The Association between Physician Involvement During Pediatric Out-of- Hospital Cardiac Arrest and Patient Outcomes: a Japanese Nation-Wide Observational Study
Article Information
Shunichi Otaka1,2*, Hiroyuki Ohbe3, Ryuhei Igeta1,2, Takuyo Chiba1,2, Shunya Ikeda4, Takashi Shiga1,2
1Department of Emergency Medicine, International University of Health and Welfare Narita Hospital, Chiba, Japan
2Graduate School of Medicine, International University of Health and Welfare, Chiba, Japan
3Department of Clinical Epidemiology and Health Economics, School of Public Health, The University of Tokyo, Tokyo, Japan
4Department of Public Health, School of Medicine, International University of Health and Welfare, Chiba, Japan
*Corresponding author: Shunichi Otaka, Department of Emergency Medicine, International University of Health and Welfare Narita Hospital, 852 Hatakeda, Narita City, Chiba 286-8520, Japan.
Received: 30 July 2022; Accepted: 2 August 2022; Published: 8 August 2022
Citation: Shunichi Otaka, Hiroyuki Ohbe, Ryuhei Igeta, Takuyo Chiba, Shunya Ikeda, Takashi Shiga. The Association between Physician Involvement During Pediatric Out-of-Hospital Cardiac Arrest and Patient Outcomes: a Japanese Nation-Wide Observational Study. Cardiology and Cardiovascular Medicine 6 (2022): 424-431.
Share at FacebookAbstract
Background: The effectiveness of physician involvement during out-ofhospital cardiac arrest has been shown in adults but remains unknown in pediatric patients. This study aimed to investigate the association between physician involvement during pediatric out-of-hospital cardiac arrest and patient outcomes.
Methods: Using a Japanese nationwide database, we identified pediatric patients with out-of-hospital cardiac arrest from January 2005 to September 2017. We used a generalized linear model to compare outcomes between patients with and without physician involvement during pediatric outof- hospital cardiac arrest. The primary outcome was neurologically favourable survival after 1 month. Secondary outcomes were the return of spontaneous circulation and 1-month survival.
Results: In total, 24,684 patients were included in this study. In the adjusted analyses, a ‘physician-present’ group showed a lower 1-month neurologically favourable survival rate compared with a ‘physicianabsent’ group (difference, -0.9%; 95% confidence interval [CI] -1.7–- 0.2; P = 0.02). The physician-present group had a higher rate of return of spontaneous circulation than the physician-absent group (difference, 1.4%; 95% CI 0.2–2.5; P = 0.02), but no difference in 1-month survival was observed between the groups (difference, -0.9%; 95% CI -2.0–0.2; P = 0.09).
Conclusions: Physician involvement during pediatric out-of-hospital cardiac arrest was associated with lower 1-month neurologically favourable survival rates, although it was associated with a better return of spontaneous circulation than that in the physician-absent group.
Cardiac Arrest articles; Database articles; Emergency Medicine articles; Out-Of-Hospital Cardiac Arrest articles; Pediatric articles; Prehospital articles
Article Details
List of Abbreviations:
OHCA- Out-of-Hospital Cardiac Arrest; ROSC- Return of Spontaneous Circulation; EMS- Emergency Medical Service; ECG- Electrocardiogram; CPR- Cardiopulmonary Resuscitation; CPC- Cerebral Performance Categories
1. Background
Out-of-hospital cardiac arrest (OHCA) is reported to be rare in the pediatric population.1 Although pediatric patients are more likely to be discharged alive than adult patients, their neurological outcome is a concern [1,2]. To achieve better survival and neurological outcomes, early involvement of physicians can be expected to improve outcomes. Several studies have shown the effectiveness of physician involvement in OHCA [3-8]. One Japanese observational study reported that patients with physician involvement had higher return of spontaneous circulation (ROSC) and 1-month survival rates and neurologically favourable outcomes compared with those without physician involvement [4]. Another nationwide observational study conducted in Denmark also showed that physician involvement was associated with higher ROSC and 1-month survival rates [5]. However, these studies mainly involved adult patients. At present, while there are reports of physician involvement in pediatric OHCA, there is no clear evidence of the associated benefits; hence, the rationale for this study. This study aimed to investigate the association between physician involvement during pediatric OHCA and patient outcomes using a nationwide prehospital database in Japan. We hypothesized that physician involvement may be associated with better overall survival and neurologically favourable outcomes in pediatric patients with OHCA, as indicated in results reported concerning adult patients with OHCA.
2. Methods
2.1 Emergency Medical Service (EMS) System in Japan: In 2019, there were 726 fire stations with dispatch centers in Japan. The Fire and Disaster Management Agency of Japan supervise the EMS system. In Japan, the emergency team is usually not permitted to terminate the resuscitation of patients with OHCA in a prehospital setting. All patients with OHCA are transported to hospital except in cases of decapitation, incineration, decomposition, and rigor mortis. Therefore, the Japanese EMS system is ideal for investigating the association between physician involvement during pediatric OHCA and patient outcomes. In a prehospital setting, the EMS team, comprising three people, can perform the following procedures for patients with OHCA: (i) obtain and assess an initial electrocardiogram (ECG), (ii) use an automated external defibrillator; place a peripheral intravenous line, administer Ringer’s lactate solution, and administer adrenaline (epinephrine) intravenously; and ventilate using a bag valve mask; and (iii) establish an advanced airway using an endotracheal tube, a laryngeal mask airway, a Combi-tube, and an esophageal gastric tube airway. Some procedures require instructions from a physician remotely. Patients with OHCA are treated according to Japanese Cardiopulmonary Resuscitation (CPR) guidelines, which are based on guidelines issued by the American Heart Association, the European Resuscitation Council, and the International Liaison Committee on Resuscitation. If an EMS team determines that it would be preferable to have a physician at the scene, the team picks up a hospital physician while enroute to the scene or calls a physician to attend the scene. However, there are no strict criteria for requesting physicians to attend a scene, with many complex factors such as the severity of the patient's illness, geographical conditions, and local medical resources influencing this decision.
2.2 Data Collection: We collected patient data from an ongoing prospective Japanese nationwide prehospital database, namely, the All-Japan Utstein Registry of the Fire and Disaster Management Agency. The database has been described in detail elsewhere [3,4,9]. Briefly, this population-based registry covers approximately 127 million residents in Japan. All patients with OHCA have been registered. Data have been collected since January 2005 and continue to be accumulated. The registry is based on the International Utstein style [10]. The database contains patients’ age (years), sex, date of the OHCA, call time to the EMS, suspected etiology of cardiac arrest, type of witness, interventions by bystanders (chest compression, rescue breathing, and defibrillation), provision of dispatcher CPR instructions, initial ECG rhythm at the time of EMS contact, interventions by healthcare providers (defibrillation, advanced airway device use, intravenous fluids, and adrenaline use), EMS arrival time at the scene, and outcomes (ROSC, 1-month survival, and 1-month cerebral performance categories [CPCs]).
2.3 Patient Selection: We collected data concerning pediatric patients with OHCA (age range, 0–18 years) from January 1, 2005 to September 9, 2017. We excluded patients with unrealistic time records, unknown outcome status, and patients who did not receive any resuscitation by bystanders or healthcare providers. We divided the patients into two groups: 1) patients who received physician involvement on site during OHCA (physician-present group), and 2) patients who did not (physician-absent group). Physician involvement was defined in the database as any physician present, regardless of whether or not a procedure was performed.
2.4 Variables and Outcomes: We considered the following variables: age, sex, year of the OHCA, season, call time to EMS, suspected etiology of cardiac arrest, type of witness, interventions by bystanders (chest compression, rescue breathing, and defibrillation), the provision of dispatcher CPR instructions, initial ECG rhythm at the time of EMS contact, and time from emergency call to EMS arrival at the scene, to perform adjustments required for these patients.
We subdivided the patients into the following age categories: new-borns and infants (0 years), toddlers to younger grade-schoolers (1–7 years), children (8–12 years), and adolescents (13–18 years).11–13 The year of the OHCA was categorized as follows: 2005–2009, 2010–2013, and 2014–2017. We also categorized the seasons as follows: January–March, April–June, July–September, and October–December. We divided the time of cardiac arrest into morning (0600 h to 0900 h), daytime (0900 h to 1700 h), evening (1700 h to 2200 h), and night-time (2200 h to 0600 h) based on previous studies and patterns of medical staffing [11,14,15]. The etiology of cardiac arrest was grouped into cardiac causes, other medical causes (respiratory disease, cerebrovascular disease, malignant tumor, hypothermia, anaphylaxis, and any medical disease), and external causes (traffic accident, suffocation, drowning, and trauma). The time from the emergency call to the EMS arrival at the scene was divided equally into four categories: <6 min, 6–7 min, 8–9 min, and >9 min. The primary outcome was 1-month neurologically favourable survival using Glasgow-Pittsburgh CPCs as measures of neurological outcomes [10]. CPCs are defined as follows: CPC1, good cerebral performance; CPC2, moderate cerebral disability; CPC3, severe cerebral disability; CPC4, coma and vegetable state; and CPC5, brain death. We defined CPC1 and CPC2 as ‘neurologically favourable survival [16]. The secondary outcomes were ROSC and 1-month survival rates.
2.5 Statistical Analysis: Continuous variables are expressed as mean and standard deviation, and categorical variables are expressed as frequencies and percentages. We used absolute standardized differences to assess differences in the variables between the groups. We regarded an absolute standardized difference of <10% as well balanced [17,18]. Unadjusted outcomes were compared using chi-square tests. In adjusted analyses, we used a generalized linear model to compare the outcomes between the groups with and without physician involvement during pediatric OHCA. We used the following independent variables: physician involvement, age, sex, year of the OHCA, season, time of arrest, suspected etiology of cardiac arrest, witness status, interventions by a bystander, provision of dispatcher CPR instructions, initial ECG rhythm at the time of EMS contact, and time from emergency call to EMS arrival at the scene. Subgroup analyses were performed for each variable to investigate its association with 1-month neurologically favourable survival. To confirm the robustness of our results, we performed a sensitivity analysis using overlap weights to balance the patients’ backgrounds between the groups [19-21]. Overlap weights are a propensity score weighting method to adjust for potential confounders [22]. The weights are defined as a value of 1 – the propensity score for treated patients and the propensity score itself for untreated patients. In this study, we calculated the propensity score for receiving physician care using a multivariable logistic regression model including the following variables: age, sex, year of the OHCA, season, time of arrest, suspected etiology of cardiac arrest, witness status, interventions by a bystander, provision of dispatcher CPR instructions, initial ECG rhythm at the time of EMS contact, and time from emergency call to EMS arrival at the scene. We then constructed an overlap-weighted generalized linear model to compare primary outcomes. We reported the results as risk differences, 95% confidence intervals (CIs), and two-sided P-values. We considered a two-sided P-value of <0.05 as statistically significant. Statistical analyses were performed using STATA (version 16.0; StataCorp, College Station, TX, USA) software.
2.6 Ethics Statement: This study was approved by the Institutional Review Board of the International University of Health and Welfare, Narita Hospital, Japan (approval number: 21-Im-043, September 28, 2021). The requirement for informed consent was waived due to the use of anonymized data.
3. Results
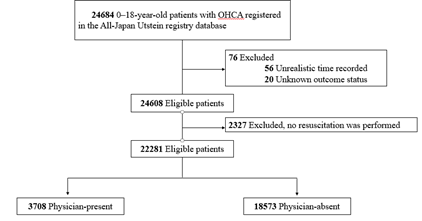
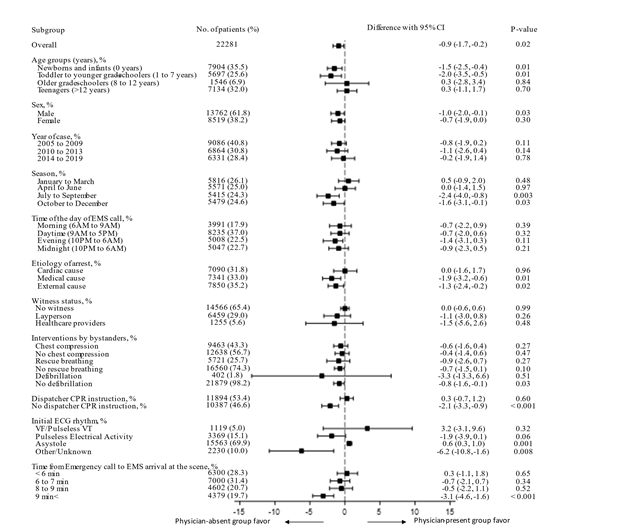
This study included 24,684 patients. A patient flowchart is shown in Figure 1. After applying the exclusion criteria, 22,281 patients qualified for the study. In total, there were 3708 patients in the physician-present group and 18,573 patients in the physician-absent group. Patient baseline characteristics are shown in Table 1. Compared with patients in the physician-absent group, patients in the physician-present group were older, experienced more cardiac arrests during the daytime, had fewer instances of dispatcher CPR instructions provided, and had a longer time from the emergency call to EMS arrival at the scene. In the unadjusted analyses, no differences were observed between the two groups in terms of 1-month neurologically favourable survival (8.3% vs. 8.4%; P = 0.84) and 1-month survival (15.7% vs. 15.3%; P = 0.55). In terms of ROSC, the physician-present group had better outcomes than the physician-absent group (20.9% vs. 18.0%; P < 0.001). The generalized linear model results are presented in Table 2. Compared with the physician-absent group, the physician-present group had a lower 1-month neurologically favourable survival rate (difference, -0.9%; 95% CI -1.7–-0.2; P = 0.02) and a higher ROSC rate (difference, 1.4%; 95% CI 0.2–2.5; P = 0.02), but with no difference in 1-month survival (difference, -0.9%; 95% CI -2.0–0.2; P = 0.09). Figure 2 shows the results of subgroup analyses for each variable. Patients with initial ECGs showing asystole had higher 1-month neurologically favourable survival rates with physician involvement than those without (difference, 0.62%; 95% CI 0.26–0.99; P < 0.001). However, in other subgroups, the physician-present group was not found to have higher 1-month neurologically favourable survival rates than the physician-absent group. Table 3 shows the results of the sensitivity analyses using the overlap weights. These results were similar to those of the main analyses.
|
Variables |
Physician-present |
Physician-absent |
Absolute |
||
|
(n = 3708) |
(n = 18573) |
standardized difference (%) |
|||
|
Age: years, mean (SD) |
6.5 |
-6.8 |
6.5 |
-7 |
0.1 |
|
Age groups: years, n (%) |
6.1 |
||||
|
New-borns and infants (0 years) |
1263 |
-34.1 |
6641 |
-35.8 |
|
|
Toddler to younger grade-schoolers (1–7 years) |
1006 |
-27.1 |
4691 |
-25.3 |
|
|
Older grade-schoolers (8–12 years) |
286 |
-7.7 |
1260 |
-6.8 |
|
|
Adolescents (>12 years) |
1153 |
-31.1 |
5981 |
-32.2 |
|
|
Sex (male), n (%) |
2335 |
-63 |
11427 |
-61.5 |
3 |
|
Year of OHCA, n (%) |
17.3 |
||||
|
2005 to 2009 |
1775 |
-47.9 |
7311 |
-39.4 |
|
|
2010 to 2013 |
982 |
-26.5 |
5882 |
-31.7 |
|
|
2014 to 2017 |
951 |
-25.6 |
5380 |
-29 |
|
|
Season, n (%) |
9.6 |
||||
|
January to March |
857 |
-23.1 |
4959 |
-26.7 |
|
|
April to June |
920 |
-24.8 |
4651 |
-25 |
|
|
July to September |
997 |
-26.9 |
4418 |
-23.8 |
|
|
October to November |
934 |
-25.2 |
4545 |
-24.5 |
|
|
Call time to EMS, n (%) |
21.1 |
||||
|
Morning (0600 h to 0900 h) |
577 |
-15.6 |
3414 |
-18.4 |
|
|
Daytime (0900 h to 1700 h) |
1669 |
-45 |
6566 |
-35.4 |
|
|
Evening (1700 h to 2200 h) |
797 |
-21.5 |
4211 |
-22.7 |
|
|
Night-time (2200 h to 0600 h) |
665 |
-17.9 |
4382 |
-23.6 |
|
|
Etiology of cardiac arrest, n (%) |
8 |
||||
|
Cardiac cause |
1075 |
-29 |
6015 |
-32.4 |
|
|
Medical cause |
1233 |
-33.3 |
6108 |
-32.9 |
|
|
External cause |
1400 |
-37.8 |
6450 |
-34.7 |
|
|
Witness status, n (%) |
6.8 |
||||
|
No witness |
2323 |
-62.7 |
12243 |
-65.9 |
|
|
Layperson |
1160 |
-31.3 |
5299 |
-28.5 |
|
|
Healthcare providers |
224 |
-6 |
1031 |
-5.6 |
|
|
Interventions by bystanders, n (%) |
|||||
|
Chest compression |
2201 |
-59.4 |
10437 |
-56.2 |
6.4 |
|
Rescue breathing |
1062 |
-28.6 |
4659 |
-25.1 |
8 |
|
Defibrillation |
79 |
-2.1 |
323 |
-1.7 |
2.8 |
|
Dispatcher CPR instruction, n (%) |
1817 |
-49 |
10077 |
-54.3 |
10.5 |
|
Initial ECG rhythm, n (%) |
9.3 |
||||
|
VF/ Pulseless VT |
208 |
-5.6 |
911 |
-4.9 |
|
|
Pulseless electrical activity |
652 |
-17.6 |
2717 |
-14.6 |
|
|
Asystole |
2471 |
-66.6 |
13092 |
-70.5 |
|
|
Other/ Unknown |
377 |
-10.2 |
1853 |
-10 |
|
|
Time from emergency call to EMS arrival at the scene, n (%) |
11.2 |
||||
|
<6 min |
1060 |
-28.6 |
5240 |
-28.2 |
|
|
6–7 min |
1091 |
-29.4 |
5909 |
-31.8 |
|
|
8–9 min |
699 |
-18.9 |
3903 |
-21 |
|
|
9 min< |
858 |
-23.1 |
3521 |
-19 |
|
Table 1: Patients' Baseline Characteristics in the ‘Physician-Present’ and ‘Physician-Absent’ Groups.
CPR, cardiopulmonary resuscitation; ECG, electrocardiogram; EMS, emergency medical services; SD, standard deviation; VF, ventricular fibrillation; VT, ventricular tachycardia
|
Outcomes |
Difference (%) |
95% CI (%) |
P-value |
||
|
1-month neurologically favourable survival |
-0.9 |
-1.7 |
to |
-0.2 |
0.02 |
|
ROSC |
1.4 |
0.2 |
to |
2.5 |
0.02 |
|
1-month survival |
-0.9 |
-2 |
to |
0.2 |
0.09 |
Table 2: Generalized Linear Models Comparing ‘Physician-Present’ and ‘Physician-Absent’ Group Outcomes after Adjusting for Prehospital Variable.
CI, confidence interval; ROSC, return of spontaneous circulation
|
Outcomes |
Physician-present (%) (n = 3708) |
Physician-absent (%) (n = 18573) |
Difference(%) |
95% CI (%) |
P-value |
||
|
1-month neurologically favourable survival |
8.2 |
9.1 |
-0.9 |
-1.7 |
to |
-0.2 |
0.02 |
|
ROSC |
20.7 |
19.3 |
1.4 |
0.3 |
to |
2.4 |
0.01 |
|
1-month survival |
15.6 |
16.5 |
-0.9 |
-1.9 |
to |
0.1 |
0.07 |
Table 3: An Overlap-Weighted Generalized Linear Model Comparing Physician-Present and Physician-Absent Group Outcomes.
CI, confidence interval; ROSC, return of spontaneous circulation
4. Discussion
We investigated the association between physician involvement during pediatric OHCA and patient outcomes using a nationwide prehospital database in Japan. The physician-present group showed lower 1-month neurologically favourable survival rates than the physician-absent group. However, pediatric patients with OHCA and an asystole rhythm showed higher 1-month neurologically favourable survival rates compared to patients with other rhythm. For secondary outcomes, there was no difference in 1-month survival rates between the groups; however, the physician-present group had a higher ROSC rate than the physician-absent group. Compared with previous studies that have focused on adult patients with OHCA, neurologically favourable survival and 1-month survival rates were inconsistent.4–7 Our results can be explained as follows. First, according to the subgroup analysis, fewer instances of dispatcher CPR instructions and longer times from the emergency call to EMS arrival at the scene may have affected our finding that the physician involvement group had poorer outcomes. One possibility is that because a physician was dispatched, the dispatcher CPR instructions might not have been emphasized for patients who would have otherwise required appropriate CPR instruction. Moreover, the extra time required to dispatch a physician to the scene may have delayed the arrival and interventions of other healthcare providers. As a result, the quality of CPR in the group with physicians present may have been diminished. Second, there were differences noted in terms of the etiology of the OHCA. Coronary artery disease is the most common cause of cardiogenic cardiac arrest in adults, whereas congenital heart disease is reported to be the most common cause in pediatric patients [23,24]. After resuscitation, acute coronary syndromes can be treated with percutaneous coronary intervention; however, treating congenital heart disease is very challenging. The resuscitation rate is high with early intervention by physicians; however, for this reason, the prognosis of OHCA at 1 month may not be satisfactory. Third, oxygenation is less important for resuscitation in patients with ventricular fibrillation/pulseless ventricular tachycardia than for those in asystole as sufficient oxygen may remain in the blood; therefore, chest compressions and defibrillation are effective during CPR [25-27]. However, in the case of patients in asystole, there is insufficient oxygen in the blood, and establishing a secured airway and administering oxygen may be necessary for resuscitation. Physician involvement in patients with asystole showed higher neurologically favourable survival rates. A similar finding was observed in a study concerning the relationship between ECG waveforms and adrenaline administration [28]. The effects of adrenaline differed depending on the initial waveform. Individualized medical interventions using ECG waveforms are expected in the future. Our study has several implications for improving the outcomes of physician involvement in OHCA in future. First, it is recommended that a dispatcher provide CPR instructions, even when physicians are being sent to the scene. Second, it may be advisable to establish an EMS that reduces the time to physician arrival at the scene. Third, in future, if technology enables the detection of ECG waveforms immediately after the occurrence of OHCA, it may be desirable to develop within the EMS the ability to choose whether to prioritize dispatching a physician or arriving at the scene earlier with the emergency team only. This study had several strengths. First, this is the first study to evaluate the association between physician involvement during pediatric OHCA and patient outcomes. Second, we adjusted for patients’ backgrounds using a generalized linear model with several prehospital information variables. Third, we performed overlap weights for the sensitivity analysis and confirmed the robustness of the analysis. The results were found to be the same as those of the main analyses. This study had some limitations. First, the database did not include information regarding patients’ backgrounds, such as past medical history, comorbidities, and socioeconomic status. Second, we could not determine why a decision to dispatch a physician was made in each case. Third, because of the nature of this prehospital database, there was no information on in-hospital care. Fourth, we could not evaluate the quality of prehospital care. These limitations could have affected patient outcomes.
5.Conclusions
In conclusion, physician involvement during pediatric OHCA was associated with lower 1-month neurologically favourable survival, although it was associated with higher ROSC compared with cases without a physician. EMS improvement and patient selection may be effective in obtaining higher quality physician involvement in future pediatric OHCA.
Declarations
Ethics Approval and Consent to Participate
:This study was approved by the Institutional Review Board of the International University of Health and Welfare, Narita Hospital, Japan (approval number: 21-Im-043, September 28, 2021). The requirement for informed consent was waived due to the use of anonymized data.
Consent for Publication
:Not applicable.
Availability of Data and Materials:
The data that support the findings of the present study are available from the All-Japan Utstein Registry; restrictions apply to the availability of data, which were used under license for the current study, and they are not publicly available. However, data are available upon reasonable request and with permission from the All-Japan Utstein Registry.
Competing Interests:
The authors declare that they have no competing interests.
Funding:
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Authors’ Contributions:
Conceptualization: SO, HO, RI, TC, SI, ST; Data curation: SO, HO; Formal analysis: SO, HO; Investigation: SO, HO; Methodology: SO, HO, TS; Project administration: SO; Resources: SO, Ryuhei Igeta, TS; Software: SO; Supervision: HO, TC, SI, TS; Writing; SO, HO; Validation: SO, HO; Visualization: SO, HO; Writing - original draft: SO; Writing - review & editing: SO, HO, TS. All authors read and approved the final manuscript. SO had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Acknowledgements:
We thank all the EMS personnel, the participating physicians, and the Fire and Disaster Management Agency for their generous cooperation in establishing and maintaining the database.
References
- Atkins DL, Everson-Stewart S, Sears GK, et al. Resuscitation Outcomes Consortium Investigators. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: The Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation 119 (2009): 1484-1491.
- Goto Y, Funada A, Nakatsu-Goto Y. Neurological outcomes in children dead on hospital arrival. Crit Care (2015): 410.
- Yasunaga H, Horiguchi H, Tanabe S, et al. Collaborative effects of bystander-initiated cardiopulmonary resuscitation and prehospital advanced cardiac life support by physicians on survival of out-of-hospital cardiac arrest: A nationwide population-based observational study. Crit Care 14 (2010) : R199.
- Hagihara A, Hasegawa M, Abe T, et al. Physician presence in an ambulance car is associated with increased survival in out-of-hospital cardiac arrest: A prospective cohort analysis. PLOS ONE 9 (2014): e84424.
- Hamilton A, Steinmetz J, Wissenberg M, et al. Association between prehospital physician involvement and survival after out-of-hospital cardiac arrest: A Danish nationwide observational study. Resuscitation 108 (2016): 95-101.
- Goto Y, Funada A, Goto Y. Impact of prehospital physician-led cardiopulmonary resuscitation on neurologically intact survival after out-of-hospital cardiac arrest: A nationwide population-based observational study. Resuscitation 136 (2019): 38-46.
- Sato N, Matsuyama T, Akazawa K, et al. Benefits of adding a physician-staffed ambulance to bystander-witnessed out-of-hospital cardiac arrest: A community-based, observational study in Niigata, Japan. BMJ Open 9 (2019): e032967.
- Knapp J, Häske D, Böttiger BW, et al. Influence of prehospital physician presence on survival after severe trauma: Systematic review and meta-analysis. J Trauma Acute Care Surg 87 (2019): 978-989.
- Nishiyama C, Kiyohara K, Matsuyama T, et al. Characteristics and outcomes of out-of-hospital cardiac arrest in educational institutions in Japan—All-Japan Utstein registry. Circ J 84 (2020): 577-583.
- Chamberlain D, Cummins RO, Abramson N, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: The ‘Utstein style.’ Prepared by a Task Force of Representatives from the European Resuscitation Council, American Heart Association, Heart and Stroke Foundation of Canada, Australian Resuscitation Council. Resuscitation 22 (1991): 1-26.
- Shinohara M, Muguruma T, Toida C, et al. Daytime admission is associated with higher 1-month survival for pediatric out-of-hospital cardiac arrest: Analysis of a nationwide multicenter observational study in Japan. PLOS ONE 16 (2021): e0246896.
- Jayaram N, McNally B, Tang F, et al. Survival After out-of-hospital cardiac arrest in children. J Am Heart Assoc 4 (2015): e002122.
- Meaney PA, Nadkarni VM, Cook EF, et al. Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics 118 (2006): 2424-2433.
- Volchenboum SL, Mayampurath A, Göksu-Gürsoy G, et al. Association between in-hospital critical illness events and outcomes in patients on the same ward. JAMA 316 (2016): 2674-2675.
- Kitamura T, Kiyohara K, Nitta M, et al. Survival following witnessed pediatric out-of-hospital cardiac arrests during nights and weekends. Resuscitation 85 (2014): 1692-1698.
- Nakahara S, Tomio J, Ichikawa M, et al. Association of bystander interventions with neurologically intact survival among patients with bystander-witnessed out-of-hospital cardiac arrest in Japan. JAMA 314 (2015): 247-254.
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28 (2009): 3083-3107.
- Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38 (2009): 1228-1234.
- Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: A primer for practitioners. BMJ 367 (2019): l5657.
- Li F, Thomas LE, Li F. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol 188 (2019): 250-257.
- Thomas LE, Li F, Pencina MJ. Overlap weighting: A propensity score method that mimics attributes of a randomized clinical trial. JAMA 323 (2020): 2417-2418.
- Yasunaga H. Introduction to applied statistics—Chapter 1 propensity score analysis. Ann Clin Epidemiol 2 (2020): 33-37.
- Meyer L, Stubbs B, Fahrenbruch C, et al. Incidence, causes, and survival trends from cardiovascular-related sudden cardiac arrest in children and young adults 0 to 35 years of age: A 30-year review. Circulation 126 (2012): 1363-1372.
- Geri G, Passouant O, Dumas F, et al. Etiological diagnoses of out-of-hospital cardiac arrest survivors admitted to the intensive care unit: Insights from a French registry. Resuscitation 117 (2017): 66-72.
- Larsen MP, Eisenberg MS, Cummins RO, et al. Predicting survival from out-of-hospital cardiac arrest: A graphic model. Ann Emerg Med 22 (1993): 1652-1658.
- Ibrahim WH. Recent advances and controversies in adult cardiopulmonary resuscitation. Postgrad Med J 83 (2007): 649-654.
- Berg RA, Hilwig RW, Kern KB, et al. Simulated mouth-to-mouth ventilation and chest compressions (bystander cardiopulmonary resuscitation) improves outcome in a swine model of prehospital pediatric asphyxial cardiac arrest. Crit Care Med 27 (1999): 1893-1899.
- Homma Y, Shiga T, Funakoshi H, et al. Association of the time to first epinephrine administration and outcomes in out-of-hospital cardiac arrest: SOS-Kanto 2012 study. Am J Emerg Med 37 (2019): 241-248.