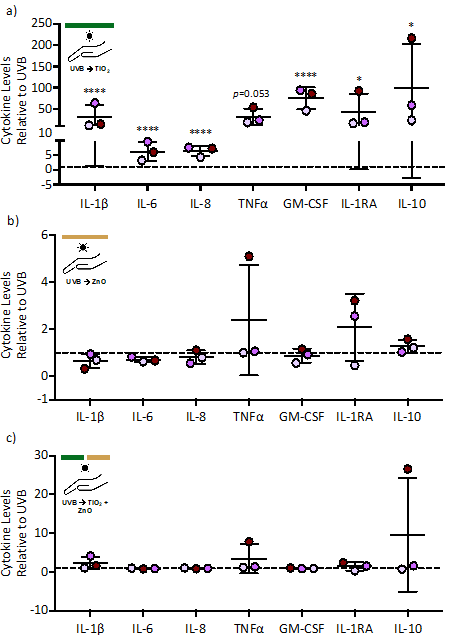
Figure 3: Cytokine levels following ZnO or TiO2 nanoparticle application post-UVB exposure. Human skin from three separate donors was exposed to a total of 1000 J/m2 of UVB light followed by treatment with 0.25 mg of TiO2 (a) or ZnO (b) or a combination of TiO2 and ZnO (c). Skin samples were processed 24 hrs following exposure and analyzed for cytokine levels via a multiplex cytokine assay. Seven of the 27 cytokines are shown (see Table 1 for all cytokine values). Cytokine levels are expressed as fold change (mean ± SD) relative to the UVB exposure group, which was set to 1 and represented by the dashed line. Statistical differences compared to the UVB was determined by a repeated measures ANOVA with a Bonferroni stepdown multiple comparison procedure. * and **** denote significance at p<0.05 and p<0.0001, respectively.
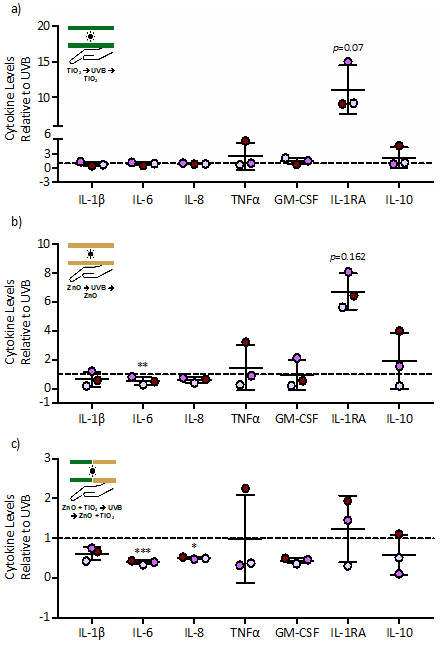
Figure 4: Cytokine levels following a pre-UVB and post-UVB application of TiO2 or ZnO nanoparticle. Human skin from three separate donors were treated with 0.25 mg of TiO2 (a) or ZnO (b) or a combination of TiO2 and ZnO (c) before and after exposure to a total of 1000 J/m2 of UVB light. Skin samples were processed 24 hrs following exposure and analyzed for cytokine levels via a multiplex cytokine assay. Seven of the 27 cytokines are shown (see Table 1 for all cytokine values). Cytokine levels are expressed as fold change (mean ± SD) relative to the UVB exposure group, which was set to 1 as represented by the dashed line. Statistical differences compared to the UVB was determined by a repeated measures ANOVA with a Bonferroni stepdown multiple comparison procedure. *, **, and *** denote p<0.05, p<0.01 and p<0.001, respectively.
3.2 Application of TiO2 after UVB exposure significantly increased cytokine levels
To simulate a probable real case scenario of applying sunscreen after sun exposure, TiO2 and/or ZnO were applied to the skin explants after exposure to UVB. TiO2 application after UVB exposure increased, to varying degrees, the level of all cytokines evaluated compared to the UVB control (Table 1 and representative cytokines in Fig. 3a). Application of ZnO after UVB exposure had a minimal effect on cytokine levels (Fig. 3b). Interestingly, the increase in cytokines induced by TiO2 was abrogated when ZnO was applied with TiO2 after UVB exposure (Fig. 3c).
3.3 Application of ZnO or a combination of ZnO and TiO2 pre- and post-UVB exposure may decrease inflammatory cytokine levels
To simulate applying sunscreen before and after sun exposure, TiO2 and/or ZnO were applied to the skin explants prior to and after UVB exposure. Except for a trend towards increased IL-1RA, application of TiO2 pre- and post-UVB exposure had little effect on cytokine levels compared to the UVB alone control (Fig. 4a). When ZnO was applied pre- and post-UVB exposure, IL-6 was significantly decreased and IL-1RA trended towards an increase compared to the UVB alone control (Fig. 4b). A combined application of TiO2 and ZnO pre- and post-UVB exposure resulted in a greater decrease in IL-6 and IL-8 but also diminished the induction of IL-1RA as compared to a pre- and post-UVB application of ZnO alone.
3.4 Histological analysis of skin exposed to UVB or nanoparticles
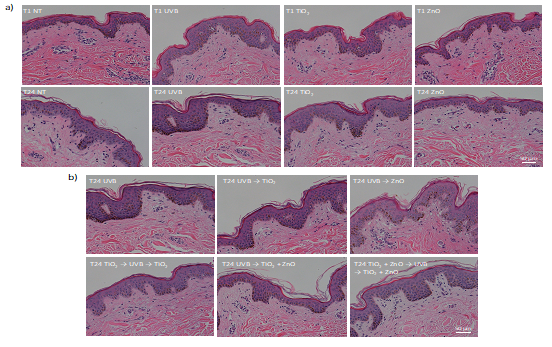
To assess the sample quality and the effects of UVB and sunscreen-associated nanoparticles on skin morphology, tissue samples were also collected for hematoxylin and eosin staining at 1 hr and 24 hrs after treatment. None of the treatment conditions induced gross morphological changes compared to the no treatment control. There was also no indication of pyknotic nuclei in the keratinocytes suggesting that the treatments did not induce cell death (Fig. 5).
Figure 5: Skin histology following different UVB and nanoparticle exposure scenarios. Human skin from three separate donors were treated as indicated on the images then embedded in paraffin at 1 hr (T1) or 24 hrs (T24) after treatment. Tissues were sectioned and stained with hematoxylin and eosin. Tissue sections from the mixed-race donor are shown and are representative of all three donors except for having more visible melanin. UVB and nanoparticle exposures are indicated with (a) showing 1 hr and 24 hrs after exposure to only 1000 J/m2 of UVB or application of 0.25 mg TiO2 or ZnO and (b) showing 24 hrs after combined UVB and nanoparticle treatment. The no treatment controls at 1 hr and 24 hrs are also represented.
4. Discussion
TiO2 and ZnO nanoparticles are commonly used in sunscreen products; however, there remains a concern regarding their safety. The aim of this study was to evaluate in human skin the effects of TiO2 and ZnO nanoparticles on a panel of cytokines under different UVB exposure scenarios. Although previous studies have assessed these nanoparticles, those relevant to dermal exposure and human models have primarily focused on penetration through the skin or in vitro toxicity studies using keratinocytes; few studies have evaluated cytokine production (reviewed by [22-27]). The current study utilized human skin explants, which allows for an assessment of the dermal cytokine reaction in the absence of infiltrating white blood cells.
Inflammatory cytokines play an important role in the body’s first line innate response to foreign bodies and environmental insults by regulating and coordinating the functions of immune cells. Keratinocytes have been shown to secrete a number of cytokines including IL-1β, IL-6, IL-8, GM-CSF, and TNFa. Cytokine expression can be induced and modulated by both intrinsic and extrinsic factors [33-37]. Our study evaluated 27 human cytokines using a multiplex cytokine panel and similar to previous studies using skin wound models or UV exposure, we observed detectable basal levels for all cytokines measured [33, 36, 38]. Compared to the no treatment control, cytokine levels were not significantly increased at 24 hr following a UVB exposure of 1000 J/m2 (10 J/cm2). Other studies using human volunteers and skin biopsies demonstrated increased cytokine expression and levels at 24 hr following approximately 4.2 to 16.8 J/cm2 of solar simulated UV radiation, which includes both UVA and UVB radiation [39, 40]. However, Barr et al [39] identified the maximal increase in IL-1b, TNFa, and IL-10 protein levels following skin exposure to solar simulated UV radiation at 15 hrs with a reduction at 24 hrs. Similar to our results showing an increase in IL-1RA at 24 hr in two of the three donor explants, solar simulated UV radiation increased IL-1RA in the majority of the biopsies by 15 hr, which was sustained through 72 hrs [39]. In vitro studies using human keratinocytes demonstrated an increase in cytokine expression and secretion at 24 hr following 20 to 1000 mJ/cm2 UVB exposure [38, 41-43]. Compared to these studies, our skin explants demonstrated a minimal cytokine response following UVB exposure. This could be due to several reasons including the use of only UVB and not solar simulated UV radiation. Solar simulated UV radiation has both UVA and UVB and has deeper penetration into the epidermis because of the longer UVA wavelengths [39, 40, 44]. Additionally, unlike skin biopsies, our explant model lacks infiltrating lymphocytes, which would likely lead to lower overall cytokine levels, but does allow for an acute assessment of the dermal response.
The aim of the current study was to directly assess the dermal response to the sunscreen-relevant nanoparticles ZnO and TiO2 in the context of pre- and post-exposure to UVB. Strikingly, nearly all the cytokines assessed were increased when TiO2 nanoparticles were applied after UVB exposure, simulating a realistic use case of applying sunscreen after already being exposed to the sun. This increase in cytokine levels was not observed with post-UVB exposure of ZnO or the combined application of TiO2 and ZnO suggesting that ZnO is more bioinert or induces a response that resolves within 24 hrs as compared to TiO2. Additionally, ZnO may provide a protective effect against the induction of cytokines induced by TiO2 after UVB exposure. Previous in vitro and in vivo studies have demonstrated the potential for TiO2 and ZnO nanoparticles to induce oxidative stress, inflammation, and cytotoxicity [25, 45-48]. However, when applied dermally, the majority of studies support their general safety and limited dermal absorption; none have specifically evaluated cytokine levels when nanoparticles are applied post-UVB [16, 22-27].
Using an in vitro porcine model, Monteiro-Riviere et al [49] identified no dermal penetration of ZnO but TiO2 penetrated seven layers, which was enhanced in UV-damaged skin. In human subjects, TiO2 has been identified in the epidermis and dermis of human subjects who applied TiO2 containing sunscreen for 2-6 weeks prior to skin surgery [50]. Additionally, the possibility of some systemic absorption of TiO2 and ZnO from dermal application of sunscreen has been suggested through human studies and evaluation of blood and urine [16, 51]. Although minimal, these studies do support some dermal penetration of TiO2 that may be increased with prior UV exposure corresponding to the increase in cytokines seen in the current study. Furthermore, opposite of the protective effect we observed with a combination of ZnO with TiO2 after UVB exposure, a previous study demonstrated a protective effect of TiO2 on ZnO-induced toxicity by binding free Zn2+ ions [52]. An additional consideration is the potential for inhalation toxicity if spray sunscreens contain nanoparticles. ZnO has been shown to induce acute lung toxicity in animal models and metal fume fever in occupational settings [28, 29]. TiO2 has also been associated with abnormal pulmonary function when inhaled [53, 54]. Additionally, it is important to consider the potential environmental impact of nanoparticles in sunscreens as they wash off into the environment through regular use. Previous studies suggest a detrimental impact of engineered nanoparticles environmental processes and organisms [55, 56]. For example, TiO2 and ZnO nanoparticles have been found to reduce microbial biomass and diversity in the environment by changing the composition of the soil bacterial community [55]. Plants also appear to be sensitive to engineered nanomaterials including TiO2 and ZnO nanoparticles and exhibit many adverse effects [56].
It is noteworthy that the concentrations of TiO2 and ZnO used in the current study (i.e. 0.25 mg or 0.01 mg/cm2) are much lower than the concentration of these nanoparticles in sunscreen when approximated to potential dose per surface area. The maximum allowable nanoparticle concentration in sunscreen is less than 25% [57]; however, Bocca et al [58] analyzed four different sunscreens and identified between 2.6% to 18.3% TiO2 and 0.05% to 0.22% ZnO. Based on these concentrations and the recommendation of the American Academy of Dermatology to use 1 oz (28.3 g) of sunscreen to adequately cover the adult body, the potential exposure to TiO2 and ZnO would be 4.6 to 43.2 mg/cm2 and 88 to 519 mg/cm2, respectively, assuming coverage of 80% of the body and a body surface area of 1.5 to 2 m2 [59]. Therefore, the marked increase in cytokines when TiO2 is applied after UVB occurs at concentrations estimated to be more than ~400 fold lower than that found in some sunscreens.
Overall, our results suggest the potential for a direct inflammatory response of the skin to UVB and sunscreen containing TiO2 depending on the sunscreen formulation and timing of application. Since sunscreens typically contain at least two organic active ingredients to offer broad protection [19] further studies are necessary to determine if current formulations of TiO2-containing sunscreens produce a similar effect on cytokine production when applied after UVB exposure and if altering the composition could mitigate this effect, such as a combined TiO2 and ZnO formulation. A combination of TiO2 and ZnO applied after UVB did not show an increase in cytokine levels as seen with application of TiO2 alone. Additionally, a combined application of TiO2 and ZnO pre- and post-UVB exposure decreased the level of some cytokines, potentially protecting against an inflammatory response.
5. Limitations of the study
The small sample size (N=3) and lack of diversity in sex and types of skin (i.e. types I – VI) is a clear limitation of this study. However, considering that the exposures were conducted when the skin was received, which was approximately two weeks apart for each donor, the remarkable consistency in the cytokine response to TiO2 post-UVB exposure supports a biologically relevant response. An additional consideration is a potential inflammatory response induced by the excision of the skin and the further cutting required to prepare the samples for the different treatment groups. In comparison to previous studies evaluating cytokine levels associated with dermal wounds and wound age, our cytokine levels at 1 hr are consistent with a response to a dermal wound, i.e. surgical excision [33, 60], and thus suggests a higher basal cytokine level than might be found in intact skin. A higher basal cytokine profile could impact the response to test treatments, highlighting the importance of time-matched no treatment controls. A final consideration is the nanoparticle formulation. The current study used nanoparticles suspended in media, which was previously shown to increase the mean diameter (i.e. 1307 ± 313.7 nm for 40 nm TiO2 and 188.9 ± 37.2 nm for 85 nm ZnO [32]). Whereas previous analysis of TiO2 and ZnO in four commercially available sunscreens identified a smaller particle mean diameter for both TiO2 (89 ± 18 to 107 ± 14) and ZnO (74 ± 8 to 98 ± 22), which is likely due to the nanoparticle protein corona formed by the more hydrophobic composition of the sunscreen [58]. Future experiments are needed to evaluate the effect of post-UVB application of commercially available TiO2 containing sunscreen on cytokine levels.
6. Conclusion
Despite the limitations noted above, the current study suggests the potential for a direct inflammatory response of the skin to UVB and sunscreen containing TiO2 depending on the sunscreen formulation and timing of application. Further studies are necessary to determine 1) if current formulations of TiO2-containing sunscreens, which we estimate to have a higher surface area TiO2 concentration than that used in this study, produce a similar effect on cytokine production when applied after UVB exposure, 2) if this effect is specific to certain skin types or sex-dependent, and 3) if altering the composition could mitigate this effect.
Acknowledgements
This work was partly funded by Battelle Memorial Institute through a student research award from the Dermal Toxicology Specialty Section of the Society of Toxicology (N.P.) and the Department of Pharmacology and Toxicology at Wright State University (N.P. and C.E.W.S). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations acknowledged above. The authors would like to acknowledge Dr. Saber Hussain (Wright Patterson Air Force Base) for providing and characterizing the nanoparticles used in this project, Dr. Richard Simman (Boonshoft School of Medicine, Dayton, OH) for providing human skin explants, Christine Rapp and Clayton Allex-Buckner for technical support, Dr. Jeffrey Travers (Boonshoft School of Medicine, Dayton, OH) for supplying the UVB exposure, the Proteomic Analysis Laboratory directed by Dr. David Cool (Boonshoft School of Medicine) for running the Bio-Plex assay, and Michael Bottomley (Wright State University) for statistical support.
Author Contributions
RT analyzed the results, drafted and critically revised the manuscript for important intellectual content.
SA analyzed the results, drafted and critically revised the manuscript for important intellectual content.
NP conceived and designed the study and acquired the data.
CS helped conceive and design the study, acquire the data, and critically revise the manuscript for important intellectual content.
Conflicts of Interest
The authors declare they have no competing interests.
Ethics approval and consent to participate
This study was exempted by the Wright State University Institutional Review Board due to using de-identified human skin discarded from abdominoplasty surgeries at Sycamore Hospital, Dayton, Ohio.
Availability of data and materials
The Bio-Plex assay data will be made available on request. Send requests to Courtney Sulentic at Courtney.sulentic@wright.edu.
References
- Romanhole RC. Update on ultraviolet A and B radiation generated by the sun and artificial lamps and their effects on skin. International Journal of Cosmetic Science 37 (2015): 366-370.
- Pal HC, Athar M, Elmets CA, Afaq F. Fisetin inhibits UVB-induced cutaneous inflammation and activation of PI3K/AKT/NFκB signaling pathways in SKH-1 hairless mice. Photochem Photobiol 2015 91 (2015): 225-234.
- Shih B, Farrar M, Cooke M, Osman J, Langton A. Fractional Sunburn Threshold UVR Doses Generate Equivalent Vitamin D and DNA Damage in Skin Types I–VI but with Epidermal DNA Damage Gradient Correlated to Skin Darkness. The Journal of Investigative Dermatology 138 (2018): 2244-2252.
- Arora S, Tyagi N, Bhardwaj A, et al. Silver nanoparticles protect human keratinocytes against UVB radiation-induced DNA damage and apoptosis: potential for prevention of skin carcinogenesis. Nanomedicine: Nanotechnology, Biology and Medicine 11 (2015): 1265-1275.
- Maddodi N, Jayanthy A, Setaluri V. Shining light on skin pigmentation: the darker and the brighter side of effects of UV radiation. Photochem Photobiol. Sep-Oct 88 (2012): 1075-82.
- Sheehan J, Young A. The sunburn cell revisited: an update on mechanistic aspects. Photochem Photobiol Sci 365 (2002): 365-377.
- Murphy G, Young AR, Wulf HC, Kulms D, Schwarz T. The molecular determinants of sunburn cell formation. Experimental Dermatology 10 (2001): 155-160.
- Yuki T, Hachiya A, Kusaka A, et al. Characterization of tight junctions and their disruption by UVB in human epidermis and cultured keratinocytes. J Invest Dermatol 131 (2011): 744-52.
- Brandner JM, Zorn-Kruppa M, Yoshida T, Moll I, Beck LA, De Benedetto A. Epidermal tight junctions in health and disease. Tissue Barriers 3 (2015): e974451.
- Green A, Williams G, Nèale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. The Lancet 354 (1999): 723-729.
- Darlington S, Williams G, Neale R, Frost C, Green A. A Randomized Controlled Trial to Assess Sunscreen Application and Beta Carotene Supplementation in the Prevention of Solar Keratoses. Archives of Dermatology 139 (2003): 451-455.
- Naylor MF, Boyd A, Smith DW, Cameron GS, Hubbard D, Neldner KH. High Sun Protection Factor Sunscreens in the Suppression of Actinic Neoplasia. Archives of Dermatology 131 (1995): 170-175.
- Mulliken JS, Russak JE, Rigel DS. The Effect of Sunscreen on Melanoma Risk. Dermatologic Clinics 30 (2012): 369-376.
- Silva ESD, Tavares R, Paulitsch FDS, Zhang L. Use of sunscreen and risk of melanoma and non-melanoma skin cancer: a systematic review and meta-analysis. Eur J Dermatol 28 (2018): 186-201.
- Tips to Stay Safe in the Sun: From Sunscreen to Sunglasses US. Food and Drug Administration.
- Pelclova D, Navratil T, Kacerova T, et al. NanoTiO(2) Sunscreen Does Not Prevent Systemic Oxidative Stress Caused by UV Radiation and a Minor Amount of NanoTiO(2) is Absorbed in Humans. Nanomaterials (Basel) 9 (2019).
- Mancebo SE, Hu JY, Wang SQ. Sunscreens: a review of health benefits, regulations, and controversies. Dermatol Clin 32 (2014): 427-438.
- Rai R, Shanmuga SC, Srinivas C. Update on photoprotection. Indian J Dermatol 57 (2012): 335-342.
- Serpone N. Sunscreens and their usefulness: have we made any progress in the last two decades? Photochem Photobiol Sci 20 (2021): 189-244.
- Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arabian Journal of Chemistry 12 (2019): 908-931.
- Santos AC, Marto J, Chá-Chá R, et al. Nanotechnology-based sunscreens—a review. Materials Today Chemistry 23 (2022): 100709.
- Schilling K, Bradford B, Castelli D, et al. Human safety review of "nano" titanium dioxide and zinc oxide. Photochem Photobiol Sci 9 (2010): 495-509.
- Robertson TA, Sanchez WY, Roberts MS. Are commercially available nanoparticles safe when applied to the skin? J Biomed Nanotechnol 6 (2010): 452-468.
- Vujovic M, Kostic E. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: A Review of Toxicological Data. J Cosmet Sci 70 (2019): 223-234.
- Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 10 (2013): 15.
- Gulson B, McCall MJ, Bowman DM, Pinheiro T. A review of critical factors for assessing the dermal absorption of metal oxide nanoparticles from sunscreens applied to humans, and a research strategy to address current deficiencies. Arch Toxicol. Nov 89 (2015): 1909-30.
- Newman MD, Stotland M, Ellis JI. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J Am Acad Dermatol 61 (2009): 685-92.
- Greenberg MI, Vearrier D. Metal fume fever and polymer fume fever. Clin Toxicol (Phila) 53 (2015): 195-203.
- Larsen ST, Da Silva E, Hansen JS, Jensen ACØ, Koponen IK, Sørli JB. Acute Inhalation Toxicity After Inhalation of ZnO Nanoparticles: Lung Surfactant Function Inhibition In Vitro Correlates with Reduced Tidal Volume in Mice. Int J Toxicol 39 (2020): 321-327.
- Tilly TB, Nelson MT, Chakravarthy KB, et al. In Vitro Aerosol Exposure to Nanomaterials: From Laboratory to Environmental Field Toxicity Testing. Chem Res Toxicol 33 (2020): 1179-1194.
- Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicology in Vitro 19 (2005): 975-983.
- Gajewicz A, Schaeublin N, Rasulev B, et al. Towards understanding mechanisms governing cytotoxicity of metal oxides nanoparticles: Hints from nano-QSAR studies. null 9 (2015): 313-325.
- Peyron P-A, Colomb S, Becas D, et al. Cytokines as new biomarkers of skin wound vitality. International Journal of Legal Medicine 135 (2021): 2537-2545.
- Kondo S. The roles of keratinocyte-derived cytokines in the epidermis and their possible responses to UVA-irradiation. J Investig Dermatol Symp Proc 4 (1999): 177-183.
- Ansel J, Perry P, Brown J, et al. Cytokine modulation of keratinocyte cytokines. The Journal of investigative dermatology 94 (1990): 101S-107S.
- Cecchi R. Estimating wound age: looking into the future. International Journal of Legal Medicine 124 (2010): 523-536.
- Grellner W. Time-dependent immunohistochemical detection of proinflammatory cytokines (IL-1beta, IL-6, TNF-alpha) in human skin wounds. Forensic Sci Int. Dec 130 (2002):90-6.
- Yoshizumi M, Nakamura T, Kato M, et al. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol Int 32 (2008): 1405-1411.
- Barr RM, Walker SL, Tsang W, et al. Suppressed alloantigen presentation, increased TNF-alpha, IL-1, IL-1Ra, IL-10, and modulation of TNF-R in UV-irradiated human skin. The Journal of investigative dermatology 112 (1999): 692-698.
- Brink N, Szamel M, Young AR, Wittern KP, Bergemann J. Comparative quantification of IL-1beta, IL-10, IL-10r, TNFalpha and IL-7 mRNA levels in UV-irradiated human skin in vivo. Inflamm Res 49 (2000): 290-296.
- Köck A, Schwarz T, Kirnbauer R, et al. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med 172 (1990): 1609-1614.
- Syed DN, Afaq F, Mukhtar H. Differential activation of signaling pathways by UVA and UVB radiation in normal human epidermal keratinocytes. Photochem Photobiol 88 (2012): 1184-1190.
- Lehmann B, Abraham S, Meurer M. Role for tumor necrosis factor-alpha in UVB-induced conversion of 7-dehydrocholesterol to 1alpha,25-dihydroxyvitamin D3 in cultured keratinocytes. J Steroid Biochem Mol Biol 89 (2004): 561-565.
- Sklar LR, Almutawa F, Lim HW, Hamzavi I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: a review. Photochem Photobiol Sci. Jan 12 (2013): 54-64.
- Tran DT, Salmon R. Potential photocarcinogenic effects of nanoparticle sunscreens. Australas J DermatolFeb 52 (2011): 1-6.
- Auttachoat W, McLoughlin CE, White KL, Smith MJ. Route-dependent systemic and local immune effects following exposure to solutions prepared from titanium dioxide nanoparticles. null 11 (2014): 273-282.
- Wright C, Iyer AKV, Wang L, et al. Effects of titanium dioxide nanoparticles on human keratinocytes. Drug Chem Toxicol 40 (2017): 90-100.
- Liou SH, Wu WT, Liao HY, et al. Global DNA methylation and oxidative stress biomarkers in workers exposed to metal oxide nanoparticles. J Hazard Mater 331 (2017): 329-335.
- Monteiro-Riviere NA, Wiench K, Landsiedel R, Schulte S, Inman AO, Riviere JE. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: an in vitro and in vivo study. Toxicol Sci 123 (2011): 264-80.
- Tan MH, Commens CA, Burnett L, Snitch PJ. A pilot study on the percutaneous absorption of microfine titanium dioxide from sunscreens. Australas J Dermatol 37 (1996): 185-7.
- Gulson B, McCall M, Korsch M, et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol Sci 118 (2010): 140-9.
- Kathawala MH, Ng KW, Loo SCJ. TiO2 nanoparticles alleviate toxicity by reducing free Zn2+ ion in human primary epidermal keratinocytes exposed to ZnO nanoparticles. Journal of Nanoparticle Research 17 (2015): 263.
- Garabrant DH, Fine LJ, Oliver C, Bernstein L, Peters JM. Abnormalities of pulmonary function and pleural disease among titanium metal production workers. Scand J Work Environ Health 13 (1987): 47-51.
- Otani N, Ishimatsu S, Mochizuki T. Acute group poisoning by titanium dioxide: inhalation exposure may cause metal fume fever. Am J Emerg Med 26 (2008): 608-11.
- Ge Y, Schimel JP, Holden PA. Evidence for Negative Effects of TiO2 and ZnO Nanoparticles on Soil Bacterial Communities. Environ Sci Technol 45 (2011): 1659-1664.
- Khan M, Khan MSA, Borah KK, Goswami Y, Hakeem KR, Chakrabartty I. The potential exposure and hazards of metal-based nanoparticles on plants and environment, with special emphasis on ZnO NPs, TiO2 NPs, and AgNPs: A review. Environmental Advances 6 (2021): 100128.
- 84 Sunscreen drug products for over-the counter human use—Proposed rule (Federal Register) 6204-6275 (2019).
- Bocca B, Caimi S, Senofonte O, Alimonti A, Petrucci F. ICP-MS based methods to characterize nanoparticles of TiO2 and ZnO in sunscreens with focus on regulatory and safety issues. Science of The Total Environment 630 (2018): 922-930.
- Mance M, Prutki M, Dujmovic A, Milosevic M, Vrbanovic-Mijatovic V, Mijatovic D. Changes in total body surface area and the distribution of skin surfaces in relation to body mass index. Burns 46 (2020): 868-875.
- Takamiya M, Fujita S, Saigusa K, Aoki Y. Simultaneous detection of eight cytokines in human dermal wounds with a multiplex bead-based immunoassay for wound age estimation. Int J Legal Med 122 (2008): 143-8.