Technical Solutions found to install the German Mandatory Breast Implant Registry
Article Information
von Fritschen U1,2*, Schiltz D1, Prantl L2, Fricke A1,3
Affiliation:
1Department of Plastic and Aesthetic Surgery, Hand Surgery, HELIOS Hospital Emil von Behring, Walterhöfer Str. 11, 14165 Berlin, Germany
2University Center for Plastic, Reconstructive, Aesthetic and Hand Surgery, University Hospital Regensburg and Caritas Hospital St. Josef, 93053 Regensburg, Germany
3Department of Plastic and Hand Surgery, University of Freiburg Medical Centre, Medical Faculty of the University of Freiburg, Germany
*Corresponding author:
Dr. med. Uwe von Fritschen, Department of Plastic and Aesthetic Surgery, Hand Surgery. HELIOS Hospital Emil von Behring, Walterhöfer Str. 11, 14165 Berlin, Germany.
Received: November 24, 2023; Accepted: December 04, 2023; Published: December 19, 2023
Citation: von Fritschen U, Schiltz D, Prantl L, Fricke A. Technical Solutions found to install the German Mandatory Breast Implant Registry. Journal of Surgery and Research. 6 (2023): 442-447
Share at FacebookAbstract
Background: Despite numerous attempts, sound data on long-term implant performance is lacking for almost every aspect of implant based procedures of the breast. Various scandals highlighting insufficient trackand- trace functions led the German Government in accordance with new EU-requirements to engage in a comprehensive device registry.
Objectives: This article aims to provide information about the challenges of implant registries to provice high evidence data and the complex structural and regulatory requirements implementating a mandatory registry
Methods: We analysed the current limitations of studies and other international registries and developed solutions to overcome these setbacks
Results: Insufficient inclusion rates and inability to verify adhearance, lack of funding, privacy issues and inefficient research methodologies, turned out to be the most common.
Conclusion: To assure longitudinal assessment of every implant-based procedure, a mandatory approach was identified as the most reasonable way. Beside limitations of personal privacy rights, extensive legislative and procedural requirements were to be met. We describe our approach, obstacles encountered, and strategies to inform future registries on structural requirements.
Keywords
Breast implant registry, Device registry, Breast reconstruction, Cosmetic augmentation, Patient safety, Registries
Breast implant registry articles; Device registry articles; Breast reconstruction articles; Cosmetic augmentation articles; Patient safety articles; Registries articles
Breast implant registry articles Breast implant registry Research articles Breast implant registry review articles Breast implant registry PubMed articles Breast implant registry PubMed Central articles Breast implant registry 2023 articles Breast implant registry 2024 articles Breast implant registry Scopus articles Breast implant registry impact factor journals Breast implant registry Scopus journals Breast implant registry PubMed journals Breast implant registry medical journals Breast implant registry free journals Breast implant registry best journals Breast implant registry top journals Breast implant registry free medical journals Breast implant registry famous journals Breast implant registry Google Scholar indexed journals Device registry articles Device registry Research articles Device registry review articles Device registry PubMed articles Device registry PubMed Central articles Device registry 2023 articles Device registry 2024 articles Device registry Scopus articles Device registry impact factor journals Device registry Scopus journals Device registry PubMed journals Device registry medical journals Device registry free journals Device registry best journals Device registry top journals Device registry free medical journals Device registry famous journals Device registry Google Scholar indexed journals Breast reconstruction articles Breast reconstruction Research articles Breast reconstruction review articles Breast reconstruction PubMed articles Breast reconstruction PubMed Central articles Breast reconstruction 2023 articles Breast reconstruction 2024 articles Breast reconstruction Scopus articles Breast reconstruction impact factor journals Breast reconstruction Scopus journals Breast reconstruction PubMed journals Breast reconstruction medical journals Breast reconstruction free journals Breast reconstruction best journals Breast reconstruction top journals Breast reconstruction free medical journals Breast reconstruction famous journals Breast reconstruction Google Scholar indexed journals Cosmetic augmentation articles Cosmetic augmentation Research articles Cosmetic augmentation review articles Cosmetic augmentation PubMed articles Cosmetic augmentation PubMed Central articles Cosmetic augmentation 2023 articles Cosmetic augmentation 2024 articles Cosmetic augmentation Scopus articles Cosmetic augmentation impact factor journals Cosmetic augmentation Scopus journals Cosmetic augmentation PubMed journals Cosmetic augmentation medical journals Cosmetic augmentation free journals Cosmetic augmentation best journals Cosmetic augmentation top journals Cosmetic augmentation free medical journals Cosmetic augmentation famous journals Cosmetic augmentation Google Scholar indexed journals Patient safety articles Patient safety Research articles Patient safety review articles Patient safety PubMed articles Patient safety PubMed Central articles Patient safety 2023 articles Patient safety 2024 articles Patient safety Scopus articles Patient safety impact factor journals Patient safety Scopus journals Patient safety PubMed journals Patient safety medical journals Patient safety free journals Patient safety best journals Patient safety top journals Patient safety free medical journals Patient safety famous journals Patient safety Google Scholar indexed journals Registries articles Registries Research articles Registries review articles Registries PubMed articles Registries PubMed Central articles Registries 2023 articles Registries 2024 articles Registries Scopus articles Registries impact factor journals Registries Scopus journals Registries PubMed journals Registries medical journals Registries free journals Registries best journals Registries top journals Registries free medical journals Registries famous journals Registries Google Scholar indexed journals breast implants articles breast implants Research articles breast implants review articles breast implants PubMed articles breast implants PubMed Central articles breast implants 2023 articles breast implants 2024 articles breast implants Scopus articles breast implants impact factor journals breast implants Scopus journals breast implants PubMed journals breast implants medical journals breast implants free journals breast implants best journals breast implants top journals breast implants free medical journals breast implants famous journals breast implants Google Scholar indexed journals German Society of Plastic, Reconstructive and Aesthetic Surgeons articles German Society of Plastic, Reconstructive and Aesthetic Surgeons Research articles German Society of Plastic, Reconstructive and Aesthetic Surgeons review articles German Society of Plastic, Reconstructive and Aesthetic Surgeons PubMed articles German Society of Plastic, Reconstructive and Aesthetic Surgeons PubMed Central articles German Society of Plastic, Reconstructive and Aesthetic Surgeons 2023 articles German Society of Plastic, Reconstructive and Aesthetic Surgeons 2024 articles German Society of Plastic, Reconstructive and Aesthetic Surgeons Scopus articles German Society of Plastic, Reconstructive and Aesthetic Surgeons impact factor journals German Society of Plastic, Reconstructive and Aesthetic Surgeons Scopus journals German Society of Plastic, Reconstructive and Aesthetic Surgeons PubMed journals German Society of Plastic, Reconstructive and Aesthetic Surgeons medical journals German Society of Plastic, Reconstructive and Aesthetic Surgeons free journals German Society of Plastic, Reconstructive and Aesthetic Surgeons best journals German Society of Plastic, Reconstructive and Aesthetic Surgeons top journals German Society of Plastic, Reconstructive and Aesthetic Surgeons free medical journals German Society of Plastic, Reconstructive and Aesthetic Surgeons famous journals German Society of Plastic, Reconstructive and Aesthetic Surgeons Google Scholar indexed journals Health insurer articles Health insurer Research articles Health insurer review articles Health insurer PubMed articles Health insurer PubMed Central articles Health insurer 2023 articles Health insurer 2024 articles Health insurer Scopus articles Health insurer impact factor journals Health insurer Scopus journals Health insurer PubMed journals Health insurer medical journals Health insurer free journals Health insurer best journals Health insurer top journals Health insurer free medical journals Health insurer famous journals Health insurer Google Scholar indexed journals
Article Details
Background
Various adverse reports [1-3] and uncertainty about the long-term risks associated with breast implants, in addition to expanded EU-requirements, led the German government to engage in a mandatory registration and longitudinal follow-up of almost all implantable devices [4]. The background and principle decisions were published elsewhere, while this article aims to provide information about the structural requirements and technical implementation [5].
Material
Various countries have set up breast implant registries. Most of them failed to provide longterm sustainable data due to various reasons. As a consequence, the German Society of Plastic, Reconstructive and Aesthetic Surgeons joined an international collaboration of breast specialist (ICOBRA), developing prerequisites for improved data on breast implant performance
[6,7]. A survey was conducted among the members to identify setbacks and critical aspects of current and historic registries, responsible for limitations in the informative value of the generated data or failure to maintain functionality. These aspects had to be reconciled with the specific German requirements, including vigilance, protection of personal rights and the legal framework. Ultimately, this preliminary work was able to contribute to defining and implementing the regulatory, structural and legal requirements of the Germany implant registry.
Results
- Missing sustainable fundings and low or incomplete capture rates could be identified as main reasons for registries to fail or not succeed to maintain quality data.
- Evaluation of long-term implications of implants as well as comparative device performance requires livelong identification of a patient and merging of data, regardless of the institutions performing the procedures.
- A next to complete inclusion rate is key for evidence based Opt-out consent based policies proved to be an advance on opt-in solutions, but still cannot guarantee adherence.
- Compliance to participate turned out to be a challenge for all registries. Various national approaches attempt to overcome this setback, but especially the private sector and revision surgeries proved to be difficult to track.
- No registry has means to reliably identify missing data- sets. This holds true even in mandatory registries.
- Adherence to include data is largely dependent on the required workload. Over-collection of data-points, burdensome or repetitive inclusion hamper readiness to
- The denominator (e.g. the total amount of implants inserted) is a vital parameter to contextualize the results. Despite ambitious national attempts, registries struggle to provide reliable means to relate outcome measures to the overall amount of implants inserted or removed.
- Missing clinical and patient related parameter may lead to a misjudgement of the reasons for a revision surgery.
- An early warning system is dependent on a sufficiently large data-pool for every type of implant, unlikely with limited national data-sets.
- Contacting patients in case of an alert requires contact data. Keeping them up-to date lays mostly in the hands of the patient with is performed rather unreliably.
- Longterm data governance and outcome reporting mechanisms are tend to be unstructured, understaffed and performed on a voluntary basis.
Specific aspects in Germany
- Germany does not provide an overall unique identifier for the German population
- Accumulating and storing of personal data above the timeframe required to treat a patient and merging of patient related information is not in keeping with German data privacy legislation
- Federal competence hinders national data pooling
Solutions found
Inclusion rate: Universally failed registries had adopted an optional participation process (opt-in). Data capture was too low to adequately enable statistically sound outcome analysis. Opt-out policy proved a major advance but opting out remains a flaw and is difficult to track [8]. It was therefore decided to oblige health providers to register all implant related procedures in a mandatory registry.
Ease of Insertion, Device library: Scanning the barcode is not sufficient, since not all relevant information is included in the code. Also different codes with different information are dispatched on the outer and inner package. Therefore manufacturer were obliged to include and up-date all relevant information in a national implantable device library. Data relevant for the registration process will be accessable online, or off-line by downloading the library. The dataset can then be inserted by scanning the UDI or by manually entering the combination of manufacturer and REF-No. To avoid redundant insertion of data-points, a link to the facility information system is foreseen. Until all institutions adapt the software and fullfill the technical requirements, a Web-based platform is provided as an interim solution.
Track-and-trace: Requirements to inform patients about potential implant related problems include a connection of a specific device to a patient and up-to-date contact data. Health insurer, covering 99% of our population, were obliged to create a life-long patient identifyer and keep contact data up to date. However, not everyone is included to this system. For federal police and armed forces, among others, a final decision is pending, but a similar connection is presently favoured. For patients without an ID like foreigners, a dummy-ID will be generated, but cannot be fully functional, since longitudinal collection of data and contact in case of adverse event will not be possible.
Data privacy rights: Data ownership is located at the Ministry of Health (MH). Responsibilities, duties and processing of data are separated between two independent administrative offices, the Trust Center (TC) and the Registry Office (RO), independent in physical, organizational, technical and personel terms. The implanting unit provides patients with a copy of all information submitted to the registry.
Patients might also claim information about submitted data at any time. Herefore, any registered health provider (HP) can send the patient-ID to the TC. The RO will provide the information recorded in the registry in closed envelopes to the TC. The TC will add their information and both are sent in another envelope to the HP, assuring, that only the patient will receive the accumulated information. The dataset must be anonymized if the purpose of the registry can be met accordingly, usually at the death of a patient.
Data workflow: The mode of communication is prescribed by law unsing the Telematic Infrastructure (TI), a Limited Company, providing a safe communication platform to exchange health related data. Propriators are representatives of the main stakeholders with the MH holding 51% [9]. Technically the HP will inquire the patient´s ID and the insurer-ID and submit the patient-identifying data and a self-generated internal-record-identifier to the TC. Here a transfer-code is generated and returned. With this code, the procedure-related information is submitted to the RO. The registry office sends the transfer-code to the TC and receives the corresponding patient- and record pseudonyms. These pseudonyms are linked to the procedure-related information. After matching the implants used with the device library, a registration confirmation and a copy of the data included is issued for the HP, necessary to prove registration to the patient and insurer.
After completion, the transfer-code is deleted by all stakeholders, since it was only necessary to “transport” and merge the data, ensuring, that at no time the RO receives patient identifying data or the TC information about the implant-based procedure. The entire procedure is performed automated in the background as soon as the software integration of the facility information system is enabled by software suppliers.
Using the TI requires a safe identification of the user and the implanting unit. The RO will issue an institution-ID after approval. For authentication in the registration process this ID and an institution ID-card (SMC-B) is reqired.[10] Applications are reviewed by national trust centers before the cards are issued by the federal press. Technical requirements for connecting to the TI require a card terminal for the SMC-B card and either a connector or a service provider operating connectors.
On site, the technical provision must be adapted by the local IT-provider to the corresponding hard-/and software situation of the facility. This includes not only the connection of the workstations, but also the release of an App, opening the authenticator and the internet browser for portal access. Additionally personal access rights and logins need to be determined. The complex IT-interaction is depicted in figure 1.
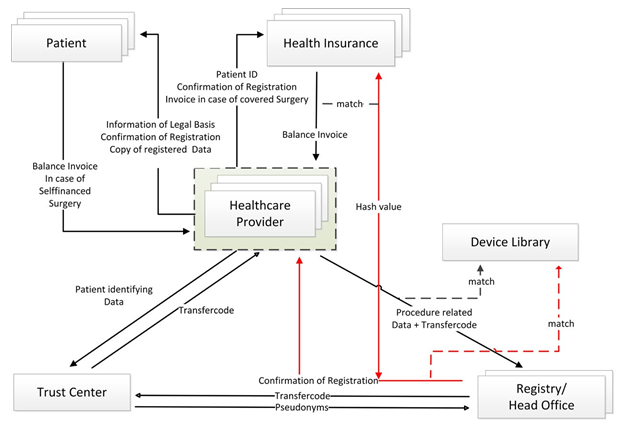
Figure 1: Interaction of stakeholders
Longitudinal follow-up: Enabling follow-up of an implant, required national data-pooling and shifting of data governance from federal to national competence. When a patient is re-operated, the new dataset will be linked to the same pseudonym by TC and RO, identified by means of the health insurance ID.
Compliance: To ensure complete inclusion, registration is linked to remuneration. In case of procedures covered by insurer, the report confirmation is attached to the online invoice procedure of the institution. Insurer will verify the validity of the report with the RO by automated means (Hash value and ID of report confirmation). In self-financed surgeries, the patient has to balance the bill only after registration is proved to them.
Identification of Datasets / Rapid alert system: If the HP has to identify specific datasets, e.g. in case of questionable data or device conspicuities, the RO will issue a list of affected record pseudonyms to the trust center. The TC will de-pseudonymise them and send a list of internal-record- identifier mentioned above to each implanting unit together with safety directions of the competent authority, allowing to assign each identifier to a patient. An approach, already routine in the context of national quality reporting.
If the contact data in the patient file is not up to date, HP can request the current details from insurer. However, healthcare providers can go out of business. The authorities are aware of this problem, but currently no conclusive procedure is prescribed for this scenario. Probably the legal successor will assum this responsibility.
Funding
To establish a reliable funding, fees are charged from the HP, manufacturers and data recipients as outlined in the law [11]. In case of procedures covered by insurer, expenses for reporting are in turn reimbursed by the cost providers. No reimbursement is foreseen in case of self-financed procedures. Additional cost include charges of software suppliers, provider of connectors and TI.
Discussion
In Germany, two previous breast implant registries, run by scientific societies [12,13] failed, mainly due to poor data capture, burdensome data collection requirements, unstructured inclusion, data privacy issues, and insufficient funding. Our survey could show, that other countries experienced similar problems [8,14-23], failing to provide quality data for the evaluation of long-term implications as well as comparative device performance or an early alert in case of implant-related conspicuities [21-24]. Despite the considerable comprehensive prerequisites required, German Health Authorities decided to engage in a national registry with mandatory inclusion policy promising to exclude many of these limits from the outset.
A conspicuous problem for precise data inclusion is the complexity of data collection requirements and the processing time. Besides using a minimum dataset, linking the facility information system and automating asset handling, facilitating the entry of implant details turned out to be challenging [6,24]. Enabling a scan-mode had to take into accout the inconsistent information in the barcodes provided by the manufacturers as well as reliably up-to date information. Providing all relevant information centrally available, required the implementation of a device library. As an alternative to a national solution, a connection to the EU database on medical devices, was discussed, but unfortunately, EUDAMED has some shortcomings with regard to implant details, among others [25]. However, in order to not interfere with EU standards and harmonization, the lawgiver cannot obligate producers of medical devices to include the specifications as a prerequisite to access the German market. As part of the sanction mechanism, medical caregivers will not be able to invoice the procedure though, unless registered products are used.
The complex data exchange between the institutions involved, will be automated in the background. Extracting details documented in the facility information system requires software adaption. However, after extensive consultations no agreement could be reached among the various software suppliers on which programming language should be used. The MH published XML-specifications, but will probably shift to FHIR after the interim phase to meet the wishes of the IT-industry [26].
Software suppliers supporting hospitals with standardized programs, are presently working on appropriate solutions, but expecially private practices with custom made systems might face challenges in the beginning.
For longitudinal follow-up, but also to install a track-and- trace function, a unique identifier for the entire population had to be created. Our health-system qualified as the most reasonable solution, not only because the current contact data is up to date, but also since the TC needs to be informed in case of death of a patient. In addition, insurer need to be informed about the registration anyway in case of surgeries covered by health insurance. Legislation was adapted accordingly and presently the population is issued new insurance certificates, if the ID is not already included.
Providing a safe platform to communicate was so far only stipulated for patients covered by the statuary health system. Expanding this system to private practices and other parties, presently not included, required registration to the TI, obtain specific personalized identifier and invest in additional hardware and software. Even though these prerequisites might not be popular, safe handling of sensitive data was a major concern. As depicted, much effort was put in safeguarding personal data under these requirements.
The implanting institution is obliged to inform insurer, even though potentially, patients with self-financed surgeries might not want their insurer being involved. However, the insurer will only be informed, that an implant related procedure was performed, without further specification of the diagnosis or the device. In the beginning, a relation to the procedure performed might be thinkable, but with more and more types of implants included, no reasonable connection will be possible.
Even though law prescribes a mandatory inclusion, compliance to adhere needs monitoring. In England, another registry with mandatory inclusion policy, inclusion rates are suspected not to reflect the espected rate of procedures, with no means to identify missing data-sets or non-adherence [27]. Monitoring is specifically difficult in revision cases, when no new implant is inserted and thus lacking an objective tracking parameter. The Austalian registry found that compliance was particulary unreliable in private practice [17]. We consider the connection of reporting to the right to invoice the procedure not only guarantees adherence, but also delivers a very reliable denominator, otherwise only difficult to access.
Limitations and solutions pending
The start of the registry was delayed for various times and reasons reaching from supply-chains during Corona to challenges in the internal data handling. This led many institutions and software supplierts to postpone engagement. 2023 was intended a trial phase, reaching full functionality in July 2024. However, many institutions only now realize the need to provide the required prerequisits.
On the technical side, the software interface to integrate the institutional information systems, depend on the readiness of software suppliers and ability to cope with the specifications proviced by the authorities, with presently no system ready to use. A timely start is assured by most suppliers, though.
Even though an interim Web-based solution is provided, the prescribed mode of transmission via TI requires connection to this system allowing for safe communication of sensitive data. Hardware, software and institutional identifyer need to be installed in institutions, currently not included to the system.
Barcodes and QR-codes are not standardized. Some scanner showed to have problems reading some codes, requiring devices providing a programming option.
An early alert system was an assignement for the registry, greatly depending on the extent of the data set. The functional aspects of pooling international ICOBRA datasets will be established at a later time.
Conclusion
Against the background of various adverse events, where German vigilance proved to be incapable to provide a timely recognition and also had no means to identify affected patients, led authorities to engage in a comprehensive approach.
The implementation process required a lot of time and resources from everyone involved, certainly partly associated with the use of breast implants as a starting device. Due to the very complex processes involved, not all scenarios could be predicted, though, and problems will only become apparent during the course of application, requiring further adjustments. However, we are convinced, that the data generated will be of significantly improved quality compared to current approaches.
Contribution to authorship
Conception and planning: UvF, LP and AF. Carrying out the research, data collection: UvF, DS. Analysing and writing up the results: UvF, AF, DS. Revision and final approval: UvF, AF, LP, DS.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research,
authorship, and publication of this article.
AI
During the preparation of this work, the authors used no tool/service. The authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
We read and followed the instructions for authors. We hereby declare our acceptance of the conditions posed. The manuscript is original, has not previously been published in either printed or electronic form and is not under consideration for publication elsewhere. All authors approved the final manuscript and its submission to JSR.
References
- Cook RR, Harrison MC, LeVier RR. The breast implant controversy. Arthritis Rheum 37 (1994): 153-157.
- Gherardini G, Zaccheddu R, Basoccu G. Trilucent breast implants: voluntary removal following the Medical Device Agency recommendation. Report on 115 consecutive patients. Plast Reconstr Surg 113 (2004): 1024-1027.
- Oulharj S, Pauchot J, Tropet Y. PIP breast implant removal: a study of 828 cases. J Plast Reconstr Aesthet Surg 67 (2014): 302-307.
- Gesetz zur Errichtung des Deutschen Implantatregisters (2023).
- Von Fritschen U, Rakhorst HA, Stark B, et al. The Mandatory German Breast Implant Registry Law: A Model for Sustainable Implant Aesthet Surg J 43 (2023): Np858-np865.
- Spronk PER, Begum H, Vishwanath S, et al. Toward international harmonization of breast implant Registries: International Collaboration of Breast Registry Activities Global Common Data Set. Plast Reconstr Surg 146 (2020): 255-267.
- Cooter RD, Barker S, Carroll SM, et al. International importance of robust breast device registries. Plast Reconstr Surg 135 (2015): 330-336.
- Brown T, Merten S, Mosahebi A, et al. Breast implant registries: The problem with ambition. Aesthet Surg J 36 (2016): 255-259.
- Telematics infrastructure (2023).
- D-Trust. SMB-C Card (2023).
- Implant Register Fee Ordinance (IRegGebV) (2023).
- Nestle-Krämling C, Thill Netz- und matrixgestützte Implantatrekonstruktion. Gynäkologe 49 (2016): 166-172.
- Renner C, Neuhann-Lorenz C. International Breast Implant Registry: a user report. Aesthetic Plast Surg 30 (2006): 616-621.
- Rakhorst HA, Mureau MAM, Cooter RD, et al. The new opt-out Dutch National Breast Implant Registry- Lessons learnt from the road to implementation. J Plast Reconstr Aesthet Surg 70 (2017): 1354-1360.
- Hopper I, Ahern S, Best RL, et al. Australian breast device registry: breast device safety transformed. ANZ J Surg 2017;87:9-10.
- Hopper I, Ahern S, Nguyen TQ, et al. Breast Implant Registries: A call to action. Aesthet Surg J 38 (2018): 807-810.
- Hopper I, Best RL, McNeil JJ, et al. Pilot for the Australian Breast Device Registry (ABDR): a national opt-out clinical quality registry for breast device BMJ Open 7 (2017): e017778.
- Song WJ, Kang SG, Seo BMF, et al. Pilot study of the Korean national breast implant registry: Experiences and lessons J Plast Reconstr Aesthet Surg 75 (2022): 1833-1841.
- Campanale A, Ventimiglia M, Minella D, et National Breast Implant Registry in Italy. Competent authority perspective to improve patients’ safety. Plastic Reconstructive and Regenerative Surgery 1 (2022): 1-11.
- Becherer BE, Spronk PER, Mureau MAM, et al. High risk device registries: Global value, costs, and sustainable funding. J Plast Reconstr Aesthet Surg 71 (2018): 1362-1380.
- Henriksen TF, Holmich LR, Friis S, et al. The Danish Registry for Plastic Surgery of the Breast: establishment of a nationwide registry for prospective follow-up, quality assessment, and investigation of breast surgery. Plast Reconstr Surg 111 (2003): 2182-2189.
- Bargon CA, Becherer BE, Young-Afat DA, et Moving breast implant registries forward: Are they fair and functional? J Plast Reconstr Aesthet Surg 8 (2020): 89-95.
- Swanson The case against the national breast implant registry. Ann Plast Surg 86 (2021): 245-247.
- Becherer BE, Hopper I, Cooter RD, et al. Improving breast implant safety through international collaboration of national registries - A review of over 85000 patients and 200000 implants from four countries. Plast Reconstr Surg 17 (2023).
- Commission, E. EUDAMED - European Database on Medical Devices (2023).
- Spezifikation IRD (2023).
- NHS Digital issues call for more data to be added to breast implant registry. British Journal of Healthcare Computing (2018).
