Temperature Dependence of Henry's Law Constants of Fenpropidin and Pyrimethanil: Impact on their Atmospheric Partitionnings and Lifetimes
Article Information
Valérie Feigenbrugel, Stéphane Le Calvé*
ICPEES – Institut de Chimie et Procédés pour l’Energie, l’Environnement et la Santé, Group of Atmospheric Physical Chemistry, UMR 7515 CNRS – Université de Strasbourg – ECPM, 25 rue Becquerel F-67087 Strasbourg cedex 2, France
*Corresponding Author: Stéphane Le Calvé, ICPEES – Institut de Chimie et Procédés pour l’Energie, l’Environnement et la Santé, Group of Atmospheric Physical Chemistry, UMR 7515 CNRS – Université de Strasbourg – ECPM, 25 rue Becquerel F-67087 Strasbourg cedex 2, France
Received: 17 March 2021; Accepted: 23 March 2021; Published: 05 April 2021
Citation:
jhjkjk
Share at FacebookAbstract
Henry's law constants (HLC) play a key role in the environmental fate of pesticides and their distribution between the different phases, i.e., between air, water and soil. For certain compounds such as fenpropidin and pyrimethanil, HLC values are poorly documented in the literature and in particular their temperature dependence. This work reports the experimental HLC values of two pesticides, namely fenpropidin and pyrimethanil, determined by means of a dynamic equilibrium system coupled to an off-line analysis performed by Gas Chromatography–Photoionisation Detection (GC–PID). The measurements were conducted over the range 278–293 K.
In pure water, the experimental average values of HLC at 293 K were: HLC293K(fenpropidin) = (10.0 ± 3.1) × 104 M atm–1, HLC293K(pyrimethanil) = (8.2 ± 0.7) × 104 M atm–1. The obtained data were used to derive the following Arrhenius expressions where the quoted errors represent 2σ: ln HLC (fenpropidin) = (6060 ± 2420)/T – (9.1 ± 8.3); ln HLC (pyrimethanil) = (14570 ± 1800)/T – (38.4 ± 6.2). The environmental implications are then discussed in terms of lifetime or partitionning between the different atmospheric compartments.
Keywords
Pesticides; Fenpropidin; Pyrimethanil; Henry’s Law Constants; Partitionning; Atmospheric lifetime
Pesticides articles; Fenpropidin articles; Pyrimethanil articles; Henry?s Law Constants articles; Partitionning articles; Atmospheric lifetime articles
Pesticides articles Pesticides Research articles Pesticides review articles Pesticides PubMed articles Pesticides PubMed Central articles Pesticides 2023 articles Pesticides 2024 articles Pesticides Scopus articles Pesticides impact factor journals Pesticides Scopus journals Pesticides PubMed journals Pesticides medical journals Pesticides free journals Pesticides best journals Pesticides top journals Pesticides free medical journals Pesticides famous journals Pesticides Google Scholar indexed journals Fenpropidin articles Fenpropidin Research articles Fenpropidin review articles Fenpropidin PubMed articles Fenpropidin PubMed Central articles Fenpropidin 2023 articles Fenpropidin 2024 articles Fenpropidin Scopus articles Fenpropidin impact factor journals Fenpropidin Scopus journals Fenpropidin PubMed journals Fenpropidin medical journals Fenpropidin free journals Fenpropidin best journals Fenpropidin top journals Fenpropidin free medical journals Fenpropidin famous journals Fenpropidin Google Scholar indexed journals Pyrimethanil articles Pyrimethanil Research articles Pyrimethanil review articles Pyrimethanil PubMed articles Pyrimethanil PubMed Central articles Pyrimethanil 2023 articles Pyrimethanil 2024 articles Pyrimethanil Scopus articles Pyrimethanil impact factor journals Pyrimethanil Scopus journals Pyrimethanil PubMed journals Pyrimethanil medical journals Pyrimethanil free journals Pyrimethanil best journals Pyrimethanil top journals Pyrimethanil free medical journals Pyrimethanil famous journals Pyrimethanil Google Scholar indexed journals Henry’s Law Constants articles Henry’s Law Constants Research articles Henry’s Law Constants review articles Henry’s Law Constants PubMed articles Henry’s Law Constants PubMed Central articles Henry’s Law Constants 2023 articles Henry’s Law Constants 2024 articles Henry’s Law Constants Scopus articles Henry’s Law Constants impact factor journals Henry’s Law Constants Scopus journals Henry’s Law Constants PubMed journals Henry’s Law Constants medical journals Henry’s Law Constants free journals Henry’s Law Constants best journals Henry’s Law Constants top journals Henry’s Law Constants free medical journals Henry’s Law Constants famous journals Henry’s Law Constants Google Scholar indexed journals Partitionning articles Partitionning Research articles Partitionning review articles Partitionning PubMed articles Partitionning PubMed Central articles Partitionning 2023 articles Partitionning 2024 articles Partitionning Scopus articles Partitionning impact factor journals Partitionning Scopus journals Partitionning PubMed journals Partitionning medical journals Partitionning free journals Partitionning best journals Partitionning top journals Partitionning free medical journals Partitionning famous journals Partitionning Google Scholar indexed journals Atmospheric lifetime articles Atmospheric lifetime Research articles Atmospheric lifetime review articles Atmospheric lifetime PubMed articles Atmospheric lifetime PubMed Central articles Atmospheric lifetime 2023 articles Atmospheric lifetime 2024 articles Atmospheric lifetime Scopus articles Atmospheric lifetime impact factor journals Atmospheric lifetime Scopus journals Atmospheric lifetime PubMed journals Atmospheric lifetime medical journals Atmospheric lifetime free journals Atmospheric lifetime best journals Atmospheric lifetime top journals Atmospheric lifetime free medical journals Atmospheric lifetime famous journals Atmospheric lifetime Google Scholar indexed journals fungicides articles fungicides Research articles fungicides review articles fungicides PubMed articles fungicides PubMed Central articles fungicides 2023 articles fungicides 2024 articles fungicides Scopus articles fungicides impact factor journals fungicides Scopus journals fungicides PubMed journals fungicides medical journals fungicides free journals fungicides best journals fungicides top journals fungicides free medical journals fungicides famous journals fungicides Google Scholar indexed journals pesticides articles pesticides Research articles pesticides review articles pesticides PubMed articles pesticides PubMed Central articles pesticides 2023 articles pesticides 2024 articles pesticides Scopus articles pesticides impact factor journals pesticides Scopus journals pesticides PubMed journals pesticides medical journals pesticides free journals pesticides best journals pesticides top journals pesticides free medical journals pesticides famous journals pesticides Google Scholar indexed journals Chemical articles Chemical Research articles Chemical review articles Chemical PubMed articles Chemical PubMed Central articles Chemical 2023 articles Chemical 2024 articles Chemical Scopus articles Chemical impact factor journals Chemical Scopus journals Chemical PubMed journals Chemical medical journals Chemical free journals Chemical best journals Chemical top journals Chemical free medical journals Chemical famous journals Chemical Google Scholar indexed journals soil articles soil Research articles soil review articles soil PubMed articles soil PubMed Central articles soil 2023 articles soil 2024 articles soil Scopus articles soil impact factor journals soil Scopus journals soil PubMed journals soil medical journals soil free journals soil best journals soil top journals soil free medical journals soil famous journals soil Google Scholar indexed journals
Article Details
1. Introduction
Fenpropidin and pyrimethanil are two fungicides. Their chemical structure and their physical properties are presented in Table 1 (Matthews, 1998 [1]). Fenpropidin is a fungicide of the piperidine family. This active substance can be used alone, or in combination with other active substances which are most often fungicides in the IBS category (Sterol Biosynthesis Inhibitors). Used alone, its fungicidal activity is mainly directed against powdery mildew in wheat and barley. Combined with other fungicides, it is used on wheat, barley, rye, triticale, and beets (ACTA, 2021 [2]). A survey of pesticides application rates on 4,500 ha crops and 50 ha vineyards performed in the Champagne area (Reims, France) for an annual average over years 2010–2015 has determined that fenpropidin was one of the most applied pesticide (∼98 g/ha/year) (Villiot et al., 2018 [3]).
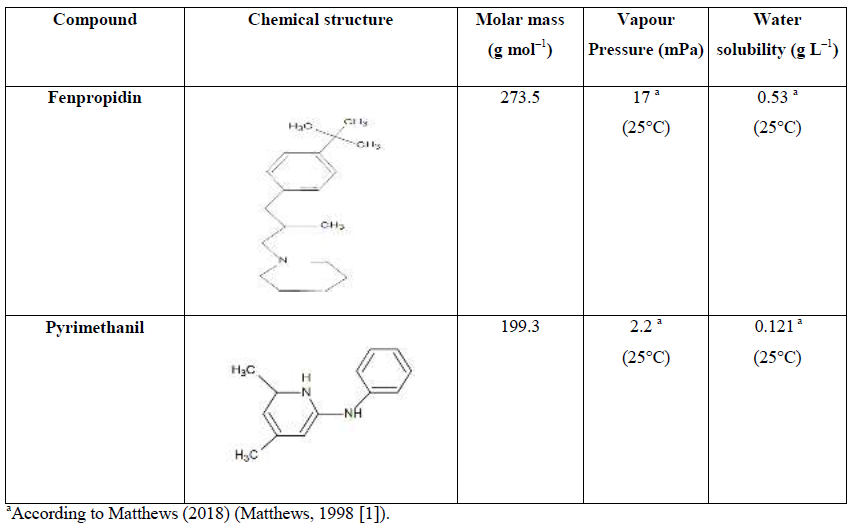
Table 1: Chemical structures and physical and chemical properties of the studied pesticides.
Pyrimethanil appeared on the phytosanitary product market in the early 2000s in various formulations. It is an anilinopyrimidine fungicide that helps reduce mutagenic and genotoxic effects while maintaining excellent fungal performance on fruits and vegetables (Liu et al., 2018 [4]). This fungicide is very active against gray rot (Botrytis cinerea) and scabs of apples and pears (Venturia inaequalis and Venturia pirina) (Gullino et al., 2000 [5]). Its mode of action aims at inhibiting the biosynthesis of methionine (Masner et al., 1994 [6]).
Once applied to targeted agricultural areas, pesticides can volatilize into the atmosphere from ground or leaf surfaces, For example, Bedos et al. (2009) reported measurements of fenpropidin volatilization on a wheat field using the aerodynamic gradient method and an inverse dispersion modeling approach (Bedos et al., 2010 [7]). Pesticides can also penetrate the soil, and then be washed into rivers or groundwater.
The partitionning of these pollutants between the different phases of the environment, i.e., soil, water and air, is a key parameter for understanding their environmental behaviour (Rice et al., 2002 [8]; Staudinger and Roberts, 1996 [9]) and Health impact. In a study performed on 53 pesticides and 75 central European arable soils, Hvezdová et al. (2018) highlighted that fenpropidin was one of the most frequently detected species (20% of soils), with values exceeding the level of 0.01 mg/kg in 13% of soils (Hvezdová et al., 2018 [10]). Once on the ground, pesticides can be degraded by biological processes (Ballesteros Martín et al., 2008 [11]; Vanni et al., 2006 [12]). Vanni et al. (2003) estimated the pyrimethanil degradation in the soil, leading to a half-time degradation (t1/2) of about 50 and 75 days, in the presence and absence of light, respectively (Vanni et al., 2003 [13]). Contamination of surface water induced by pesticides use is mainly related to runoff, usually within a few weeks after application. For instance, pyrimethanil concentration up to 2.9 µg L-1 was quantified in surface stream water of Cachapoal River basin (central Chile) (Climent et al., 2019 [14]). In water, a pesticide is subject to volatilization, hydrolysis, or photolysis (Katagi, 2018 [15]). Pesticides can reach the atmosphere both as gas or aerosols after spray drift during the application, the post-application volatilization and wind erosion of soil particles containing sorbed pesticides. For instance, Coscollà et al. (2013) shown that fenpropidin was accumulated in the fine (0.1 – 1 mm) particle size fraction (Coscollà et al., 2013 [16]) with concentration of 0.2 ng m–3. In the atmospheric gas-phase, pesticides can be oxidized by chemical reactions (Atkinson et al., 1999 [17]; Klöpffer, 1992 [18]) or eliminated by wet and dry deposition (Bidleman, 1988 [19]; Potter et al., 2014 [20]).
Because of their intensive use, these pesticides have been measured in the environment. Désert et al. (2018) monitored 59 current-use pesticides in ambient air samples collected from 2012 to 2017, at two rural and six urban sites in South of France (Désert et al., 2018 [21]). Pyrimethanil was rarely detected since it was present in 3 to 22% of samples depending on the sites with maximum values varying from 0.004 to 0.212 ng m–3 on 7 of the 8 sites investigated.
Nevertheless, one site exhibited an occurrence of 77% and a maximum concentration of 5.58 ng m–3 (Désert et al., 2018 [21]). More surprising, Córdoba Gamboa et al. (2020) reported that pyrimethanil was very frequently detected in schools in Costa-Rica with mean values of 5.4 ng m–3 in indoor air (Córdoba Gamboa et al., 2020 [22]).
Besides, Villiot et al. (2018) [3] recently reported fenpropidin concentrations ranged between 0.13 and 5.88 ng m–3 close to Reims (France) with average value of 0.68 ng m–3 over 4 years when this fungicide was quantified (quantification frequencies higher than 50%) (Villiot et al., 2018 [3]).
In equilibrium between the gas and liquid phases, the water/air partitioning coefficient corresponds to the Henry’s Law Constant, usually defined as HLC = [X] / PX where [X] is the aqueous concentration of X (mol L–1) and PX is its atmospheric partial pressure (atm). Inverse value (H) of HLC is also widely used, the relationship between these two expressions being: H (Pa m3 mol–1) = 101.325 / HLC (M atm–1) (Sander, 2015 [23]; Trapp and Matthies, 1998 [24]).
Compounds with low HLC values have long atmospheric residence times and can be transported over long distances, whereas compounds with high HLC values will rather be washed away by rain. Although rain can be an effective mechanism for removing gas species from the troposphere, it is often difficult to estimate its efficiency as many Henry’s law constants of pesticide are either unknown or roughly estimated from calculations.
Indeed, HLC values can be either experimentally determined or calculated based on Structure Activity Relationships (SAR) (Meylan and Howard, 1991 [25]). When the component has a low water solubility, H (Pa m3 mol–1) is defined as being equal to the ratio of its saturated vapor pressure (Pl) in the liquid state and its solubility (s) in water. For compounds of low volatility and poorly soluble in water, such as pesticides targeted in this work (see Table 1), the uncertainties in vapor pressure and solubility are often very high, which leads to unreliable estimates made by SAR. This reinforces therefore the need of experimental measurements.
In this work, Henry’s law constants of two pesticides, namely fenpropidin and pyrimethanil, have been measured between 278 and 293 K with a dynamic equilibrium system. To our knowledge, we report the first experimental determination and the first temperature dependence of their experimental Henry law constants for both investigated fungicides (see Table 2).
|
T (K) |
10–4 × HLC (Fenpropidin) (M atm–1) |
10–4 × HLC (Pyrimethanil) (M atm–1) |
|
278.25 |
- |
131 ± 17 |
|
283.15 |
20.8 ± 4.6 |
34.2 ± 4.2 |
|
283.25 |
18.7 ± 4.1 |
46.0 ± 5.9 |
|
287.95 |
- |
19.8 ± 2.7 |
|
288.05 |
15.7 ± 3.7 |
24.0 ± 3.0 |
|
288.15 |
20.2 ± 4.4 |
- |
|
292.95 |
- |
7.3 ± 0.9 d |
|
293.15 |
8.8 ± 2.1 a |
8.3 ± 1.7 a |
|
8.1 ± 2.1 c |
8.3 ± 1.2 a |
|
|
11.8 ± 2.7 b |
8.5 ± 1.2 |
|
|
10.0 ± 2.5 |
7.7 ± 1.0 b |
|
|
11.1 ± 2.5 d |
- |
By default, the experiments were carried out with a flow rate of 0.2 L min–1. Experiments were carried out with an air flow rate of: a 0.1 L min–1; b 0.3 L min–1; c 0.4 L min–1; d 0.5 L min–1.
Table 2: Henry’s Law Constants (in units of Mol L–1 atm–1) of fenpropidin and pyrimethanil in pure water, as a function of the temperature determined by using the dynamic equilibrium system.
2. Experimental Section
This section details the experimental conditions used to perform the measurements of Henry’s law constants followed by the pesticide analysis.
2.1 Reagents
Aqueous solutions of fenpropidin and pyrimethanil were prepared with Milli-Q Water (18 mW cm). Pure fenpropidin (97.8%) and pyrimethanil (99.9%) were obtained from Riedel de Haen, both of them being used without further purification. The aqueous concentrations used for the experiments performed in deionized water were in the range 4.3–13.1 mg L–1 (14.7–47.9 × 10–6 M) for fenpropidin and 2.5–12 mg L–1 (12.5–60.2 × 10–6 M) for pyrimethanil given that their water solubilities at 298 K are 530 and 121
mg L–1, respectively (Matthews, 1998 [1]). The concentrations used for the aqueous solutions were thus 40–123 and 10–48 times lower than the expected solubility for fenpropidin and pyrimethanil, respectively. In fact, the experiments tend to demonstrate that the real water solubility of fenpropidin is probably lower than 530 mg L–1 (Matthews, 1998 [1]), since we could not dissolve neither 98 mg nor 43 mg in 1 L of water after 4 days of continuous stirring.
2.2 Dynamic equilibrium system
The experiments aiming at determining the Henry’s law constants were performed by means of a dynamic equilibrium system which has already been extensively used for HLC measurements of Volatile Organic Compounds (Allou et al., 2011 [26]; Feigenbrugel et al., 2004b [27]; Katrib et al., 2002 [28]) or Semi-Volatile Organic Compounds (Feigenbrugel et al., 2004a [29]; Gautier et al., 2003 [30]; Xie et al., 2004 [31]). The experimental set up is shown in Figure 1. The reactor has a double jacket in which circulates a liquid regulated in temperature with an accuracy of ±0.1 °C. A microporous PTFE membrane tube (160 cm × 0.8 cm i.d., Sumitomo Corporation) was immersed in about 0.6 L of the diluted aqueous solution containing the pesticide and placed into the reactor. Compressed air was passed through this porous tube at a controlled flow rate ranging between 0.1 and 0.5 L min–1, insuring a contact time of 5.4–27 s between the two aqueous and gas phases. For the dissolved pesticide, phase equilibrium was achieved at the gas/water interface along the inner surface of the tube. At the outlet of the reactor, the gas stream containing the pesticide at equilibrium concentration was then diluted using clean dry air (0.5 L min–1) before to cross two tubes connected in series and filled with an adsorbent, namely XAD–2 Amberlite resin (Supelco), to trap the targeted pesticide. Before its use, this adsorbent was purified by Soxhlet extraction with n-hexane/dichloromethane (50/50) for 24 h and dried at room temperature (Feigenbrugel et al., 2004a [29]; Schummer et al., 2012 [32]).
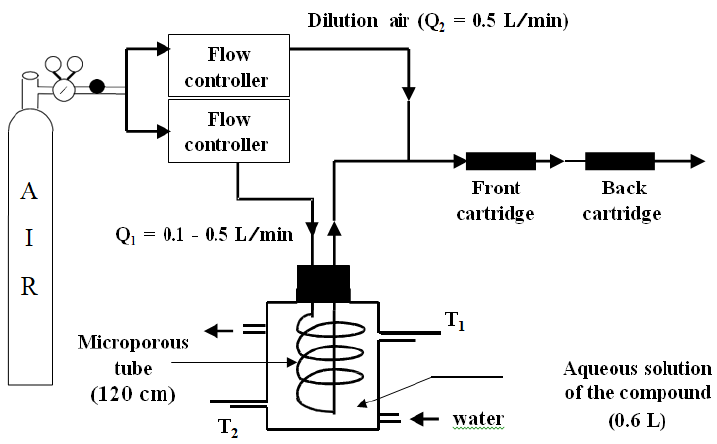
Figure 1: Scheme of the dynamic equilibrium system used to determine directly Henry's law constants as a function of the temperature which was measured by two temperature sensors T1 and T2.
After sampling, the pesticide was extracted three times by eluting the adsorbent tube with approximately 20, 10 and 10 mL of solvent. Cyclohexane and dichloromethane were used to extract fenpropidin and pyrimethanil, respectively. Prior to pesticide quantification, the solvent collected in the different sampling bottles was weighted to determine the precise mass of solvent used for each extraction, and therefore its corresponding precise volume.
The experiments revealed that no pyrimethanil was trapped in the second adsorbent tube. For fenpropidin, the mass trapped in the first adsorbent tube ranged between 84% and 100%.
One to eight days were required to concentrate fenpropidin and pyrimethanil in the adsorbent tubes. The total amount of pesticide entrained by the air flow and trapped in the adsorbent tubes was always negligible compared to its total mass in the aqueous solution. Therefore, the aqueous pesticide concentration could be considered constant during the whole experiment.
2.3 Pesticide analysis
Fenpropidin and pyrimethanil were analysed by Gas Chromatography equipped with a photoionization Detector (GC–PID, Thermo Finnigan Trace GC) and a 100%–dimethylpolysiloxane capillary column (HP–1MS, 30 m·× 0.25 mm i.d. × 1 µm film thickness). For this, 4 µL of the solution obtained after extraction was injected in the pulsed splitless mode by an automatic sampler (AS3000). Helium (UHP certified to >99.9995% from Air Liquide) was used as carrier gas with a flowrate set at 1 mL min–1 and the injector temperature was maintained at 250 °C. The column temperature started at 80 °C and was increased to
250 °C at a rate of 15 °C min–1. This final temperature was then maintained 7 and 18 minutes for fenpropidin and pyrimethanil, respectively.
The extracted pesticide solutions and therefore the gas-phase concentrations of the pesticides were quantified from external calibration curves. For this, known liquid pesticide concentrations were prepared either in cyclohexane for fenpropidin or in dichloromethane for pyrimethanil. The corresponding standard solutions were then injected into GC–PID to plot concentration curves vs. peak areas. The calibration curves were linear in the concentration ranges used in this study, i.e., 70–450 µg L–1 for fenpropidin and 120–4900 µg L–1 for pyrimethanil, the corresponding correlation coefficients being always greater than 0.99. For each studied species, two analytical blanks were performed and confirmed that none of the investigated pesticides was present either in compressed air or purified
XAD–2 resin as expected.
2.4 Determination of the Henry’s law constant
The Henry's law constant (HLC) of compound A in dilute aqueous solution is given by the following relationship:

where [A]aq is the known concentration of the aqueous solution and PA the partial pressure of the compound. The calculation of this constant is done by assuming that the compound in the gas phase is an ideal gas and that [A]aq is constant in the reactor for the duration of the experiment. PA can then be expressed as follows:

nA is calculated from the mass mA of pesticide trapped in the adsorbent tube and V is determined from the air flow rate Q1 passing through the microporous tube and the duration t of the experiment. So, Henry's law constant (HLC) can be expressed as follows:
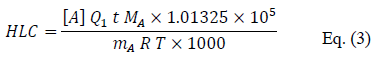
where HLC is expressed in mol L–1 atm–1, [A] in mol L–1, Q1 in L min–1, t in min, MA in g mol–1, mA in g,
R = 8.314 J K–1 mol–1, T in K. Knowing the concentration [A] of the compound in the liquid phase in the reactor, it is then possible to deduce its Henry's law constant at the temperature considered.
3. Results and Discussion
The experimental values of the Henry’s law constants measured in this work in deioized water are gathered in Table 2. The reported uncertainties take into account the error due to the solvent extraction from the adsorbent, the error due to the GC analysis which is estimated at 5 % for all of them, the error on the air flowmeter, given at 2.5 mL min–1 by the manufacturer, i.e., an error ranging between 0.5 and 2.5% for flow rates ranging respectively from 0.5 to 0.1 L min–1 and finally the error on the aqueous concentrations, generally equal to less than 2 %. The error on time is always considered negligible. The uncertainty related to the adsorbent extraction has been estimated at 5% for pyrimethanil while it is roughly around 15 % for fenpropidin regarding its very low amount trapped in the adsorbent tubes even after several days of experiment. The resulting relative uncertainties quoted on HLC values vary in the ranges 22 – 26 % and 12 – 25% for fenpropidin and pyrimethanil, respectively (see Table 2).
3.1 Gas/liquid equilibrium state at the reactor outlet
Given that Henry's law constant describes an equilibrium state between gas and liquid phases, it is necessary to check that this equilibrium is reached at the outlet of the microporous tube by varying the gas flow rate at a constant temperature. Indeed, a too fast gas flow would not allow equilibrium causing a disruption of the distribution equilibrium between the gas phase and the aqueous phase at the outlet of the microporous tube, and thus distort the results. Within the experimental errors, Figure 2 shows that the values of HLC obtained at 293 K are independent of the change in air flow rate in the range 0.1–0.5 L min–1 for the two pesticides studied in this work. This demonstrates that the equilibrium between solution and vapour is achieved at the reactor outlet.
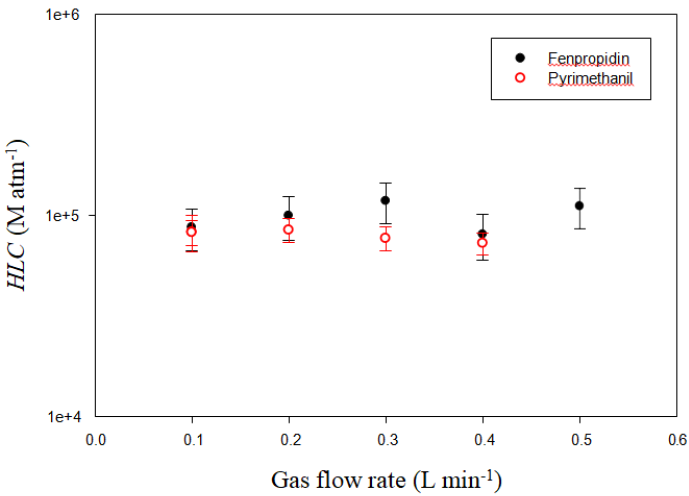
Figure 2: Plot of Henry’s Law Constants (HLC) versus the gas flow rate ranging between 0.1 and 0.5 L min–1 for fenpropidin (black full circle) and pyrimethanil (red open circle), at room temperature, i.e., between 292.95 and 293.15 K. The relative errors on HLC were determined as mentionned in the text and varied between 22 and 26 % for fenpropidin, 12 and 25 % for pyrimethanil.
3.2 Values of henry’s law constant at 293 K
From the measurements conducted at 293K (see Table 2), the average values of HLC are equal to (10.0 ± 3.1) × 104 and (8.2 ± 0.7) × 104 M atm–1, for fenpropidin and pyrimethanil respectively, the quoted errors representing 2σ.
3.3 Temperature dependence and Van’t Hoff equation of Henry’s law constants
The Arrhenius plots of the Henry's Law Constants measured in the range 278–293 K for fenpropidin and pyrimethanil are presented in Figure 3.
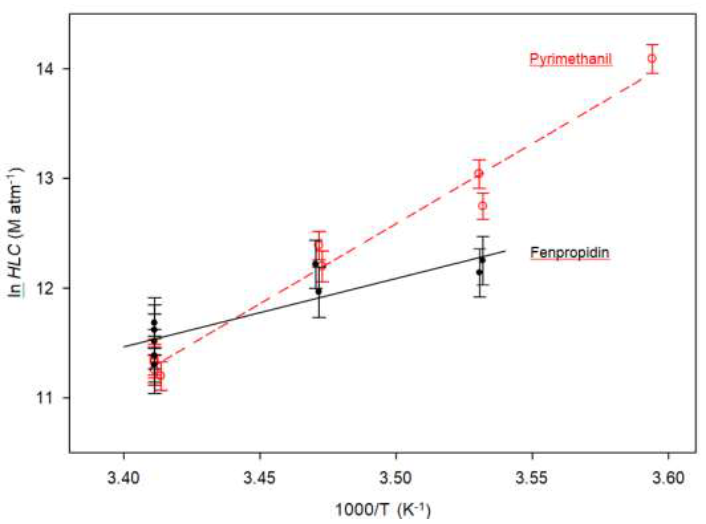
Figure 3: Plot of ln HLC versus 1000/T for fenpropidin (black full circle) and pyrimethanil (red open circle) in the temperature ranges of 283 – 293 K and 278 – 293 K, respectively. The relative errors on HLC were determined as mentioned in the text and varied in the ranges 22 – 26 % and 12 – 25 % for fenpropidin and pyrimethanil, respectively. The continuous black line and red dashed line corresponds to the linear fits made in accordance with Van't Hoff's equation, for fenpropidin and pyrimethanil, respectively.
For fenpropidin and pyrimethanil, the temperature dependencies of their Henry’s law constants were derived to be:
ln HLC (fenpropidin) = (6,060 ± 2,420)/T – (9.1 ± 8.3)
ln HLC (pyrimethanil) = (14,570 ± 1,800)/T – (38.4 ± 6.2)
where the quoted errors correspond to 2σ.
From these two equations, we can calculate the corresponding HLC values at 293 K, i.e., HLC293K = 10.72 × 104 M atm–1 and HLC293K = 8.30 × 104 M
atm–1, for fenpropidin and pyrimethanil, respectively. These calculated values are consistent with the average ones reported above.
Besides, the temperature dependence of Henry's law constant has been modeled in many cases by an equation expressed according to Shepson et al. (1996) (Shepson et al., 1996 [33]):
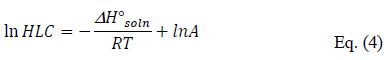
where ΔH°soln is the enthalpy of solvation (J mol–1), T is the temperature (K), R is the ideal gas constant and A is a constant (mol L–1 atm–1).
The values of ΔH°soln have been determined for the two studied pesticides from the plot of ln (HLC) vs. 1/T, where the slope is equal to – ΔH°soln /R according to eq. (4). As illustrated in Figure 3, these plots are linear within experimental uncertainties which means that ΔH°soln can be considered as constant in the investigated range of temperature, i.e., between 278 and 293 K for all the pesticides in deionized water.
The derived enthalpies of solvation obtained for the two pestidides in deionized water are the following (in units of kJ mol–1): – 50 ± 20 (fenpropidin); – 121 ± 15 (pyrimethanil) where the quotted errors for ΔH°soln are given at the 2σ level from the least-squares analysis.
The commonly applied rule for estimating HLC at lower temperature consists of doubling HLC value for a decrease of 10 °C (Staudinger and Roberts, 2001 [34]). This method is relevant for fenpropidin with a very limited underestimate of 3.7 % compared to the experimental determination of 2.23 × 105 M atm–1 at 283 K deduced from the Arrhenius expression. Conversely, when applied to our HLC values between 293 K and 283 K, the calculated value for pyrimethanil is 65% lower than the real one, i.e., 1.66 × 105 M atm–1 instead of 4.81 × 105 M atm–1.
3.4 Comparison with the literature
Our values of HLC at 293K and corresponding solvatation enthalpies are gathered in Table 3 where they can be compared to those found in the literature including the data reported by Sander (2015) in its Compilation of Henry’s law constants (version 4.0) for water (Sander, 2015 [23]). As shown in Table 3, we report in this work the first experimental measurements and the first temperature dependencies of their Henry constants for these two compounds.
|
Compound |
T (K) |
HLC (M atm–1) |
T range (K) |
ΔH°solv (kJ mol–1) |
Type a |
Technique |
Reference |
|
Fenpropidin |
293 |
(88.0 ± 19.2) × 103 |
278 – 293 |
– 50 ± 20 |
M |
DES b |
This work |
|
298 |
1.16 × 103 |
- |
- |
C |
c |
(Maniere et al., 2011 [35]) |
|
|
- |
0.01 × 103 |
- |
- |
C |
c |
(Matthews, 1998 [1]) |
|
|
Pyrimethanil |
293 |
(80.3 ± 4.3) × 103 |
283 – 293 |
– 121 ± 15 |
M |
DES b |
This work |
|
- |
0.40 × 103 |
- |
- |
C |
d |
(HSDB: Hazardous Substances Data Bank, 2021 [36]) |
|
|
- |
28 × 103 |
- |
- |
C |
c |
(Matthews, 1998 [1]) |
a M: Experimental Measurement, C: calculated value; b DES: Dynamic Equilibrium System; c Calculated value from the ratio between the vapour pressure and the solubility; d calculated value based on the method proposed by Meylan and Howard (1991).
Table 3: Comparaison of experimental HLC values in pure water with those reported in the literature for fenpropidin and pyrimethanil.
For fenpropidin, only two calculated values of HLC, derived from their estimated water solubilty and vapour pressure, have been reported in the literature. The Henry's law constant given in the Manual of Pesticides (Matthews, 1998 [1]) is a calculated value of 10 Pa m3 mol–1, equivalent to 10.1 M atm–1.
AGRITOX, the database on active plant protection substances from INRA (National Institute of Agronomic Research, France), indicates a HLC value of 1,165 M atm–1 (equivalent to 8.7 × 10-2 Pa m3 mol–1 or 11.5 mol Pa–1 m–3) at 298 K (Maniere et al., 2011 [35]). The Pesticides Manual indicates a solubility in water, at pH 7 and 298 K, of 0.53 g L–1.
However, we have observed in our experiments that this solubility value was probably significantly overestimated. The calculated HLC value would thus be distorted, explaining the huge difference observed between our experimental value and the calculated ones.
As for fenpropidin there are no experimental measurements of HLC for pyrimethanil. Only two calculated values have been reported so far. First, a value of 2.8 × 104 M atm–1 (276 mol Pa–1 m–3) can be found in the Pesticides Manual (Matthews, 1998 [1]) which is of the order of magnitude of our experimental value (8.0 × 104 M atm–1).
A much smaller estimation of 4.0 × 102 M atm–1 obtained by using the method of Meylan and Howard (1991) has been reported more recently (HSDB: Hazardous Substances Data Bank, 2021 [36]). This latter value is two order of magnitude lower than our experimental determination.
4. Environmental Implications
This section aims at better understanding the behavior of the studied pesticides in the environment based on
the experimental Henry’s law constants determined in this work. The Henry’s law is therefore applied to gas-liquid equilibrium for water droplets in tropospheric clouds.
4.1 Removal from the atmosphere by wet deposition
The removal of pesticides from the atmosphere by wet deposition can be done in two ways: either by precipitating a contaminated cloud, or by leaching out the lower layers of the atmosphere. In this section, we do not consider the leaching of particles present under the cloud. The processes that form precipitation are very complex processes that encompass reactions that occur both inside the cloud and under the cloud, between water droplets, gases, and aerosols (Seinfeld et al., 1998 [37]).
Brimblecombe and Dawson (1984) proposed a simple model to predict the lifetime of a compound in the atmosphere with respect to wet deposition. According to these authors, the time required for the removal of pesticides from precipitation can be estimated by assuming a first order disappearance mechanism. In the absence of reaction in the aqueous phase and assuming a rapid equilibrium between the atmospheric liquid and gas phases, the first order rate constant kwd defining the wet deposition can be calculated from the equation (Brimblecombe and Dawson, 1984 [38]):
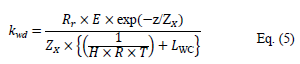
where Rr is the annual rate of precipitation (estimated at 0.96 m / year at our latitudes (Haurwitz et al., 1944 [39]) or Rr = 3.2 × 10-8 m s–1).
E is a corrective factor due to the evaporation of water droplets (of the order of 1.33), z is the characteristic height of the clouds (of the order of 3.5 km), Zx is the average height where the studied compounds are found (of around 2.3 km at our latitudes (Oort and Rasmusson, 1971 [40]), HLC is the Henry's law constant (mol L–1 atm–1), R is the ideal gas constant (0.08205 atm L mol–1 K–1) and LWC is the dimensionless constant representing the average quantity of liquid water contained in clouds (4.2 × 10–7) (Kolb et al.,1995 [41]).
This relationship is only valid for compounds exhibiting a Henry's law constant greater than 105 M atm–1 at 283K according to Brimblecombe and Dawson (1984), which is the case for fenpropidin and pyrimethanil with respective values of 2.23 × 105 M atm–1 and 4.81 × 105 M atm–1.
This rate constant kwd makes it possible to calculate the corresponding lifetime twd of the compound related to the wet deposition according to the following equation:

The lifetime twd is in fact defined as the time required for the wet deposition to cause a reduction by a factor of 1/e in the initial concentratin of the compound (i.e., 37% of its initial value). Considering Henry's law constants of 2.18 × 105 and 4.81 × 105 M atm-1 determined at 283 K in this work for fenpropidin and pyrimethanil respectively, the rate constants kwd are respectively equal to 6.48 × 10–6 s and 7.86 ×10–6 s, which implies respective lifetimes of 42.8 h and 35.4 h.
4.2 Chemical reactivity in the atmosphere
This following section aims at evaluating the cloud impact on the atmospheric chemistry of the studied pesticides by comparing their atmospheric lifetimes under clear sky (τgas), and cloudy conditions (τmultiphase).
Once in the atmospheric compartment, pesticides can react in the gas phase or can be taken up by water droplets where they can also be degraded. For most of the organic compounds, OH radical is the most efficient oxidizing species in both the gas phase (Atkinson, 2000 [42]) and the aqueous phase (Ervens et al., 2003 [43]). Direct photolysis in both the gas and liquid phases can also occur when the molecules significantly absorb at wavelengths higher than 290 nm. For the two fungicides considered in this work, the photolysis rates in air and water have not been reported in the literature.
Feigenbrugel et al. (2006) reported only their absorption spectra in water with maximum value of
? = 90 M–1 cm–1 at 310 nm for fenpropidin and ? decreasing from 19,800 to 2,400 M–1 cm–1 between 280 and 320 nm for pyrimethanil (Feigenbrugel et al., 2006 [44]). If fenpropidin poorly absorbs the sunlight, it can not be excluded that pyrimethanil will be partially photodissociated under sunny conditions.
The lifetime of A in the gas phase (τA,gas) taking only into account the reaction of A with OH radicals, is defined as follow:
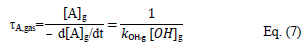
where kOH,g is the rate constant of the gas phase reaction of OH radicals with A, [OH]g is the mean concentration of OH radicals and [A]g is the tropospheric concentration of A.
If we consider now the cloudy atmosphere as a multiphase reactor where both the gas and aqueous phases coexist, then the multiphase lifetime of A can be defined as follows (Monod et al., 2005 [45]):
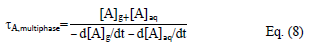
where [A]aq is the concentration of A in the aqueous phase. At equilibrium, the fractions of A in the gas (fA,g) and aqueous (fA,aq) phases are the following:
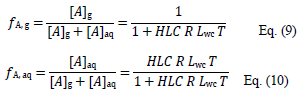
where R is the ideal gas constant (in L atm mol–1 K–1), and Lwc the dimensionless liquid water content of the cloud (typically 4.2×10–7).
Assuming that equilibrium is rapidly reached, eq. (8) can be written as follows:
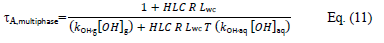
where kOH,aq is the rate constant of OH reaction with A in aqueous phase, [OH]aq is the mean concentration of OH radicals in the tropospheric aqueous phase.
For the two fungicides studied here, kOH values in both aqueous and gas phases are not readily available in the literature but can be estimated in gas phase by using Structure Activity Relationships (SAR method) (Kwok and Atkinson, 1995 [46]). In gas phase at 298 K, the rate coefficients of OH reactions with fenpropidin and pyrimethanil are estimated to be 1.1×10–10 and 2.0×10–10 cm3 molecule–1 s–1, respectively (US EPA, 2012 [47]).
In aqueous phase, the rate constants of OH reactionswith both fungicides can be estimated from those in the gas phase, using the relationship determined from 47 experimental measurements of rates constants for OH reaction with VOCs in both phases (Monod et al., 2005 [46]):
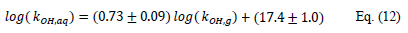
The rate coefficients of OH reaction with fenpropidin and pyrimethanil in the aqueous phase were then estimated to 1.4×1010 M–1 s–1 and 2.1×1010 M–1 s–1, respectively.
Together with the Henry's law constants determined in this work, these data have then been used to estimate τgas and τmultiphase, by using typical concentrations of OH radicals in the gas and aqueous phases: [OH]g=1×106 molecule cm–3 and [OH]aq= 1×10–13 M (Monod et al., 2005 [46]). It was assumed here that the gas phase concentration of OH radicals is not significantly reduced under cloudy conditions and is the same under clear sky and cloudy conditions (Monod and Carlier, 1999 [48]).
At 298 K which is the temperature where most of experimental rate constants were measured, the calculated aqueous fractions are 0.44 for fenpropidin, and 0.27 for pyrimethanil.
The corresponding atmospheric lifetimes (in units of hours) are reduced in the cloud compared to clear sky conditions (in parentheses): fenpropidin, 0.4 (2.5); pyrimethanil, 0.4 (1.4).
However, the average temperature of tropospheric clouds is 283 K and therefore, the calculated aqueous fractions of fenpropidin and pyrimethanil rise respectively from 0.44 to 0.68 and from 0.27 to 0.82 when the temperature decreases from 298 K to 283 K, temperature range where the HLC values of fenpropidin and pyrimethanil increase respectively from 0.76×105 to 2.23×105 M atm–1 and from 0.36×105 to 4.81×105 M atm–1.
Assuming that the rate constants of OH reactions with both pesticides in the gas and aqueous phases do not vary significantly between 283 and 298 K, as it was already observed for many oxygenated VOCs (Atkinson, 2000 [42]; Le Calvé et al., 1998 [49], 1997 [50]), we can then estimate the multiphase lifetimes of these compounds at 283 K. These lifetimes τmultiphase at 283 K (in units of hours) are lower than those calculated at 298 K (in parentheses): fenpropidin, 0.3 (0.4); pyrimethanil, 0.2 (0.4).
4. Conclusion
Although the environmental fates of Fenpropidin and Pyrimethanil have been widely studied in the literature either in air (Córdoba Gamboa et al., 2020 [22]; Désert et al., 2018 [21]; Villiot et al., 2018 [3]) or in soil and water (Agüera et al., 2000 [51]; Vanni et al., 2003 [13], 2000 [52]), this works reports the first experimental determination of their Henry’s law constants, a key parameter to understand their environmental behavior. The HLC values of these two fungicides were determined in the range 278–293 K by means of a Dynamic Equilibrium System, which permits to derive the enthalpy of solvation from the Van’t Hoff expression. From the HLC measurements performed at a typical temperature of tropospheric clouds of 283 K, it was estimated that 68 and 82 % of fenpropidin and pyrimethanil were in atmospheric liquid droplets under standard cloudy conditions. The experimental Henry’s law constants obtained in this work have also been used to estimate either the wet removal efficiency for fenpropidin and pyrimethanil. The associated lifetimes of 42.8 h and 35.4 h for fenpropidin and pyrimethanil respectively, indicate that these two fungicides will be efficiently scavenged by atmospheric wet deposition.
Considering their atmospheric reactivity toward OH radicals in both gas and liquid phases, their multiphase atmospheric lifetimes at 283 K were estimated to be 0.3 and 0.2 hours for fenpropidin and pyrimethanil, respectively. Considering all the uncertainties associated with the above calculations, it can be concluded that the oxidation of these pesticides in the gas and aqueous phases can compete. Kinetic data in aqueous and gas phase are needed for a better evaluation of the atmospheric fate of these pesticides.
Therefore, these compounds will not be persistent in the atmosphere, unlike what has been observed in soils, with half-lifes in the ranges 13.4–16.5 days (Zhao et al., 2012 [53]) and 45–60 days (Vanni et al., 2003 [13]), for fenpropidin and pyrimethanil, respectively.
Acknowledgment
Financial support for this work has been provided by the French Ministry of Environment through the PRIMEQUAL 2 program and the region of Alsace. This work was sponsored in part by the French Agency for Environment and Energy Management (ADEME).
References
- Matthews G. The Pesticide Manual: By Tomlin C. British Crop Protection Council EU (11th Edition) (1998): 1606.
- Index acta phytosanitaire (2021).
- Villiot A, Chrétien E, Drab-Sommesous E, et al. Temporal and seasonal variation of atmospheric concentrations of currently used pesticides in Champagne in the centre of Reims from 2012 to 2015. Atmospheric Environment 174 (2018): 82-91.
- Liu L, Zhang D, Cui Y, et al. Measurement and Correlation of the Solubility of Pyrimethanil in Seven Monosolvents and Two Different Binary Mixed Solvents. J. Chem. Eng. Data 63 (2018): 2804-2812.
- Gullino ML, Leroux P, Smith CM. Uses and challenges of novel compounds for plant disease control. Crop Protection 19 (2000): 1-11.
- Masner P, Muster P, Schmid J. Possible methionine biosynthesis inhibition by pyrimidinamine fungicides. Pesticide Science 42 (1994): 163-166.
- Bedos C, Rousseau-Djabri MF, Loubet B, et al. Fungicide Volatilization Measurements: Inverse Modeling, Role of Vapor Pressure, and State of Foliar Residue. Environ. Sci. Technol 44 (2010): 2522-2528.
- Rice CP, Nochetto CB, Zara P. Volatilization of Trifluralin, Atrazine, Metolachlor, Chlorpyrifos, α-Endosulfan, and β-Endosulfan from Freshly Tilled Soil. J. Agric. Food Chem 50 (2002): 4009-4017.
- Staudinger J, Roberts PV. A critical review of Henry’s law constants for environmentalapplications. Null 26 (1996): 205-297.
- Hvezdová M, Kosubová P, Košíková M, et al. Currently and recently used pesticides in Central Europeanarable soils. Sci Total Environ 613-614 ( 2018): 361-370.
- Ballesteros Martín MM, Sánchez Pérez JA, García Sánchez JL, et al. Degradation of alachlor and pyrimethanil by combined photo-Fenton and biological oxidation. Journal of Hazardous Materials 155 (2008): 342-349.
- Vanni A, Anfossi L, Cignetti A, et al. Degradation of Pyrimethanil in Soil: Influence of Light, Oxygen, and Microbial Activity. Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes 41 (2006): 67-80.
- Vanni A, Fontana F, Cignetti A, et al. Behaviour of pyrimethanil in soil: abiotic and biotic processes. Presented at the Pesticide in air, plant, soil and water system. Proceedings of the XII Symposium Pesticide Chemistry, Piacenza, Italy, 4-6 June 2003, La Goliardica Pavese s.r.l., Pavia (2003): 233-238.
- Climent MJ, Herrero-Hernández E, Sánchez-Martín MJ, et al. Residues of pesticides and some metabolites in dissolved and particulate phase in surface stream water of Cachapoal River basin, central Chile. Environmental Pollution 251 (2019): 90-101.
- Katagi T. Direct photolysis mechanism of pesticides in water. J Pestic Sci 43 (2018): 57-72.
- Coscollà C, Yahyaoui A, Colin P, et al. Particle size distributions of currently used pesticides in a rural Atmosphere of France. Atmospheric Environment 81 (2013).
- Atkinson R, Guicherit R, Hites RA, et al. Transformations of Pesticides in the Atmosphere: A State of the Art. Water, Air, and Soil Pollution 115 (1999): 219-243.
- Klöpffer W. Photochemical degradation of pesticides and other chemicals in the environment: a critical assessment of the state of the art. Science of The Total Environment 123-124 (1992): 145-159.
- Bidleman TF. Atmospheric processes. Environ. Sci. Technol 22 (1988): 361-367.
- Potter TL, Hapeman CJ, McConnell LL, et al. Endosulfan wet deposition in Southern Florida (USA). Science of the total environment 468-469 (2014): 505-513.
- Désert M, Ravier S, Gille G, et al. Spatial and temporal distribution of current-use pesticides in ambient air of Provence-Alpes-Côte-d’Azur Region and Corsica, France. Atmospheric Environment 192 (2018): 241-256.
- Córdoba Gamboa L, Solano Diaz K, Ruepert C, et al. Passive monitoring techniques to evaluate environmental pesticide exposure: Results from the Infant’s Environmental Health study (ISA). Environmental Research 184 (2020): 109243.
- Sander R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmospheric Chemistry and Physics 15 (2015): 4399-4981.
- Trapp S, Matthies M. Substance Data, in: Chemodynamics and Environmental Modeling. Springer, Berlin, Heidelberg (1998).
- Meylan WM, Howard PH. Bond contribution method for estimating henry’s law constants. Environmental Toxicology and Chemistry 10 (1991): 1283-1293.
- Allou L, El Maimouni L, Le Calvé S. Henry’s law constant measurements for formaldehyde and benzaldehyde as a function of temperature and water composition. Atmospheric Environment 45 (2011): 2991-2998.
- Feigenbrugel V, Le Calvé S, Mirabel P, et al . Henry’s law constant measurements for phenol, o-, m-, and p-cresol as a function of temperature. Atmospheric Environment 38 (2004b): 5577-5588.
- Katrib Y, Deiber G, Mirabel P, et al. Atmospheric Loss Processes of Dimethyl and Diethyl Carbonate. Journal of Atmospheric Chemistry 43 (2002): 151-174.
- Feigenbrugel V, Le Calvé S, Mirabel P. Temperature dependence of Henry’s law constants of metolachlor and diazinon. Chemosphere 57 (2004a): 319-327.
- Gautier C, Le Calvé S, Mirabel P. Henry’s law constants measurements of alachlor and dichlorvos between 283 and 298K. Atmospheric Environment 37 (2003): 2347-2353.
- Xie Z, Le Calvé S, Feigenbrugel V, et al. Henry’s law constants measurements of the nonylphenol isomer 4(3′,5′-dimethyl-3′-heptyl)-phenol, tertiary octylphenol and γ-hexachlorocyclohexane between 278 and 298K. Atmospheric Environment 38 (2004): 4859-4868.
- Schummer C, Tuduri L, Briand O, et al. Application of XAD-2 resin-based passive samplers and SPME–GC–MS/MS analysis for the monitoring of spatial and temporal variations of atmospheric pesticides in Luxembourg. Environmental Pollution 170 (2012): 88-94.
- Shepson PB, Mackay E, Muthuramu K. Henry’s Law Constants and Removal Processes for Several Atmospheric β-Hydroxy Alkyl Nitrates. Environ. Sci. Technol 30 (1996): 3618-3623.
- Staudinger J, Roberts PV. A critical compilation of Henry’s law constant temperature dependence relations for organic compounds in dilute aqueous solutions. Chemosphere 44 (2001): 561-576.
- Maniere I, Bouneb F, Fastier A, et al. AGRITOX-Database on pesticide active substances. Fuel and Energy Abstracts 205 (2011).
- HSDB: Hazardous Substances Data Bank. TOXicology data NETwork (TOXNET) (2021).
- Seinfeld JH, Seinfeld PDCJH, Pandis SN. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, Wiley-Interscience publication. Wiley (1998).
- Brimblecombe P, Dawson GA. Wet removal of highly soluble gases. Journal of Atmospheric Chemistry 2 (1984): 95-107.
- Haurwitz B, Austin JM, Austin JB. Climatology. McGraw-Hill Book Company, Incorporated (1944).
- Oort A, Rasmusson E. Atmospheric circulation statistics, in: NOAA Professional Paper 5, U.S. Dept. of Commerce,. Nat. Ocean and Atmos. Admin., Rockville, Md., (1971): 323.
- Kolb CE, Worsnop DR, Zahniser MS, et al (1995).
- Atkinson R. Atmospheric chemistry of VOCs and NOx. Atmospheric Environment 34 (2000): 2063-2101.
- Ervens B, Gligorovski S, Herrmann H. Temperature-dependent rate constants for hydroxyl radical reactions with organic compounds in aqueous solutions. Phys. Chem. Chem. Phys 5 (2003): 1811-1824.
- Feigenbrugel V, Le Calvé S, Mirabel P. Molar absorptivities of 2,4-D, cymoxanil, fenpropidin, isoproturon and pyrimethanil in aqueous solution in the near-UV. Spectrochim Acta A Mol Biomol Spectrosc 63 (2006): 103-110.
- Monod A, Poulain L, Grubert S, et al. Kinetics of OH-initiated oxidation of oxygenated organic compounds in the aqueous phase: new rate constants, structure–activity relationships and atmospheric implications. Atmospheric Environment 39 (2005): 7667-7688.
- Kwok ESC, Atkinson R. Estimation of hydroxyl radical reaction rate constants for gas-phase organic compounds using a structure-reactivity relationship: An update. Atmospheric Environment 29 (1995): 1685-1695.
- US EPA. Estimation Programs Interface SuiteTM for Microsoft® Windows, v 4.11. AOPWIN v1.92. United States Environmental Protection Agency, Washington, DC, USA (2012).
- Monod A, Carlier P. Impact of clouds on the tropospheric ozone budget: Direct effect of multiphase photochemistry of soluble organic compounds. Atmospheric Environment 33 (1999): 4431-4446.
- Le Calvé S, Hitier D, Le Bras G, et al. Kinetic Studies of OH Reactions with a Series of Ketones. J. Phys. Chem. A 102 (1998): 4579-4584.
- Le Calvé S, Le Bras G, Mellouki A,. Kinetic Studies of OH Reactions with a Series of Methyl Esters. J. Phys. Chem. A 101 (1997): 9137-9141.
- Agüera A, Almansa E, Tejedor A, et al. Photocatalytic Pilot Scale Degradation Study of Pyrimethanil and of Its Main Degradation Products in Waters by Means of Solid-Phase Extraction Followed by Gas and Liquid Chromatography with Mass Spectrometry Detection. Environ. Sci. Technol 34 (2000): 1563-1571.
- Vanni A, Gamberini R, Calabria A, et al. Determination and identification ofmetabolites of the fungicides Iprodione and Procymidone in compost. Chemosphere 41 (2000): 1431-1439.
- Zhao H, Xue J, Jiang N, et al. Dissipation and residue of fenpropidin in wheat and soil under field conditions. Ecotoxicol Environ Saf 77 (2012): 52-56.
