The Effect of Montelukast Sodium on the Reduction of Relapse Rates in Children with Steroid Dependent Nephrotic Syndrome
Article Information
Abeyagunawardena S1, Jayaweera H2 and Abeyagunawardena A S2
1Department of Medicine, Faculty of Medicine, University of Peradeniya, Sri Lanka
2Department of Paediatrics, Faculty of Medicine, University of Peradeniya, Sri Lanka
*Corresponding Author: Asiri Samantha Abeyagunawardena, Department of Paediatrics, Faculty of Medicine, University of Peradeniya, Sri Lanka
Received: 01 December 2020; Accepted: 14 January 2021; Published: 26 January 2021
Citation: Abeyagunawardena S, Jayaweera H, Abeyagunawardena AS. The Effect of Montelukast Sodium on the Reduction of Relapse Rates in Children with Steroid Dependent Nephrotic Syndrome. Archives of Nephrology and Urology 4 (2021): 009-017.
Share at FacebookAbstract
Background: Montelukast sodium (MS) a leukotriene receptor antagonist is known to reduce upper respiratory tract infections in children with asthma. A single centre study was conducted in Sri Lanka to evaluate the efficacy of MS in reducing the relapse rate of nephrotic syndrome (NS).
Methods and Findings: Patients with minimal-change disease, who were already on low-dose (<0.6 mg/kg) alternate-day prednisolone for a minimum of 12 months with or without adjuvant therapy were prescribed MS (4 mg for <6 years and 5 mg for >6 years) for 12 months. Urine protein excretion was recorded daily, by parents. Patients were on monthly follow-up, to assess relapse of proteinuria, episodes of infection and side effects of MS. 118 children were prescribed MS and 112 completed the 12 months of therapy. Median age was 6.2 years. Age range was 3.5-14.7 years. 79 were male and 33 were female. Significant reduction (p<0.001) in relapses was observed during MS therapy (mean=2.52 ± SD 0.60) compared to that of the previous year (mean=1.41 ± SD 0.87). The difference of relapse rates before and after the completion of MS therapy (mean=1.72 ± SD1.05) was also significant (p<0.001). The type of adjuvant therapy (Levamisole, Mycophenolate mofetil or cyclosporin A) did not have an impact on relapse rates during MS treatment. No significant side-effects were encountered with MS therapy.
Conclusion: The prescription MS is a potential therapeutic strategy in maintaining remission in childhood NS.
Keywords
Montelukast sodium; Leukotriene receptor antagonist; Childhood nephrotic syndrome; Relapse
Montelukast sodium articles; Leukotriene receptor antagonist articles; Childhood nephrotic syndrome articles; Relapse articles
Montelukast sodium articles Montelukast sodium Research articles Montelukast sodium review articles Montelukast sodium PubMed articles Montelukast sodium PubMed Central articles Montelukast sodium 2023 articles Montelukast sodium 2024 articles Montelukast sodium Scopus articles Montelukast sodium impact factor journals Montelukast sodium Scopus journals Montelukast sodium PubMed journals Montelukast sodium medical journals Montelukast sodium free journals Montelukast sodium best journals Montelukast sodium top journals Montelukast sodium free medical journals Montelukast sodium famous journals Montelukast sodium Google Scholar indexed journals Leukotriene receptor antagonist articles Leukotriene receptor antagonist Research articles Leukotriene receptor antagonist review articles Leukotriene receptor antagonist PubMed articles Leukotriene receptor antagonist PubMed Central articles Leukotriene receptor antagonist 2023 articles Leukotriene receptor antagonist 2024 articles Leukotriene receptor antagonist Scopus articles Leukotriene receptor antagonist impact factor journals Leukotriene receptor antagonist Scopus journals Leukotriene receptor antagonist PubMed journals Leukotriene receptor antagonist medical journals Leukotriene receptor antagonist free journals Leukotriene receptor antagonist best journals Leukotriene receptor antagonist top journals Leukotriene receptor antagonist free medical journals Leukotriene receptor antagonist famous journals Leukotriene receptor antagonist Google Scholar indexed journals Childhood nephrotic syndrome articles Childhood nephrotic syndrome Research articles Childhood nephrotic syndrome review articles Childhood nephrotic syndrome PubMed articles Childhood nephrotic syndrome PubMed Central articles Childhood nephrotic syndrome 2023 articles Childhood nephrotic syndrome 2024 articles Childhood nephrotic syndrome Scopus articles Childhood nephrotic syndrome impact factor journals Childhood nephrotic syndrome Scopus journals Childhood nephrotic syndrome PubMed journals Childhood nephrotic syndrome medical journals Childhood nephrotic syndrome free journals Childhood nephrotic syndrome best journals Childhood nephrotic syndrome top journals Childhood nephrotic syndrome free medical journals Childhood nephrotic syndrome famous journals Childhood nephrotic syndrome Google Scholar indexed journals Relapse articles Relapse Research articles Relapse review articles Relapse PubMed articles Relapse PubMed Central articles Relapse 2023 articles Relapse 2024 articles Relapse Scopus articles Relapse impact factor journals Relapse Scopus journals Relapse PubMed journals Relapse medical journals Relapse free journals Relapse best journals Relapse top journals Relapse free medical journals Relapse famous journals Relapse Google Scholar indexed journals steroid-dependant nephritic syndrome articles steroid-dependant nephritic syndrome Research articles steroid-dependant nephritic syndrome review articles steroid-dependant nephritic syndrome PubMed articles steroid-dependant nephritic syndrome PubMed Central articles steroid-dependant nephritic syndrome 2023 articles steroid-dependant nephritic syndrome 2024 articles steroid-dependant nephritic syndrome Scopus articles steroid-dependant nephritic syndrome impact factor journals steroid-dependant nephritic syndrome Scopus journals steroid-dependant nephritic syndrome PubMed journals steroid-dependant nephritic syndrome medical journals steroid-dependant nephritic syndrome free journals steroid-dependant nephritic syndrome best journals steroid-dependant nephritic syndrome top journals steroid-dependant nephritic syndrome free medical journals steroid-dependant nephritic syndrome famous journals steroid-dependant nephritic syndrome Google Scholar indexed journals upper respiratory tract infection articles upper respiratory tract infection Research articles upper respiratory tract infection review articles upper respiratory tract infection PubMed articles upper respiratory tract infection PubMed Central articles upper respiratory tract infection 2023 articles upper respiratory tract infection 2024 articles upper respiratory tract infection Scopus articles upper respiratory tract infection impact factor journals upper respiratory tract infection Scopus journals upper respiratory tract infection PubMed journals upper respiratory tract infection medical journals upper respiratory tract infection free journals upper respiratory tract infection best journals upper respiratory tract infection top journals upper respiratory tract infection free medical journals upper respiratory tract infection famous journals upper respiratory tract infection Google Scholar indexed journals Mycophenolate mofetil articles Mycophenolate mofetil Research articles Mycophenolate mofetil review articles Mycophenolate mofetil PubMed articles Mycophenolate mofetil PubMed Central articles Mycophenolate mofetil 2023 articles Mycophenolate mofetil 2024 articles Mycophenolate mofetil Scopus articles Mycophenolate mofetil impact factor journals Mycophenolate mofetil Scopus journals Mycophenolate mofetil PubMed journals Mycophenolate mofetil medical journals Mycophenolate mofetil free journals Mycophenolate mofetil best journals Mycophenolate mofetil top journals Mycophenolate mofetil free medical journals Mycophenolate mofetil famous journals Mycophenolate mofetil Google Scholar indexed journals Minimal change disease articles Minimal change disease Research articles Minimal change disease review articles Minimal change disease PubMed articles Minimal change disease PubMed Central articles Minimal change disease 2023 articles Minimal change disease 2024 articles Minimal change disease Scopus articles Minimal change disease impact factor journals Minimal change disease Scopus journals Minimal change disease PubMed journals Minimal change disease medical journals Minimal change disease free journals Minimal change disease best journals Minimal change disease top journals Minimal change disease free medical journals Minimal change disease famous journals Minimal change disease Google Scholar indexed journals †Leukotriene receptor antagonist articles †Leukotriene receptor antagonist Research articles †Leukotriene receptor antagonist review articles †Leukotriene receptor antagonist PubMed articles †Leukotriene receptor antagonist PubMed Central articles †Leukotriene receptor antagonist 2023 articles †Leukotriene receptor antagonist 2024 articles †Leukotriene receptor antagonist Scopus articles †Leukotriene receptor antagonist impact factor journals †Leukotriene receptor antagonist Scopus journals †Leukotriene receptor antagonist PubMed journals †Leukotriene receptor antagonist medical journals †Leukotriene receptor antagonist free journals †Leukotriene receptor antagonist best journals †Leukotriene receptor antagonist top journals †Leukotriene receptor antagonist free medical journals †Leukotriene receptor antagonist famous journals †Leukotriene receptor antagonist Google Scholar indexed journals Nephrotic syndrome articles Nephrotic syndrome Research articles Nephrotic syndrome review articles Nephrotic syndrome PubMed articles Nephrotic syndrome PubMed Central articles Nephrotic syndrome 2023 articles Nephrotic syndrome 2024 articles Nephrotic syndrome Scopus articles Nephrotic syndrome impact factor journals Nephrotic syndrome Scopus journals Nephrotic syndrome PubMed journals Nephrotic syndrome medical journals Nephrotic syndrome free journals Nephrotic syndrome best journals Nephrotic syndrome top journals Nephrotic syndrome free medical journals Nephrotic syndrome famous journals Nephrotic syndrome Google Scholar indexed journalsArticle Details
Abbreviations:
CyA: Cyclosporin A; IL-13: Interleukin 13; LEV: levamisole; LTC4: leukotriene C4; LTD4: Leukotriene D4; LTE4: Leukotrienes E4; LTRA: Leukotriene receptor antagonist; MCD: Minimal change disease; MMF: Mycophenolate mofetil; MS: Montelukast sodium; NS: nephrotic syndrome; SD: standard deviation; SDNS: steroid-dependant nephritic syndrome; TH-2: T-helper 2; URTI: upper respiratory tract infection
1. Introduction
Nephrotic syndrome (NS) is the most common glomerular disease in children characterised by nephrotic range proteinuruia, hypoalbuminemia, hyperlipidemia and generalized oedema [1]. Studies have shown that incidence of NS is particularly high in South Asian children [2, 3]. Minimal-change nephropathy (MCD) is the most common histological pattern and a majority of these children respond favourably to corticosteroid therapy. However, about 70-80 % of children are likely to relapse after the initial oral corticosteroid therapy of 8 weeks, necessitating further courses of steroids and other immunosuppressive agents. These children are considered to have steroid dependant nephrotic syndrome (SDNS) [4]. Relapses are associated with life threatening risks such as sepsis, thrombosis, malnutrition and hypovolemia [5-8]. The aetiopathology of MCD is not fully understood and remain elusive. Some studies have suggested that MCD is a T helper 2-dominated (Th2) disease, because higher levels of serum interleukin-13 (IL-13) were observed in patients with MCD [9, 10]. Studies done on rats have also shown that over-expression of IL-13 leads to podocyte injury inducing a minimal-change-like nephropathy [11]. Infections, mainly viral upper respiratory tract infections (URTIs) are a frequent cause of relapse in NS [12, 13] which has led to the hypothesis that the up-regulation of T lymphocytes and cytokine release accompanying an URTI mediates a relapse [10]. Major cytokines that are involved in this process are interleukin (IL) 2, 4 and 13 [14]. Being a tropical country, viral respiratory tract infections are common in Sri Lanka. Patients with SDNS who are on immunosuppressive therapy, are likely to be more susceptible to viral infections due to the compromise in the immunity. Environmental and socio-economic conditions could further increase the risk of infections in these patients.
Montelukast sodium (MS), a selective reversible cys-leukotriene-1 receptor (LTD4 receptor) antagonist is used in the treatment of asthma and is reported to reduce airway inflammation associated with this disease [15-17]. Cysteinylleukotrienes(CysLT), leukotrienes C4, D4, and E4 (LTC4, LTD4, LTE4) are secreted mainly by eosinophils, mast cells, monocytes and macrophages, and exert a variety of actions such as inflammatory cell recruitment, bronchoconstriction, vasodilatation, increased microvascular permeability, exudation of macromolecules, and edema which emphasizes their importance as pathogenic elements in inflammatory states [3, 18, 19]. Several studies show MS is effective in reducing URTIs and delaying exacerbations of asthma [20-22]. The observation of relapses precipitated by viral episodes in the described tertiary centre [23], prompted the authors to test effectiveness of MSin reducing the upper respiratory tract infections which could potentially reduce the viral induced NS relapses.
2. Materials and Method
2.1 Study population
All patients recruited for this study were attending the nephrotic syndrome clinic at Teaching Hospital, Peradeniya, which is a tertiary nephrology referral centre in Sri Lanka. As an institutional policy the medications could be provided only for a month, and as such all these patients were routinely reviewed on a monthly basis. Demographic data and clinical data was entered in a data base while patients too had patient held records. The parents were all trained to test and record urinary protein excretion. Patients receiving low dose (<0.6 mg/kg) prednisolone on alternate-day for a minimum of 12 months with or without adjuvant therapy as maintenance prior to the study were considered for this study. Having had two or more relapse episodes during the past 12 months was an essential criterion for recruitment. Levamisole (LEV), Mycophenolate Mofetil (MMF) or Cyclosporin A (CyA) were used as adjuvant immunosuppressive drugs. One hundred eighteen patients (81 males and 37 females) who satisfied the inclusion criteria (Table 1) were prescribed MS for a period of 12 months of which 112 patients were considered suitable for analysis. Age at entry ranged from 3.5 to 14.7 years (median 6.2). At the NS clinic, patients were reviewed on monthly basis focusing on urine protein excretion and potential side effects of MS therapy. Following the completion of 12 months of MS therapy these children were monitored for a further 12 month period focusing on viral infections and the relapse of the disease.
|
Inclusion Criteria |
Exclusion criteria |
|
· Patients with primary Steroid-sensitive Nephrotic syndrome (SSNS) · Two or more viral infection-induced relapses during the preceding 12 months · Presence of Minimal-change nephropathy (MCD) if biopsy had been performed · Patients on a minimum of 12 months of alternate-day Prednisolone treatment prior to the recruitment to the study. |
· NS presenting secondary to another disease · Presence of conditions other than MCD upon biopsy. |
Table 1: Inclusion and exclusion criteria to the study.
2.2 Study design
Montelukast sodium was prescribed for a period of one year at a dose of 4 mg for children below 6 years and 5 mg for above 6 years. The number of relapse episodes was compared with the twelve months before, during therapy and after completing MS therapy. Each patient had a patient health record and parents of the patients were trained to test and record urine protein excretion. A freshly voided urine sample was tested each morning and the presence of 3+ proteinuria, for three consecutive days was regarded as a relapse. In the case of a relapse the nephrology team was contacted by the parents or through their local healthcare clinic, and the patient was commenced on the standard relapse regimen. At each clinic visit the parents were interviewed to ascertain whether any side effects were observed namely cough, diarrhea, fever, headache, abdominal pain, runny nose and throat irritation. Full blood count, serum creatinine and liver enzymes (SGPT) were performed on all patients, every 6 months. All procedures performed in the study were in accordance with the ethical standards of the Scientific and Ethics committee, Faculty of Medicine of the University of Peradeniya and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed, written consent was obtained from the parents and/or the patients where appropriate prior to enrolment.
2.3 Statistical analysis
Results were described as the mean ± SD, as appropriate. The significance of the data was assessed using SPSS version 20.0 (SPSS Inc, Chicago, IL, U.S.A.). P values < 0.05 were considered significant. The rates of relapse episodes and mean steroid doses were analyzed by using t-tests.
3. Results
There was a significant reduction in the rate of relapse during the 12 months of MS therapy when compared to the 12 months prior to MS therapy (p<0.001). The mean yearly relapse rate was 1.41 during MS therapy, compared with 2.51 for the 12 months before, suggesting that prescribing MS can reduce the risk of relapse in frequently relapsing NS (Table 2 and Figure 1). The mean relapse rate for the 12 months post MS therapy is 1.72 which is significantly lower than the rate prior to therapy (p<0.001), suggesting a natural improvement to the disease with age or an alteration of the disease course by MS. The results also confirm that MS therapy has significantly reduced the number of viral URTI rate during MS therapy (Figure 2). No significant side-effects were encountered. Six children were excluded due to non-compliance.
|
Summary |
12 months prior to MS therapy |
12 months with MS therapy |
12 months after MS therapy |
|
Total number of relapses |
282 |
158 |
193 |
|
Mean relapse rate |
2.52 |
1.41 |
1.72 |
|
Number of patients (out of 112): ? Who did not relapse ? With one relapse ? With 2 relapses ? With 3 relapses ? With 4 relapses |
0 0 60 46 6 |
19 38 45 10 0 |
14 34 38 21 5 |
|
Mean steroid dose (mg/kg/year) |
156.52 |
87.87 |
107.17 |
Table 2: Summary of results in the years prior to, during and after MS therapy.
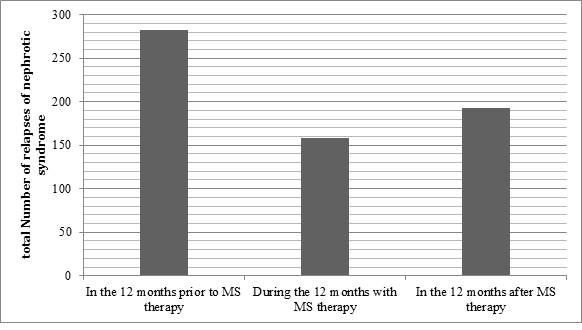
Figure 1: Comparison of the number of relapses during the three stages of the study.
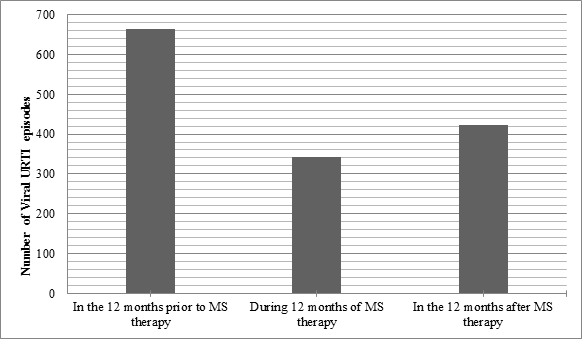
Figure 2: Comparison of the number of episodes of viral URTIencountered during the three stages of the study.
During the therapy some patients were under additional immunosuppressive drugs namely LEV, MMF and CyA. There was no significant difference of the outcome during MS therapy depending on the type of immunosuppressant drug used (p> 0.5). The sex and age of the patients did not have an effect on the relapse rates during treatment. The mean annual steroid dose during the 12-month period of MS therapy and after were significantly lower than mean annual steroid dose during the 12 months prior to MS therapy (p<0.001 and p<0.001 respectively). Moreover, the increase of mean annual steroid dose following MS therapy from that of during the therapy was also significant (p<0.001).
4. Discussion
Several studies reveal that MS is effective in delaying exacerbations of asthma arising due to viral respiratory tract infections [20, 21, 24] and also in reducing incidences of viral infections in adult asthmatic patients [22]. Kloepfer et al. found that MS attenuates eosinophilic inflammation in the airway and thereby it contributes to clinical benefits during a viral infection [25]. In another study MS attenuated initial response to primary respiratory syncytial virus infection and altered consequences of re-infections in mice [26]. This evidence points to a significant role of MS in reducing the incidences of viral infections. The results of this study too supported this observation with a significant reduction in the number of episodes of viral URTI observed during MS therapy. Hence it could be argued that this role of MS could be beneficial to patients with nephrotic syndrome as well. There is strong evidence that proteinuria in MCD is mediated by cytokines [27]. Several studies have shown the up regulation of IL-13 gene is correlated with relapses and long term outcome of children with NS [10, 28, 29]. Kimata et al. state that IL-13 is involved with the enhanced production of IgE and IgG in NS.[28]. Receptors for IL-13 have been demonstrated in podocytes, with evidence favouring direct effects of this cytokine on podocytes [30]. Considering these facts, a study by Lai et al. in 2007 revealed that IL-13 overexpression in the rat could lead to podocyte injury [11]. Although not yet studied extensively in the field of nephrology, IL-13 is known to influence leukotriene levels [31] and there is evidence of a cross-talk between IL-13 and leukotrienes in exerting the inflammatory effects of IL-13 [4, 32]. Leukotrienes are pro-inflammatory, arachidonic acid metabolites produced by leukocytes, eosinophils, mast cells, and macrophages via 5-lipoxygenase [33]. IL-13 can up-regulate both the leukocyte biosynthesis of leukotriene D4 (LTD4) and the expression of cellular type 1 cysteinyl leukotriene receptors (CysLT1). Moreover, LTD4 can up-regulate production of interleukin-13, as well as the expression of its receptor. The result of this cross-talk is a self-perpetuating circuit in which IL-13 and its receptor mediate some of the actions of LTD4 and LTD4-CysLT1 mediates some of the actions of IL-13 [34]. Based on these findings it is possible that apart from the reduction in URTI episodes during MS therapy, MSis potentially useful in inhibiting the pro-inflammatory effects of leukotrienes and thereby IL-13 resulting in fewer relapse episodes in NS.
The results of this study showed that the relapse rate in children with frequently relapsing NS decreased significantly after MS treatment with no significant side-effects. This could be mainly due reduction of viral upper respiratory infections by MS, which in turn resulted in a reduced relapse rate. An additional possibility is that MS acted to inhibit the pro-inflammatory effects of LTD4 and thereby IL-13. Thus reduced expression ofIL-13 could have restored permeability of the injured podocytes as well. Future in vitro studies could investigate such possibilities in order to establish mechanisms and pathways of Leukotriene receptor antagonists action in renal disease. These results suggest that the use of MS could be one of the potential therapeutic strategies in minimal change nephrotic syndrome, with the added advantage of reducing the steroid burden. Our results would be particularly of interest to South Asian and African countries where similar environmental and clinical settings to Sri Lanka are often found.
Funding
The study was self-funded.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Park SJ, Shin JI. Complications of nephrotic syndrome.KoreanJ Pediatr 54 (2011): 322-328.
- McKinney PA, Feltbower RG, Brocklebank JT, et al. Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire. UK.Pediatr Nephrol 16 (2001): 1040-1044.
- Banh T, Hussain-Shamsy N, Patel V, et al. Ethnic Differences in Incidence and Outcomes of Childhood Nephrotic Syndrome. Clin J Am Soc Nephrol 11 (2016): 1760-1768.
- Sinha M, MacLeod R, Rigby E, et al. Treatment of severe steroid-dependent nephrotic syndrome (SDNS) in children with tacrolimus. Nephrol Dial Transplant 21 (2006): 1848-1854.
- Uwaezuoke SN. Steroid-sensitive nephrotic syndrome in children: Triggers of relapse and evolving hypotheses on pathogenesis.Ital J Pediatr 41 (2015): 19.
- Niaudet P. Long-Term Outcome of Children with Steroid-Sensitive Idiopathic Nephrotic Syndrome. Clin J Am Soc Nephro 4 (2004): 1547-1548.
- Hall AS, Thorley G, Houtman PN. The effects of corticosteroids on behavior in children with nephrotic syndrome.Pediatr Nephrol 18 (2003): 1220-1223.
- Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet 362 (2003): 629-639.
- Cheung W, Wei CL, Seah CC, et al. Atopy, serum IgE, and interleukin-13 in steroid-responsive nephrotic syndrome. Pediatr Nephrol 19 (2004): 627-632.
- Yap HK, Cheung W,Murugasu B,et al. Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: evidence for increased IL-13 mRNA expression in relapse. Clin J Am Soc Nephrol 10 (1999): 529-537.
- Lai KW, Wei CL, Tan LK, et al. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J Am Soc Nephrol 18 (2007): 1476-1485.
- Macdonald N, Wolfish N, Maclane P, et al. Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J Pediatr 108 (1986): 378-382.
- Moorani KN. Infections are common cause of relapse in children with nephrotic syndrome. Pak Pediatr J 35 (2011): 213-219.
- Hulton SA, Shah V, Byrne MR, et al. Lymphocyte sub populations, interleukin-2 and interleukin-2 receptor expression in childhood nephrotic syndrome. Pediatr nephrol 8 (1994): 135-139.
- Aharony D. Pharmacology of leukotriene receptor antagonists.Am J Respir CritCareMed 157 (1998): 214-219.
- O’Byrne PM. Asthma treatment: antileukotriene drugs.Can Respir J5 (1998): 64-70.
- Wenzel SE. Leukotriene receptor antagonists and related compounds.Can Respir J6 (1999): 189-193.
- Damon M, Chavis C, Godard P, et al. Purification and mass spectrometry identification of leukotriene D4 synthesized by human alveolar macrophages.Biochem Biophys Res Commun111 (1983): 518-524.
- Williams JD, Czop JK, Austen KF. Release of leukotrienes by human monocytes on stimulation of their phagocytic receptor for particulate activators.J Immunol132 (1984): 3034-3040.
- Bisgaard H. A Randomized Trial of MS in Respiratory Syncytial Virus Postbronchiolitis.Am J Respir CritCareMed 167 (2003): 379-383.
- Tomita K, Sano H, Iwanaga T, et al. Association between episodes of upper respiratory infection and exacerbations in adult patients with asthma.J Asthma 49 (2012): 253-259.
- Matsuse H, Tsuchida T, Fukahori S, et al. Retrospective cohort study of leukotriene receptor antagonist therapy for preventing upper respiratory infection-induced acuteasthma exacerbations.Allergy Rhinol (Providence)4 (2013): 127-131.
- Abeyagunawardena AS, Trompeter RS. Increasing the dose of Prednisolone during viral infections reduces the risk of relapse in Nephrotic Syndrome: a randomised controlled trial. Arch Dis Child 93 (2008): 226-228.
- Leonardi S, Marchese G, Marseglia GL, et al. Montelukast sodium in allergic diseases beyond asthma. Allergy Asthma Proc 28 (2007): 287-291.
- Wu AY, Chik SC, Chan AW, et al. Anti-inflammatory effects of high-dose MS in an animal model of acute asthma.Clin ExpAllergy33 (2003): 359-366.
- Kloepfer K, DeMore J, Vrtis R, et al. Effects of MS on patients with asthma after experimental inoculation with humanrhino virus16. Ann Allergy Asthma Immunol 106 (2011): 252-257.
- Han J, Jia Y, Takeda K, et al. Montelukast sodium during Primary Infection Prevents Airway Hyperresponsiveness and Inflammation after Reinfection with Respiratory Syncytial Virus.Am J Respir CritCareMed 82 (2010): 455-463.
- Schnaper HW: The immune system in minimal change nephrotic syndrome.Pediatr Nephrol3 (1989): 101-
- Kimata H, Fujimoto M, Furusho K. Involvement of interleukin (IL)-13, but not IL-4, in spontaneous IgE and IgG4 production in nephrotic syndrome.Eur J Immunol 25 (1995): 1497-1501.
- Wei CL, Cheung W, Heng CK, et al. Interleukin-13 genetic polymorphisms in Singapore Chinese children correlate with long-term outcome of minimal-change disease.Nephrol Dial Transplant20 (2005): 728-734.
- van den Berg JG, Aten J, AnwarChand M, et al. Interleukin-4 and interleukin-13 act on glomerular visceral epithelial cells.J Am Soc Nephrol11 (2000): 413-422.
- Kang MJ, Lee SY, Kim HB, et al. Association of IL-13 polymorphisms with leukotriene receptor antagonist drug responsiveness in Korean children with exercise-induced bronchoconstriction. Pharmacogenet Genomics 18 (2008): 551-558.
- Vargaftig BB, SingerM. Leukotrienes mediate murine bronchopulmonary hyperreactivity, inflammation, and part of mucosal metaplasia and tissue injury induced by recombinant murine interleukin-13. Am J Respir Cell MolBiol 28 (2003): 410-419.
- Niimi A. Cough, asthma, and cysteinyl-leukotrienes.Pulm Pharmacol Ther26 (2013): 514-519.
- Peters-Golden M, Henderson W. Leukotrienes.N Engl J Med 357 (2007): 1841-1854.
