The live birth rate improved when the endometrial thickness decreased from the HCG day to the embryo transfer day. A retrospective cohort study of 9572 fresh embryo transfer cycles
Article Information
Miao Wang1, Xinglin Wang1, Ying Meng1, Fang Wang1, Hong Ye1*
1 Chongqing Health Center for Women and Children, Women and Children's Hospital of Chongqing Medical
University, Chongqing, China
*Corresponding Author: Hong Ye, Chongqing Health Center For Women and Children, Women and Children's Hospital of Chongqing Medical University, Chongqing, China
Received: 13 February 2023; Accepted: 16 February 2023; Published: 08 March 2023
Citation: Miao Wang. The live birth rate improved when the endometrial thickness decreased from the HCG day to the embryo transfer day. A retrospective cohort study of 9572 fresh embryo transfer cycles. Obstetrics and Gynecology Research. 6 (2023): 100-106
Share at FacebookAbstract
Aim:
To investigate whether decreased endometrial thickness from the HCG trigger day to the embryo transfer day affects the pregnancy outcomes of IVF/ICSI cycles.
Methods:
A retrospective single-center cohort study was conducted. In total, 9572 first IVF/ICSI embryo transfer cycles involving the long protocol at Chongqing Reproductive and Genetic Institute from January 1, 2016, to June 30, 2020were included. The 9572 cycles were divided into 2 groups based on whether the endometrial thickness decreased from the HCG trigger day to the embryo transfer day. The outcomes of the two groups were compared. The primary outcome was live birth rate (LBR). The secondary outcomes were clinical pregnancy rate (CPR), pregnancy loss rate (PLR) and ectopic pregnancy rate (EPR).
Results:
The LBR of participants in the decreased endometrial thickness group was higher than that in the non-decreased group (54.26% vs. 51.67%, P=0.023, after adjusting the confounding factors P=0.032), and the CPR in the decreased group was also higher than that in the non-decreased group (61.36% vs. 58.88%, P=0.027, after adjusting confounding factors P=0.037). There was no significant difference in the EPR (1.65%vs. 1.82%, P = 0.513) or pregnancy loss rate (6.85%vs. 7.42% P = 0.771) between two groups.
Conclusions:
A properly decreased of endometrial thickness from the HCG trigger day to the embryo transfer day in IVF/ICSI cycles will increases the LBR and CPR. Therefore, the decreased endometrial thickness from the HCG trigger day to the embryo transfer day cannot be the determined factor of cancelling embryo transfer.
Keywords
In Vitro Fertilization; Endometrial Thickness, Ultrasound, Progesterone
In Vitro Fertilization articles In Vitro Fertilization Research articles In Vitro Fertilization review articles In Vitro Fertilization PubMed articles In Vitro Fertilization PubMed Central articles In Vitro Fertilization 2023 articles In Vitro Fertilization 2024 articles In Vitro Fertilization Scopus articles In Vitro Fertilization impact factor journals In Vitro Fertilization Scopus journals In Vitro Fertilization PubMed journals In Vitro Fertilization medical journals In Vitro Fertilization free journals In Vitro Fertilization best journals In Vitro Fertilization top journals In Vitro Fertilization free medical journals In Vitro Fertilization famous journals In Vitro Fertilization Google Scholar indexed journals Endometrial Thickness articles Endometrial Thickness Research articles Endometrial Thickness review articles Endometrial Thickness PubMed articles Endometrial Thickness PubMed Central articles Endometrial Thickness 2023 articles Endometrial Thickness 2024 articles Endometrial Thickness Scopus articles Endometrial Thickness impact factor journals Endometrial Thickness Scopus journals Endometrial Thickness PubMed journals Endometrial Thickness medical journals Endometrial Thickness free journals Endometrial Thickness best journals Endometrial Thickness top journals Endometrial Thickness free medical journals Endometrial Thickness famous journals Endometrial Thickness Google Scholar indexed journals Ultrasound articles Ultrasound Research articles Ultrasound review articles Ultrasound PubMed articles Ultrasound PubMed Central articles Ultrasound 2023 articles Ultrasound 2024 articles Ultrasound Scopus articles Ultrasound impact factor journals Ultrasound Scopus journals Ultrasound PubMed journals Ultrasound medical journals Ultrasound free journals Ultrasound best journals Ultrasound top journals Ultrasound free medical journals Ultrasound famous journals Ultrasound Google Scholar indexed journals Progesterone articles Progesterone Research articles Progesterone review articles Progesterone PubMed articles Progesterone PubMed Central articles Progesterone 2023 articles Progesterone 2024 articles Progesterone Scopus articles Progesterone impact factor journals Progesterone Scopus journals Progesterone PubMed journals Progesterone medical journals Progesterone free journals Progesterone best journals Progesterone top journals Progesterone free medical journals Progesterone famous journals Progesterone Google Scholar indexed journals IVF articles IVF Research articles IVF review articles IVF PubMed articles IVF PubMed Central articles IVF 2023 articles IVF 2024 articles IVF Scopus articles IVF impact factor journals IVF Scopus journals IVF PubMed journals IVF medical journals IVF free journals IVF best journals IVF top journals IVF free medical journals IVF famous journals IVF Google Scholar indexed journals ICSI articles ICSI Research articles ICSI review articles ICSI PubMed articles ICSI PubMed Central articles ICSI 2023 articles ICSI 2024 articles ICSI Scopus articles ICSI impact factor journals ICSI Scopus journals ICSI PubMed journals ICSI medical journals ICSI free journals ICSI best journals ICSI top journals ICSI free medical journals ICSI famous journals ICSI Google Scholar indexed journals embryo transfer articles embryo transfer Research articles embryo transfer review articles embryo transfer PubMed articles embryo transfer PubMed Central articles embryo transfer 2023 articles embryo transfer 2024 articles embryo transfer Scopus articles embryo transfer impact factor journals embryo transfer Scopus journals embryo transfer PubMed journals embryo transfer medical journals embryo transfer free journals embryo transfer best journals embryo transfer top journals embryo transfer free medical journals embryo transfer famous journals embryo transfer Google Scholar indexed journals Ovarian Hyperstimulation Syndrome articles Ovarian Hyperstimulation Syndrome Research articles Ovarian Hyperstimulation Syndrome review articles Ovarian Hyperstimulation Syndrome PubMed articles Ovarian Hyperstimulation Syndrome PubMed Central articles Ovarian Hyperstimulation Syndrome 2023 articles Ovarian Hyperstimulation Syndrome 2024 articles Ovarian Hyperstimulation Syndrome Scopus articles Ovarian Hyperstimulation Syndrome impact factor journals Ovarian Hyperstimulation Syndrome Scopus journals Ovarian Hyperstimulation Syndrome PubMed journals Ovarian Hyperstimulation Syndrome medical journals Ovarian Hyperstimulation Syndrome free journals Ovarian Hyperstimulation Syndrome best journals Ovarian Hyperstimulation Syndrome top journals Ovarian Hyperstimulation Syndrome free medical journals Ovarian Hyperstimulation Syndrome famous journals Ovarian Hyperstimulation Syndrome Google Scholar indexed journals Vascular Endothelial Growth Factor articles Vascular Endothelial Growth Factor Research articles Vascular Endothelial Growth Factor review articles Vascular Endothelial Growth Factor PubMed articles Vascular Endothelial Growth Factor PubMed Central articles Vascular Endothelial Growth Factor 2023 articles Vascular Endothelial Growth Factor 2024 articles Vascular Endothelial Growth Factor Scopus articles Vascular Endothelial Growth Factor impact factor journals Vascular Endothelial Growth Factor Scopus journals Vascular Endothelial Growth Factor PubMed journals Vascular Endothelial Growth Factor medical journals Vascular Endothelial Growth Factor free journals Vascular Endothelial Growth Factor best journals Vascular Endothelial Growth Factor top journals Vascular Endothelial Growth Factor free medical journals Vascular Endothelial Growth Factor famous journals Vascular Endothelial Growth Factor Google Scholar indexed journals Antimullerian Hormone articles Antimullerian Hormone Research articles Antimullerian Hormone review articles Antimullerian Hormone PubMed articles Antimullerian Hormone PubMed Central articles Antimullerian Hormone 2023 articles Antimullerian Hormone 2024 articles Antimullerian Hormone Scopus articles Antimullerian Hormone impact factor journals Antimullerian Hormone Scopus journals Antimullerian Hormone PubMed journals Antimullerian Hormone medical journals Antimullerian Hormone free journals Antimullerian Hormone best journals Antimullerian Hormone top journals Antimullerian Hormone free medical journals Antimullerian Hormone famous journals Antimullerian Hormone Google Scholar indexed journals
Article Details
Abbreviations
HCG: Human Chorionic Gonadotropin; IVF: In Vitro Fertilization; ICSI: Intracytoplasmic Sperm Injection; LBR: Live Birth Rate; CPR: Clinical Pregnancy Rate; PLR: Pregnancy Loss Rate; EPR: Ectopic Pregnancy Rate; GnRH-a: Gonadotropin Releasing Hormone Agonist; AMH: Antimullerian Hormone; BMI: Body Mass Index; OHSS: Ovarian Hyperstimulation Syndrome; VEGF: Vascular Endothelial Growth Factor
INTRODUCTION
Previous studies suggested that pregnancy outcome is closely related to endometrial thickness [12]. KE Liu reported that the clinical pregnancy rate declined with the poor endometrial thickness within a certain range [3]. Therefore, we often measured endometrial thickness using transvaginal ultrasound before embryo transfer. Most of doctors decided to cancel the embryo transfer when endometrial thickness is less than 7 or 8 mm. However, we found that endometrial thickness changed between the HCG trigger day and embryo transfer day in this study. In some cases, the endometrial thickness decreased after the HCG trigger, while in others, it remained the same or continued to thicken. Does this mean that the current embryo transfer cycle should be cancelled for patients with decreasing endometrial thickness? Jokubkiene reported that sub-endometrial vascularization changes markedly during the normal menstrual cycle. It increased throughout the follicular phase, decreased to a nadir 2 days after ovulation and then increased again during the luteal phase [4]. Does a slight decrease in endometrial thickness during the stimulation cycle means changes in sub-endometrial blood vessels, and does this change affects the outcomes of embryo implantation? With these questions, we explored in this study whether the decreasing endometrial thickness from the HCG trigger day to the embryo transfer day would affect pregnancy outcomes.
Materials and methods
Data preparation
The data of 9572 IVF/ICSI embryo transfer cycles carried out with long gonadotropin releasing hormone agonist (GnRH-a) protocol stimulation at Chongqing Reproductive and Genetics Institute were obtained from January 1, 2016, to June 30, 2020. Trials were considered for inclusion with the following criteria: (1) participants who underwent controlled ovarian stimulation (COS) with the long GnRH-a protocol; (2) patients who registered for fresh embryo transfer cycles; and (3) cases who had their first IVF/ICSI cycles. Exclusion criteria: (1) age over 40 years; (2) endometrial thickness <7 mm on embryo transfer day; and (3) blastocyst transfer cycles.
Ovarian stimulation protocol and embryo transfer strategy
Patients with long protocol were treated with GnRH-a to downregulate the functions of the pituitary gland on the day 21 of the previous menstrual cycle. After downregulation of the pituitary gland, gonadotropin was initiated with a starting dose of recombinant FSH ranging from 112.5 to 300 IU, and gonadotropin was modulated according to the patient’s ovarian response. Recombinant HCG (250 µg, Merck Serono) were used to trigger ovulation in all cases after at least two mature follicles were observed to reach 18 mm. The oocytes were retrieved 34-36 hours after the HCG trigger, and progesterone gel (90 mg, Crinone 8%, Merck Serono) was administered vaginally on the day of egg collection. Embryo transfer was performed 3 days after oocyte retrieval and the usual IVF or ICSI procedure. Endometrial thickness was measured in the morning on both the HCG day and the transfer day. In our center, three doctors specializing in vaginal ultrasound measurement performed this work. They underwent unified ultrasonic measurement training. The coefficient variation of the measurement results obtained by the different doctors was less than 5%.After embryo transfer , the vaginal progesterone gel was continued for luteal support. Venous blood-HCG levels were measured 14 days after embryo transplantation. Transvaginal ultrasonography of the uterus was performed 4 weeks after embryo transplantation.
Outcomes and definitions
The primary outcome was live birth rate. The secondary outcomes were clinical pregnancy rate, pregnancy loss rate and ectopic pregnancy rate. Live birth was defined as delivery of any neonate after 28 weeks of gestation. Clinical pregnancy was defined as a viable pregnancy with fetal heart activity under ultrasonography. Ectopic pregnancy was defined as the detection of a gestational sac outside the uterus. Pregnancy loss was defined as the spontaneous loss of the embryo or fetus before 28 weeks of gestation.
Statistical analysis
Statistical Package for the Social Science software (version 24, SPSS Inc., Chicago. IL, USA) was used to analyze data in this study. Continuous variables are expressed as the mean ± SD. The 9572 cycles were divided into two groups based on whether their endometrial thickness was decreased. Decreased group: the endometrial thickness on embryo transfer day was thinner than that on the HCG day (n = 2663).Non-decreased group: the endometrial thickness on the embryo transfer day was equal to or thicker than that on the HCG day (n = 6909). Student’s t-test was used to detect differences between continuous variables, and the chi-square test was used for categorical variables. Categorical variables are presented as the number of cases (n) and the percentage (%). Binomial logistic regression analysis was used to adjust for the baseline characteristics between the two groups. P < 0.05 was considered statistically significant.
Results
A total of 41,909 cycles were screened and assessed. Finally, a total of 9572 patients with the first IVF/ICSI cycle and met the study eligibility criteria, among that, 2663 patients in the decreased group and 6909 patients in the non-decreased group (Figure 1). The demographic characteristics were shown in Table 1. There were significant differences in patients' age (30.87±3.82 vs. 31.05±3.79 years, P=0.044), and AMH was higher in the decreased group (3.07±2.45 vs. 2.96±2.27, P=0.039). There was no significant difference in BMI, infertility type or infertility reason between the two groups.
The duration of ovarian stimulation (days) in the decreased group was greater than that in the non-decreased group (10.84± 1.33 vs. 10.70±1.34, P< 0.001). There was no significant difference between the two groups with regard to estradiol on the trigger day, progesterone on the trigger day, total dose of gonadotropin, number of oocytes retrieved, fertilization type or top-quality embryos (Table 2).
The clinical outcomes in the two groups are shown in Table 3. After adjusting for potential confounders, including age, anti-Mullerian hormone (AMH), duration of ovarian stimulation (days), binomial logistic regression analysis demonstrated that there was significant difference in the live birth rate (54.26% vs. 51.67%, P=0.023, after adjusting for confounding factors P=0.032) and the clinical pregnancy rate (61.36% vs. 58.88%, P=0.027, after adjusting for confounding factors P=0.037). However, there were no significant difference in the ectopic pregnancy rate (1.65%vs. 1.82%, P=0.569, after adjusting for confounding factors P= 0.513) or the pregnancy loss rate (6.85%vs. 7.42%, P= 0.722, after adjusting for confounding factors, P = 0.771) (Table 3).
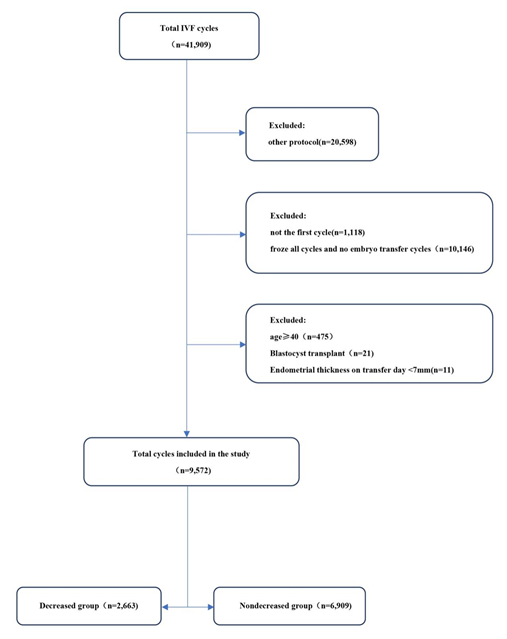
Figure 1: Flow chart of the patients’ allocation
Discussion
In this study, we found that the endometrial thickness after HCG trigger is not invariable. It can also be seen from Figure 2 that the pregnancy outcome changed with the reduction of endometrial thickness. It seems that when endometrial thickness reduction, the CPR and LBR do not decrease but tend to increase.This conclusion is consistent with that of study conducted by Jigal Haas [5].
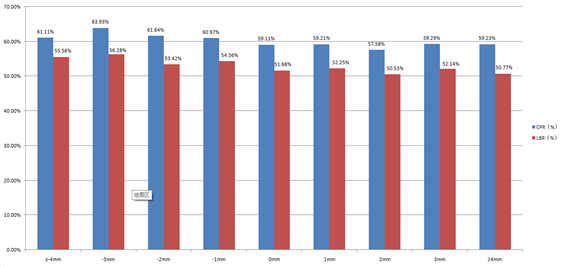
Figure 2: CPR and LBR among groups with different endometrial changes
|
|
Decreased group (n=2663) |
Nondecreased group (n=6909) |
P |
|
Age(years) |
30.87±3.82 |
31.05±3.79 |
0.044* |
|
Infertility years(years) |
5.091±3.66 |
5.03±3.57 |
0.453 |
|
BMI |
22.01±2.75 |
22.01±2.80 |
0.951 |
|
AMH |
3.07±2.45 |
2.96±2.27 |
0.039* |
|
Infertility type |
|
||
|
Primary infertility%(n) |
44.09% (1174) |
43.80% (2998) |
0.540 |
|
Secondary infertility%(n) |
55.91% (1489) |
56.20% (3911) |
0.540 |
|
Infertility reason |
|
||
|
Tubal factor%(n) |
78.67% (2095) |
79.42% (5487) |
0.419 |
|
Endometriosis%(n) |
0.86%(23) |
0.91%(63) |
0.823 |
|
Male factor%(n) |
9.13%(243) |
8.57%(592) |
0.387 |
|
Multiple factors%(n) |
6.27%(167) |
6.37%(440) |
0.861 |
|
Unexplained infertility%(n) |
5.07%(135) |
4.73%(327) |
0.491 |
*Statistically significant P-value < 0.05
Table 1: Characteristics of the basic parameters of patients in the two groups
Jokubkiene studied the changes in endometrial blood flow in 14 volunteers during the natural cycle and found that during the follicular phase, the sub-endometrial blood vessel index increased with follicular growth and that the thickness and volume of the endometrium increased rapidly. The sub-endometrial blood vessel index decreased to the lowest point 2 days after ovulation and then increased again during the luteal phase [4]. This finding seems to explain the decrease in endometrial thickness during the stimulation cycle between the HCG trigger day and the embryo transfer day. We hypothesize this endometrial change is very similar with that of in the natural cycle, which might contribute to improving endometrial receptivity. Sarani SA proposed that 1 to 5 days after ovulation is the period of the menstrual cycle when endometrial vascularization is at its lowest, and it is also the period when endometrial receptivity is thought to be at its maximum in terms of hypoxia [6]. It has been demonstrated in animal studies that near-atmospheric oxygen concentrations reduce embryo viability and compromise embryo development and that oxygen tension in the uterus is lowest during the implantation period [7-8]. Popovici RM suggested that endometrial hypoxia stimulates the production of vascular endothelial growth factor (VEGF) in endometrial stromal cells [9]. Tsuzuki T reported that hypoxic stress stimulates the generation of VEGF through hypoxia-inducing factor-1 [10]. The increase in VEGF in turn regulates the angiogenesis of the endometrium and stimulates further growth of the endometrium [11]. In a single-center retrospective control study, Zhiqin Bu found that an increased endometrial thickness after progesterone administration was associated with better pregnancy outcomes for thawed blastocyst cycles [12]. The possible reason for this conflicting result is that all of our research objects involved cleavage-stage embryo transfer, with the transfer period being in the early luteal phase, in contrast to that of the blastocyst transfer, which is closer to the middle luteal phase. In addition, the effects of high estrogen levels on the endometrium during stimulation cycles may lead to different results.
We assume that the transformation of the endometrium from the hyperplasia stage to the secretion stage is accompanied by a decrease in the vascularization degree and uterine thickness, which leads to a decrease in the endometrial oxygen concentration. Hypoxic stress stimulates the expression of VEGF through hypoxia-inducing factor-1, which leads to an increase in the endometrial vascularization degree and further stimulates the endometrium to thicken again. The reason why this change is not obvious on ultrasound is that the endometrial thickness during the luteal phase changes within a small range. Even so, different patterns of changes in endometrial thickness within a small range were associated with different pregnancy outcomes. In addition, in our study, we found that the endometrium continued to thicken in some patients from the HCG trigger day to the embryo transfer day. Does the endometrium in this population continue to thicken due to exogenous progesterone deficiency or endogenous progesterone deficiency? According to Usadi et al., this process is independent of the actual circulating progesterone concentration; whether serum progesterone levels are normal or abnormally low, secretory endometrial development is similar [13]. According to Yoo J, some infertile women have defects in progesterone receptor or endometrial resistance, and the related reasons include overexpression of bcl-6 and sirt-1[14], chronic endometrial inflammation, progesterone receptor gene polymorphism, altered microRNA expression, and epigenetic modification of the progesterone receptor [15,16]. Another theory is that the ratio of estrogen to progesterone plays a role, with too much estrogen causes the endometrium to continue to grow. This conjecture may also explain why the endometrial receptivity of fresh embryo transplantation during the ovulatory cycle is lower than that during freeze-thaw embryo transplantation [17]. Since patients who retrieved more than 20 oocytes in our center would receive whole embryo cryopreservation, we cannot analyze the pregnancy outcome after fresh embryo transfer for patients with this type of super-estrogen level, so we cannot confirm its hypothesis. However, our data revealed that there was no significant difference between the two groups in terms of estrogen levels on the HCG trigger day and progesterone levels on the HCG trigger day. It may be suggested that the levels of estrogen and progesterone in cycles are not the main factors determined whether the endometrial thickness decreases. However, the expression of estrogen and progesterone receptors and related inflammatory factors may be the key factors.
|
|
Decreased group (n=2663) |
Non-decreased group (n=6909) |
P |
|
Estradiol on the trigger day(pmol/L) |
3106.31±1188.04 |
3067.96±1282.35 |
0.166 |
|
Progesterone on the trigger day(ng/mL) |
0.518±0.280 |
0.522±0.284 |
0.492 |
|
Duration of ovarian stimulation (days) |
10.84±1.33 |
10.70±1.34 |
0.000* |
|
Total dose of gonadotropin (IU) |
2386.83±762.37 |
2369.21±738.80 |
0.3 |
|
No. of oocytes retrieved |
10.05±3.90 |
9.97±3.93 |
0.329 |
|
Endometrial thickness on HCG day |
11.11±1.53 |
9.62±1.49 |
0.000 |
|
Endometrial thickness on transfer day |
9.63±1.41 |
10.58±1.65 |
0.000 |
|
Endometrial thickness change from HCG day to transfer day |
-1.48±0.76 |
0.96±1.03 |
0.000 |
|
Fertilization type |
|
||
|
IVF%(n) |
83.59%(2226) |
84.05%(5807) |
0.583 |
|
ICSI %(n) |
12.65%(337) |
12.64%(873) |
0.979 |
|
IVF+ICS I%(n) |
3.76%(100) |
3.31%%(229) |
0.289 |
|
No. of implanted embryos |
1.94±0.25 |
1.93±0.26 |
0.274 |
|
Top-quality embryos |
1.77±1.21 |
1.84±1.20 |
0.140 |
T-tests and chi-square tests were used* Statistically significant P-value < 0.05
Table 2: Treatment characteristics in the two groups
Top-quality embryos: All transferable embryos were assessed on day 2 or 3 for blastomere number and regularity as well as for the presence and volume of cytoplasmic fragmentation. Day 3 embryos with eight equal size cells,no cytoplasmic fragments, from Day 2 embryos with four equal size cells, no cytoplasmic fragments.
|
|
Decreased group (n=2663) |
Non-decreased group (n=6909) |
Before adjustment |
After adjustment |
||
|
95%CI |
P |
95%CI |
P |
|||
|
LBR%(n) |
54.26% (1445) |
51.67(3570) |
1.014~1.214 |
0.023* |
1.009~1.209 |
0.032* |
|
CPR %(n) |
61.36%(1634) |
58.88% (4068) |
1.109~1.012 |
0.027* |
1.006~1.210 |
0.037* |
|
EPR%(n) |
1.65%(44) |
1.82%(126) |
0.640~1.279 |
0.569 |
0.630~1.260 |
0.513 |
|
PLR%(n) |
6.85%(112) |
7.42%(302) |
0.770~1.199 |
0.722 |
0.775~1.209 |
0.771 |
*Statistically significant P-value < 0.05
Table 3: Clinical outcomes in the two groups
The advantage of this study lies in it’s the first relevant report on the effect of endometrial changes on live birth rate from HCG day to embryo transfer day. At the same time, this study has a large sample size and reliable results. In order to reduce the deviation of results caused by different ovulation stimulation program, we only studied long gonadotropin releasing hormone agonist (GnRH-a) protocol. At the same time, we conducted a logistic regression to adjust the confounding factors that may affect the pregnancy outcome. The results of this study are consistent with previous studies, indicating that endometrial thickness is an important factor affecting pregnancy outcome. Its innovative discovery is that, contrary to our previous conjecture, the reduction of endometrium in a few days from the HCG day to the embryo transfer day will not adversely affect the pregnancy outcome
The limits of this study include that above conclusions were obtained in the long protocol, and it is unknown whether the same conclusions were obtained in other protocol. As mentioned in Figure 1, we conducted a retrospective analysis of 41,909 cycles, and 20,598 of them were other protocol than the long protocol, so the long plan was not the only plan we used. And we found that the Cumulative live birth rates of GnRH-a group was higher than that of GnRH-ant group in suboptimal responders; no significant difference was observed in other patients between different protocols[18], which was the reason the long protocol become the mainstream program in our center. In addition, blastocyst embryos were not included in our study. Some studies suggested that extended culture may impact offspring via differences in gene expression and epigenetic mutations, particularly regarding the blastocyst stage [19-24]. Extending the duration of embryo culture to the blastocyst stage for assisted reproduction may decrease the number of embryos transferred. Patients who received assisted reproduction treatment in China pay for themselves and did not receive any government subsidies, so most of them were not willing to risk a reduction in the number of embryos transferred after blastocyst culture. Since there were few patients with blastocyst transplantation during the egg retrieval cycle in our center, which could not be analyzed as a single group, therefore, the study with the relevant population could be considered in the future. Meanwhile, it also remains to be seen that whether this hypothesis could be applied to freeze-thaw embryo transfer.
Conclusion
In summary, the endometrial thickness from the HCG trigger day to the embryo transfer day is not static in fresh embryo transfer cycle, and we cannot use the decreased endometrial thickness as determined factor to cancel the embryo transfer. In contrast, the decreased endometrial thickness may indicate a better pregnancy outcome.
Ethics approval and consent to participate
The study has been approved by the Ethics Committee of Chongqing Maternal and Child Health Hospital. Approval reference number:(No.2019-1206) for retrospective analysis and clinical data reporting. Since the anonymous data were respectively extracted from the electronic record without any information that could identify particular individual, A waiver of informed consent was granted by the Institutional Review Board of Chongqing Health Center for Women and Children. All methods were performed in accordance with the relevant guidelines and regulations (accordance with the Declaration of Helsinki).
Consent for publication
Not applicable.
Availability of data and materials
The data sets analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
Miao W designed the study and drafted the manuscript. Xinlin W collected the data. Ying M contributed to the analysis and interpretation on data. Fang W contributed to the statistical analysis. Hong Y give the critical revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to thank Dr. Xiaodong Z and Jinwei Y for their assistance in the data analysis.
References
- Zhang Q , Li Z , Wang Y , et al (2021) The relationship and optimal threshold of endometrial thickness with early clinical pregnancy in frozen embryo transfer cycles[J]. International Journal of Gynecology & Obstetrics..
- Fang T , Chen M , Yu W , et al (2021) The predictive value of endometrial thickness in 3117 fresh IVF/ICSI cycles for ectopic pregnancy. Journal of Gynecology Obstetrics and Human Reproduction 50(4): 102072.
- Liu K, Hartman M, Hartman A, Luo Z, Mahutte N (2018) The impact of a thin endometrial lining on fresh and frozen–thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod33:1883-1888.
- Jokubkiene L, Sladkevicius P, Rovas L, Valentin L (2006) Assessment of changes in endometrial and subendometrial volume and vascularity during the normal menstrual cycle using threedimensional power Doppler ultrasound. Ultrasound Obstet Gynecology 27: 672-679.
- Haas J, Smith R, Zilberberg E, Nayot D, Meriano J, Barzilay E, Casper R (2019) Endometrial compaction (decreased thickness) in response to progesterone results in optimal pregnancy outcome in frozen-thawed embryo transfers. Fertility and Sterility 112: 503-509.e1.
- Sarani S, Ghaffari-Novin M, Warren M, Dockery P, Cooke I (1999) Morphological evidence for the `implantation window' in human luminal endometrium. HumReprod14: 3101-3106.
- Karagenc L, Sertkaya Z, Ciray N, Ulug U, Bahçeci M (2004) Impact of oxygen concentration on embryonic development of mouse zygotes. ReproductiveBioMedOnline 9:409-417.
- Fischer B, Bavister B (1993) Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J ReprodFertil99: 673-679.
- Popovici R, Irwin J, Giaccia A, Giudice L (1999) Hypoxia and cAMP Stimulate Vascular Endothelial Growth Factor (VEGF) in Human Endometrial Stromal Cells: Potential Relevance to Menstruation and Endometrial Regeneration. JClin EndocrinolMetab84: 2245-2245.
- Tsuzuki T, Okada H, Cho H, Tsuji S, Nishigaki A, Yasuda K, Kanzaki H (2011) Hypoxic stress simultaneously stimulates vascular endothelial growth factor via hypoxia-inducible factor-1and inhibits stromal cell-derived factor-1 in human endometrial stromal cells. Hum Reprod 27: 523-530.
- Moller B, Lindblom B, Olovsson M (2002) Expression of the vascular endothelial growth factors B and C and their receptors in human endometrium during the menstrual cycle. Acta ObstetGynecolScand81: 817-824.
- Bu Z, Yang X, Song L, Kang B, Sun Y (2019) The impact of endometrial thickness change after progesterone administration on pregnancy outcome in patients transferred with single frozen-thawed blastocyst. ReprodBiol Endocrinol17(1): 99.
- Usadi R, Groll J, Lessey B, Lininger R, Zaino R, Fritz M, Young S (2008) Endometrial Development and Function in Experimentally Induced Luteal Phase Deficiency. J Clin Endocrinol &Metab93: 40584064.
- Yoo J, Kim T, Fazleabas A, Palomino W, Ahn S, Tayade C, Schammel D, Young S, Jeong J, Lessey B (2017) KRASActivation and over-expression of SIRT1/BCL6 Contributes to the Pathogenesis of Endometriosis and Progesterone Resistance. Sci Rep28;7(1): 6765.
- Hu M, Li J, Zhang Y, Li X, Billig,H, et al (2018) Endometrial progesterone receptor isoforms in women with polycystic ovary syndrome. Am J Transl Res10: 2696–2705.
- Patel B, Rudnicki M, Yu J, Shu Y, Taylor R. (2017) Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstetricia et GynecologicaScandinavica96:623-632.
- Pandit R, Biliangady R, Tudu N, Kinila P, Maheswari U, Gopal I, Swamy A (2019) Is it time to move toward freeze-all strategy? – A retrospective study comparing live birth rates between fresh and first frozen blastocyst transfer. J hum Reprod Sci12: 321-326.
- Yang J , Zhang X , Ding X , et al. (2021) Cumulative live birth rates between GnRH-agonist long and GnRH-antagonist protocol in one ART cycle when all embryos transferred: real-word data of 18,853 women from China. Reproductive Biology and Endocrinology, 19(1).
- Dar S, Lazer T, Shah PS, Librach CL.( 2014) Neonatal outcomes among singleton births after blastocyst versus cleavage stage embryo transfer: a systematic review and meta-analysis.Hum Reprod Update20: 439–448.
- Cox GF, Burger J, Lip V, Mau UA, Sperling K, Wu BL, et al. (2002) Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet71: 162–164.
- DeBaun MR, Niemitz L, Feinberg AP. (2003) Association of in vitro fertilization with Beckwith-Weidemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet 72: 156–160.
- Gicquel C, Gaston V, Mandelbaum J, Siffroi JP, Flahault A, Le Bouc YL. (2003) In vitro fertilization may increase the risk of Beckwith-Weidemann syndrome related to the abnormal imprinting of the KCNQ10T gene. Am J Hum Genet72:1338–1341.
- Maher ER, Brueton LA, Bowdin SC, Luharia A, Cooper W, Cole TR, et al. (2003) Beckwith-Weidemann syndrome and assisted reproductive technology (ART). J Med Genet40: 62–64.
- Moll AC, Imhof SM, Cruysberg JR, Schouten-van Meeteren AY, Boers M, van Leeuwen FE. (2003) Incidence of retinoblastoma in children born after in vitro fertilisation. Lancet36: 309–310.
