The Differential Effects of Radiofrequency Ablation and Hepatic Resection on Serum IL-10 Level in Patients with Liver Cancer
Article Information
Nona Janikashvili1*, Nino Kikodze1, Manana Iobadze1, Ketevan Mazmishvili1, Natela Paksashvili2, Ia Pantsulaia1, Ani Gvajaia1, Malkhaz Mizandari2, Tinatin Chikovani1
1Department of Immunology, Tbilisi State Medical University, 0186 Tbilisi, Georgia
2Department of Interventional Radiology, Tbilisi State Medical University, 0186 Tbilisi, Georgia
*Corresponding Author: Dr. Nona Janikashvili, Department of Immunology, Tbilisi State Medical University, 0186 Tbilisi, Georgia
Received: 24 July 2019, Accepted: 14 August 2019, Published: 28 August 2019
Citation: Janikashvili N, Kikodze N, Iobadze M, Mazmishvili K, Paksashvili N, Pantsulaia I, Gvajaia A, Mizandari M, Chikovani T. The Differential Effects of Radiofrequency Ablation and Hepatic Resection on Serum IL-10 Level in Patients with Liver Cancer. Journal of Cancer Science and Clinical Therapeutics 3 (2019): 137-142.
Share at FacebookAbstract
Liver cancer is a rapidly lethal cancer and is on the rise worldwide. Surgery is a gold standard treatment for primary liver cancer and liver metastases, however, non-surgical locoregional treatment options are of increasing clinical interest when tumor size, number and sites permit. Previous studies underline immunomodulatory effects of radiofrequency ablation (RFA) in patients with liver cancer. RFA seems to promote effector immune cell frequencies and diminishing immunosuppressive leukocytes. Nevertheless its impact on extracellular tumor matrix is practically uncovered. Herein we report on the differential effects of RFA and surgical liver resection on serum level of anti-inflammatory marker IL-10 in patients with hepatic cancer.
Keywords
Radiofrequency; Immunosuppressive; Liver; Anti-inflammatory
Radiofrequency articles, Immunosuppressive articles, Liver articles, Anti-inflammatory articles
Radiofrequency articles Radiofrequency Research articles Radiofrequency review articles Radiofrequency PubMed articles Radiofrequency PubMed Central articles Radiofrequency 2023 articles Radiofrequency 2024 articles Radiofrequency Scopus articles Radiofrequency impact factor journals Radiofrequency Scopus journals Radiofrequency PubMed journals Radiofrequency medical journals Radiofrequency free journals Radiofrequency best journals Radiofrequency top journals Radiofrequency free medical journals Radiofrequency famous journals Radiofrequency Google Scholar indexed journals Immunosuppressive articles Immunosuppressive Research articles Immunosuppressive review articles Immunosuppressive PubMed articles Immunosuppressive PubMed Central articles Immunosuppressive 2023 articles Immunosuppressive 2024 articles Immunosuppressive Scopus articles Immunosuppressive impact factor journals Immunosuppressive Scopus journals Immunosuppressive PubMed journals Immunosuppressive medical journals Immunosuppressive free journals Immunosuppressive best journals Immunosuppressive top journals Immunosuppressive free medical journals Immunosuppressive famous journals Immunosuppressive Google Scholar indexed journals Liver articles Liver Research articles Liver review articles Liver PubMed articles Liver PubMed Central articles Liver 2023 articles Liver 2024 articles Liver Scopus articles Liver impact factor journals Liver Scopus journals Liver PubMed journals Liver medical journals Liver free journals Liver best journals Liver top journals Liver free medical journals Liver famous journals Liver Google Scholar indexed journals Anti-inflammatory articles Anti-inflammatory Research articles Anti-inflammatory review articles Anti-inflammatory PubMed articles Anti-inflammatory PubMed Central articles Anti-inflammatory 2023 articles Anti-inflammatory 2024 articles Anti-inflammatory Scopus articles Anti-inflammatory impact factor journals Anti-inflammatory Scopus journals Anti-inflammatory PubMed journals Anti-inflammatory medical journals Anti-inflammatory free journals Anti-inflammatory best journals Anti-inflammatory top journals Anti-inflammatory free medical journals Anti-inflammatory famous journals Anti-inflammatory Google Scholar indexed journals Liver cancer articles Liver cancer Research articles Liver cancer review articles Liver cancer PubMed articles Liver cancer PubMed Central articles Liver cancer 2023 articles Liver cancer 2024 articles Liver cancer Scopus articles Liver cancer impact factor journals Liver cancer Scopus journals Liver cancer PubMed journals Liver cancer medical journals Liver cancer free journals Liver cancer best journals Liver cancer top journals Liver cancer free medical journals Liver cancer famous journals Liver cancer Google Scholar indexed journals lethal tumor articles lethal tumor Research articles lethal tumor review articles lethal tumor PubMed articles lethal tumor PubMed Central articles lethal tumor 2023 articles lethal tumor 2024 articles lethal tumor Scopus articles lethal tumor impact factor journals lethal tumor Scopus journals lethal tumor PubMed journals lethal tumor medical journals lethal tumor free journals lethal tumor best journals lethal tumor top journals lethal tumor free medical journals lethal tumor famous journals lethal tumor Google Scholar indexed journals radiofrequency thermal ablation articles radiofrequency thermal ablation Research articles radiofrequency thermal ablation review articles radiofrequency thermal ablation PubMed articles radiofrequency thermal ablation PubMed Central articles radiofrequency thermal ablation 2023 articles radiofrequency thermal ablation 2024 articles radiofrequency thermal ablation Scopus articles radiofrequency thermal ablation impact factor journals radiofrequency thermal ablation Scopus journals radiofrequency thermal ablation PubMed journals radiofrequency thermal ablation medical journals radiofrequency thermal ablation free journals radiofrequency thermal ablation best journals radiofrequency thermal ablation top journals radiofrequency thermal ablation free medical journals radiofrequency thermal ablation famous journals radiofrequency thermal ablation Google Scholar indexed journals microwave ablation articles microwave ablation Research articles microwave ablation review articles microwave ablation PubMed articles microwave ablation PubMed Central articles microwave ablation 2023 articles microwave ablation 2024 articles microwave ablation Scopus articles microwave ablation impact factor journals microwave ablation Scopus journals microwave ablation PubMed journals microwave ablation medical journals microwave ablation free journals microwave ablation best journals microwave ablation top journals microwave ablation free medical journals microwave ablation famous journals microwave ablation Google Scholar indexed journals cryoablation articles cryoablation Research articles cryoablation review articles cryoablation PubMed articles cryoablation PubMed Central articles cryoablation 2023 articles cryoablation 2024 articles cryoablation Scopus articles cryoablation impact factor journals cryoablation Scopus journals cryoablation PubMed journals cryoablation medical journals cryoablation free journals cryoablation best journals cryoablation top journals cryoablation free medical journals cryoablation famous journals cryoablation Google Scholar indexed journals immunomodulatory articles immunomodulatory Research articles immunomodulatory review articles immunomodulatory PubMed articles immunomodulatory PubMed Central articles immunomodulatory 2023 articles immunomodulatory 2024 articles immunomodulatory Scopus articles immunomodulatory impact factor journals immunomodulatory Scopus journals immunomodulatory PubMed journals immunomodulatory medical journals immunomodulatory free journals immunomodulatory best journals immunomodulatory top journals immunomodulatory free medical journals immunomodulatory famous journals immunomodulatory Google Scholar indexed journals
Article Details
1. Introduction
Liver cancer is a highly lethal tumor and the third most common cancer of digestive system according to SEER data. Surgery has always been regarded as a gold standard treatment for liver cancer. In recent years, however, patients with liver cancer are often managed by non-surgical locoregional treatments including ablative therapy (radiofrequency ablation, microwave ablation, cryoablation and so on). Among them radiofrequency thermal ablation (RFA) represents a minimal invasive treatment option and demonstrates good survival rate over the surgical resection when the tumor size is <3 cm [1-3]. RFA results in higher rate of tumor necrosis, nevertheless tumor size, number and sites are of central importance when performing RFA. In addition to its clinical benefits, experimental studies report that RFA provides adjuvant/”danger” signals to the immune cells, as a consequence it stimulates CD4+ T helpers and causes a drastically increase of antigen-specific CD8+ T cells within the tumor microenvironment and tumor-draining lymph node [4-6]. In light of its immunomodulatory effects, we have recently demonstrated than RFA application correlates with the diminished NLR and PLR and reduced frequencies of CD4+CD39+ lymphocytes in patients with liver cancer [7]. We and others have additionally shown that the decrease of certain immunoregulatory markers corresponds to a better survival of patients with hepatic cancer [7, 8]. Therefore, RFA, by influencing tumor microenvironment, potentially imposes the immune surveillance of cancer calls. Importantly, however, cellular composition of tumor microenvironment and immune cell recruitment at tumor beds are largely guided by extracellular matrix proteins such as cytokines and chemokines which are negligibly explored in regards to RFA treatment [9, 10].
Among several regulatory cytokines, numerous studies have uncovered the cardinal role of IL-10 not only in deviating anti-inflammatory immune responses in patients with liver cancer, but also in influencing the malignant transformation of hepatocytes [11, 12]. IL-10 triggers the upregulation of Bcl-2 and the downregulation of apoptosis [12]. Interestingly, HCV patients with high serum level of IL-10 are more susceptible to develop HCC than ones with low IL-10 [13]. Though, liver fibrosis and necrosis support lowering IL-10 in such patients [13-15]. Studies also support to the knowledge that IL-10 assists the detachment of tumor cells from their primary sites and promotes the development of metastases [13]. Thus, IL-10 represents an important target of intervention and treatment of hepatic cancer.
Herein we report on the differential effects of RFA and LR on serum IL-10 levels in patients with hepatic cancer. Such findings should facilitate the patients selections for effective treatment options and could broaden the armamentarium of successful application of RFA.
2. Material and Methods
2.1 Patients
A total of 17 patients with primary (HCC and Cholangiocarcinoma) and secondary (metastatic) liver cancer were enrolled in our study. The study was approved by the Ethic Committee of the Tbilisi State Medical University and informed consent obtained from each recruited patient in accordance with the Declaration of Helsinki. Out of 17 patients, 7 were referred to RFA procedure and another 10 underwent surgical liver resection (LR). All decisions regarding procedures were tabled by MDT (Multidisciplinary Team). The inclusion criteria for the RFA patients selection were as follows: a) extensive liver disease or medical co-morbidities associated with tumor vascular invasion and thromboses, b) fewer than three nodules without extrahepatic metastasis, c) largest tumor size of 3-4 cm in diameter, d) visualization of the nodule during the planning of RFA by ultrasonography. The exclusion criteria included treatment by chemotherapy or TACE, RFA or LR within previous one month. Within LR group, 3 patients underwent anatomic segmental hepatectomy and non-anatomical resection was done in 7 patients. None of the patient in either group had received chemotherapy or any other treatment a month before or 3 months after the LR and RFA procedure. Healthy age-matched 14 volunteers without a history of cancer, recent acute or chronic infectious disease, or autoimmune disease, were used as controls.
2.2 Treatment techniques
An image-guided RFA procedure was performed as described in [2]. Briefly, the tumor was localized and RF antenna introduced into the target tissue under ultrasound guidance. RF processing increases temperature into the target tissue up to 1020C leading to the irreversible damage by coagulative necrosis. Abdominal contrast computed tomography (CT) was performed to document completeness of the procedure. Liver resection (LR), both anatomic segmental hepatectomy and non-anatomical liver resection, were carried out under the general anaesthesia using an upper middle incision, using non-RF based liver resection devices.
2.3 Cytokine measurement
Blood samples were collected from each patient at the diagnosis and after 1 and 3 months of treatment (RFA or LR). The following serum cytokines: IL-10, IL-17, INF-γ, TGF-β were assayed by ELISA (ebiosciences, USA). Data were analyzed using Graph Pad Prizm software and the Student’s t- test was applied to compare data between RFA and LR groups. Furthermore, both study groups were compared with healthy control subjects. P values less than 0.05 were considered as statistically significant.
3. Results and Discussion
We and others have previously reported that the destruction of tumor microenvironment by RFA results in accumulation of necrotic cancer cells, release of a wide spectrum of tumor antigens and adjuvants therefore favoring effector immune responses [4-6]. We have also demonstrated that besides the elevation of pro-inflammatory immune cells, RFA triggers the diminution of immunosuppressive cells in blood of patients with hepatic cancer [7]. However, changes in immune cell characteristics largely reflect the altered extracellular matrix composition. Data on pro- vs. anti-inflammatory cytokines and chemokines are still limited in patients receiving RFA.
A total of 17 patients with liver cancer was included in the study. Patients’ demographic characteristics have been listed and compared between treatment groups in Table 1. The mean age of patients in RFA and LR group was 55.1 ± 11.2 years and 58.6 ± 8.1 years respectively (p>0.05). There were 3 women (43%) and 4 men (57%) in the RFA cohort whilst, 5 women (50%) and 5 (50%) men in the LR group. No significant differences were observed between groups regarding the number of tumors, tumor size, tumor stage, HBsAg, Anti-HCV positivity.
Serum levels of IL-10 were elevated in patients referred to RFA compare to healthy age-matched volunteers (6.97 ± 2.23 vs. 3.6 ± 0.12, P<0.05). 1 month after RFA procedure serum level of IL-10 declined (4.23 ± 0.42, P=0.01) and was comparable with control. 3 months after RFA procedure serum level of IL-10 continued the reduction (2.4 ± 0.76, P=0.03). In the patients before the liver resection serum level of IL-10 was comparable with indices in healthy volunteers. 1 month after liver resection (LR) serum level of IL-10 was increased (4.78 ± 1.07, P=0.018) and continued enhancement at the point of 3 months post - LR (4.97+0.78, P=0.006) (Figure 1). No significant effect of either treatment (RFA and LR) was observed with other pro- or anti-inflammatory cytokines: IFNγ, IL-17 and TGFβ evaluated in the same patients (data not shown).
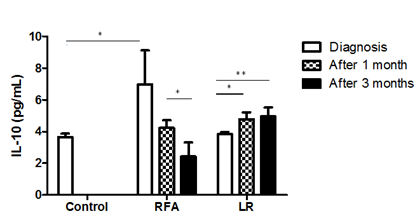
Figure 1: Serum IL-10 levels in patients with liver cancer before and after 1 and 3 months of RFA and Liver resection. (LR group- Diagnosis vs. after 1 month - P=0.018; Diagnosis vs. after 3 months - P =0.006; RFA group - Diagnosis vs. after 1 month - P=0.01; After 1 month vs. after 3 months - P=0.03).
Cytokines are the main mediators for the growth, invasion and metastasis of cancer. They are produced by cancer cells as well as immune and stromal cells in the affected area and are responsible for further uncontrolled proliferation of malignant cells, remodeling of the tumor microenvironment, triggering of intrinsic inflammation, recruiting of cells, angiogenesis and cancer cells spread [16]. Pre-treatment serum level of cytokines also widely varies in patients with liver cancer and highly determines prognosis of diseases. Il-10 is a key anti-inflammatory cytokine which is often correlated with the disease prognosis in patients with solid tumors, including those with primary or metastatic hepatic cancer. Tumor cells and tumor-in?ltrating immune cells are the major source of IL-10 in the extracellular matrix [17]. In tumor microenvironment, it exerts immunosuppressive effect on antigen presentation and recruitment of effector lymphocytes therefore supporting escape of tumor cells from immune recognition. IL-10, together with TNF-a, stimulates the expression of negative costimulatory molecule B7-H1(PDL-1) on macrophages in an autocrine manner, thereby impairs CD8+ T cell activity and supports tumor progression [17].
Previous reports demonstrate that IL-10 modulates liver ?brogenesis and promotes carcinogenesis [13-15]. Interestingly, Aroucha et al reported that the severe necro-in?ammatory state is associated with low IL-10 production in HCC patients [18]. In this line, our results revealed that, RFA, which establishes severe necro-in?ammatory state, significantly lowers serum levels of IL-10, in contrast to LR. This data indicate that tumor removal itself does not impact IL-10 and this process is more complex involving microenvironmental remodeling.
Noteworthy to highlight that opposite to RFA, patients in LR group showed elevated IL-10 levels that kept increasing 3 month post surgery. It has long supposed that local immune responses to surgery lead to systemic proinflammatory and immunosuppressive phases: production of a variety of cytokines, leading to a general inflammation: stress, activation of hypothalamic-pituitary-adrenal axis, release of steroids, such as cortisol, facilitates to the healing of injured tissues. Immunosuppressive phase avoids autoreactivity but on the other hand, it can inhibit antitumor Th1 immune response, provoke development of postoperative immune suppression and stimulate tumor cells growth.
In conclusion, RFA and LR have a distinct impact on tumor microenvironment and its further exploration should, in a long term, improve the guidance of treatment in clinical cases.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
Acknowledgments
This work is supported by Shota Rustaveli National Science Foundation (Grant # DI/3/8-335/13).
References
- Cho YK, Rhim H, Noh S. Radiofrequency ablation versus surgical resection as primary treatment of hepatocellular carcinoma meeting the Milan criteria: A systematic review. J Gastroenterol Hepatol 26 (2011): 1354-1360.
- Mizandari M, Ao G, Zhang Y, et al. Novel percutaneous radiofrequency ablation of portal vein tumor thrombus: Safety and feasibility, Cardiovascular and interventional radiology 36 (2013): 245-248.
- Mizandari M, Pai M, Xi F, et al. Percutaneous intraductal radiofrequency ablation is a safe treatment for malignant biliary obstruction: Feasibility and early results, Cardiovascular and interventional radiology 36 (2012): 814-189.
- Napoletano C, Taurino F, Biffoni M, et al. RFA strongly modulates the immune system and anti-tumor immune responses in metastatic liver patients, International journal of oncology 32 (2008): 481-490.
- Zerbini A, Pilli M, Penna A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses, Cancer Res 66 (2006): 1139-1146.
- Liu Q, Zhai B, Yang W, et al., Abrogation of local cancer recurrence after radiofrequency ablation by dendritic cell-based hyperthermic tumor vaccine, Mol Ther 17 (2009): 2049-2057.
- Mazmishvili K, Jayant K, Janikashvili N, et al. Study to evaluate the immunomodulatory effects of radiofrequency ablation compared to surgical resection for liver cancer. J Cancer 9 (2018): 3187-3195.
- Mizandari M, Paksashvili N, Kikodze N, et al. Long-term survival in a patient with low-level inflammatory markers and liver metastasis, converted resectable by TACE. Immunotherapy 9 (2017):1067-1069.
- Hernandez-Gea V, Toffanin S, Friedman SL. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology (2013): 512-527.
- Wang X, Hassan W, Jabeen Q. Interdependent and independent multidimensional role of tumor microenvironment on hepatocellular carcinoma. Cytokine 103 (2017): 150-159.
- Birgani MT, Carloni V. Tumor Microenvironment, a Paradigm in Hepatocellular Carcinoma Progression and Therapy, Int. J. Mol. Sci 18 (2017): 405.
- Shuai Zhao, Dang Wu, Pin Wu, et al. Serum IL-10 Predicts worse outcome in cancer patients: A Meta-analysis, Plos One 10 (2015): e013959.
- Sghaier I, Mouelhi L, Rabia NA, et al. Genetic variants in IL-6 and IL-10 genes and susceptibility to hepatocellular carcinoma in HCV infected patients. Cytokine 89 (2017): 62-67.
- Wei YG, Liu F, Li B, et al. Interleukin-10 gene polymorphisms and hepatocellular carcinoma susceptibility: a meta-analysis. World J Gastroenterol 17 (2011): 3941-3947.
- Zhang LJ, Wang XZ. Interleukin-10 and chronic liver disease. World J Gastroenterol 12 (2006): 1681-1685.
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 140 (2010): 883-899.
- Capece D, Gaggiano A, Verzella D, et al. The inflammatory microenvironment in hepatocellular carcinoma: A pivotal role for tumor-associated macrophages, BioMed Research International (2013): ID187204.
- Aroucha DC, Carmo RF, Vasconcelos LR, et al. TNF-α and IL-10 polymorphisms increase the risk to hepatocellular carcinoma in HCV infected individuals. J Med Virol 88 (2016): 1587-1595.
