Taiyuanostachya: An Abominable Angiosperm from the Early Permian of China
Article Information
Xin Wang*, Qiang Fu
State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology and CAS Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China
*Corresponding author: Xin Wang
Received: 23 August 2023; Accepted: 28 August 2023; Published: 06 September 2023
Citation: Xin Wang, Qiang Fu. Taiyuanostachya: An Abominable Angiosperm from the Early Permian of China. Journal of Biotechnology and Biomedicine. 6 (2023): 371-379.
Share at FacebookAbstract
Although angiosperms are clearly and strictly defined by their enclosed seeds and enclosed ovules, how old angiosperms are remains controversial. To solve this problem, the only reliable way is digging fossils. The currently widely accepted age for angiosperms is the Early Cretaceous, although this view is facing increasing challenges from pre-Cretaceous fossil evidence of angiosperms as well as molecular clock estimates. Here we report a Palaeozoic angiosperm, Taiyuanostachya gen. nov. This fossil plant has both enclosed ovules and enclosed seeds, features characteristic of angiosperms. Especially, enclosed ovules are idiosyncratic of angiosperms. The occurrences of both characters in Taiyuanostachya declare that angiosperms, the single most diversified plant group on the Earth, have occurred in the Palaeozoic, and the origination of angiosperm appears to be much earlier than assumed previously. Although appearing astonishing, this conclusion is in line with the outcomes of molecular clock estimates done decades ago. The discovery of Taiyuanostachya gen. nov is an abominable challenge for botanists who believe groundlessly that angiosperms cannot exist before the Cretaceous.
Keywords
Angiosperm; Permian; Palaeozoic; Evolution; China
Angiosperm articles Angiosperm Research articles Angiosperm review articles Angiosperm PubMed articles Angiosperm PubMed Central articles Angiosperm 2023 articles Angiosperm 2024 articles Angiosperm Scopus articles Angiosperm impact factor journals Angiosperm Scopus journals Angiosperm PubMed journals Angiosperm medical journals Angiosperm free journals Angiosperm best journals Angiosperm top journals Angiosperm free medical journals Angiosperm famous journals Angiosperm Google Scholar indexed journals Permian articles Permian Research articles Permian review articles Permian PubMed articles Permian PubMed Central articles Permian 2023 articles Permian 2024 articles Permian Scopus articles Permian impact factor journals Permian Scopus journals Permian PubMed journals Permian medical journals Permian free journals Permian best journals Permian top journals Permian free medical journals Permian famous journals Permian Google Scholar indexed journals Palaeozoic articles Palaeozoic Research articles Palaeozoic review articles Palaeozoic PubMed articles Palaeozoic PubMed Central articles Palaeozoic 2023 articles Palaeozoic 2024 articles Palaeozoic Scopus articles Palaeozoic impact factor journals Palaeozoic Scopus journals Palaeozoic PubMed journals Palaeozoic medical journals Palaeozoic free journals Palaeozoic best journals Palaeozoic top journals Palaeozoic free medical journals Palaeozoic famous journals Palaeozoic Google Scholar indexed journals Evolution articles Evolution Research articles Evolution review articles Evolution PubMed articles Evolution PubMed Central articles Evolution 2023 articles Evolution 2024 articles Evolution Scopus articles Evolution impact factor journals Evolution Scopus journals Evolution PubMed journals Evolution medical journals Evolution free journals Evolution best journals Evolution top journals Evolution free medical journals Evolution famous journals Evolution Google Scholar indexed journals China articles China Research articles China review articles China PubMed articles China PubMed Central articles China 2023 articles China 2024 articles China Scopus articles China impact factor journals China Scopus journals China PubMed journals China medical journals China free journals China best journals China top journals China free medical journals China famous journals China Google Scholar indexed journals angiosperm chaotic articles angiosperm chaotic Research articles angiosperm chaotic review articles angiosperm chaotic PubMed articles angiosperm chaotic PubMed Central articles angiosperm chaotic 2023 articles angiosperm chaotic 2024 articles angiosperm chaotic Scopus articles angiosperm chaotic impact factor journals angiosperm chaotic Scopus journals angiosperm chaotic PubMed journals angiosperm chaotic medical journals angiosperm chaotic free journals angiosperm chaotic best journals angiosperm chaotic top journals angiosperm chaotic free medical journals angiosperm chaotic famous journals angiosperm chaotic Google Scholar indexed journals Taiyuanostachya ovuilifera articles Taiyuanostachya ovuilifera Research articles Taiyuanostachya ovuilifera review articles Taiyuanostachya ovuilifera PubMed articles Taiyuanostachya ovuilifera PubMed Central articles Taiyuanostachya ovuilifera 2023 articles Taiyuanostachya ovuilifera 2024 articles Taiyuanostachya ovuilifera Scopus articles Taiyuanostachya ovuilifera impact factor journals Taiyuanostachya ovuilifera Scopus journals Taiyuanostachya ovuilifera PubMed journals Taiyuanostachya ovuilifera medical journals Taiyuanostachya ovuilifera free journals Taiyuanostachya ovuilifera best journals Taiyuanostachya ovuilifera top journals Taiyuanostachya ovuilifera free medical journals Taiyuanostachya ovuilifera famous journals Taiyuanostachya ovuilifera Google Scholar indexed journals Crosszamia minor articles Crosszamia minor Research articles Crosszamia minor review articles Crosszamia minor PubMed articles Crosszamia minor PubMed Central articles Crosszamia minor 2023 articles Crosszamia minor 2024 articles Crosszamia minor Scopus articles Crosszamia minor impact factor journals Crosszamia minor Scopus journals Crosszamia minor PubMed journals Crosszamia minor medical journals Crosszamia minor free journals Crosszamia minor best journals Crosszamia minor top journals Crosszamia minor free medical journals Crosszamia minor famous journals Crosszamia minor Google Scholar indexed journals Sporophylls uniform articles Sporophylls uniform Research articles Sporophylls uniform review articles Sporophylls uniform PubMed articles Sporophylls uniform PubMed Central articles Sporophylls uniform 2023 articles Sporophylls uniform 2024 articles Sporophylls uniform Scopus articles Sporophylls uniform impact factor journals Sporophylls uniform Scopus journals Sporophylls uniform PubMed journals Sporophylls uniform medical journals Sporophylls uniform free journals Sporophylls uniform best journals Sporophylls uniform top journals Sporophylls uniform free medical journals Sporophylls uniform famous journals Sporophylls uniform Google Scholar indexed journals organ articles organ Research articles organ review articles organ PubMed articles organ PubMed Central articles organ 2023 articles organ 2024 articles organ Scopus articles organ impact factor journals organ Scopus journals organ PubMed journals organ medical journals organ free journals organ best journals organ top journals organ free medical journals organ famous journals organ Google Scholar indexed journals
Article Details
Introduction
Although Stebbins [1] has stated that “the evolutionary line leading to the angiosperms entered a dark tunnel of ignorance at the end of the Paleozoic until the early Cretaceous”, this statement was never corroborated by any fossil evidence, since Scott et al. [2] annihilated all pre-Cretaceous records of angiosperms, and thenceforth the status of study on origin of angiosperms remains unchanged [3]. The reason underlying such an academic stagnancy includes 1) a lack of well documented fossil evidence, which is the key to the problem [4], and 2), more importantly, a lack of consensus of criterion identifying angiosperms in the fossil world made the study of early angiosperm chaotic. A merit of a recent review on early angiosperms [3] is that the authors clearly listed several features unique of angiosperms, which could be used as criterion identifying a fossil angiosperm. Unfortunately, the authors of the paper rejected themselves by listing five exemplar fossil angiosperms that did not meet their own criterion [3,5]. The rejection was further consolidated by Friis et al. [6], who published another fossil angiosperm that did not honor this criterion [6,7]. “The unique character of angiosperms is that the ovules are completely enclosed in a carpel” [8]. This stricter criterion for angiosperms applied by palaeobotanists was favored by a survey done by Tomlinson and Takaso [9], who distinguish angiosperms from gymnosperms by enclosed ovules before pollination. Here we adopt this stricter criterion (ovules enclosed before pollination) as a criterion identifying fossil angiosperms. Following this criterion, we pin down the angiosperm affinity of Taiyuanostachya ovuilifera gen. et sp. nov from the Lower Shihhotse Formation of Shanxi, China. Considering its Permian age, recognizing Taiyuanostachya as an angiosperm at least doubles the widely accepted age of angiosperms in the geological history. Such a discovery topples the existing stereotype of angiosperm evolution and history.
Materials and Methods
Most of the specimens (GP0093, GP0094, GP0094-A, GP0095) were collected by Gao from exposures of the Lower Shihhotse Formation, Lower Permian, in Simugedong approximately 5 km northeast of East Hill Mine, Taiyuan, Shanxi, China. The flora assemblage are composed of pteridophytes, cycadophytes, and Noeggerathiales [10]. One additional specimen (B461) was collected in 1980 by Zhu and Du [11] from the Lower Shihhotse Formation, Lower Permian of East Hill, Taiyuan, Shanxi, China. The primary result of Zhu and Du’s outcome is the publication of Primocycas chinensis Zhu and Du. The specimen is in the same specimen case as Primocycas chinensis. During both excursions, the collectors found that the Taiyuanostachya gen. nov specimens were associated with Taniopteris spp., Tingia hamaguchii, Sphenopteris spp., Emplectopteris triangularis, Sphenophyllum sp., Cathaysiopteris whitei, Primocycas chinensis, Crosszamia minor, C. cucullata, C. spadicia, Tianbaolinia circinalis, Discinites dentilongus [10-14]. This assemblage is typical of the Lower Shihhotse Formation, Lower Permian [15]. The specimens are preserved as coalified compressions embedded in siltstone. The specimens were photographed using a Nikon D300S digital camera. Details of the specimens were examined using a Nikon SMZ1500 stereomicroscope equipped with a Digital Sight DS-Fi1 camera. Afterward a replica of nitro cellulose [16] was made on the specimen GP0094-A, and the replica was cleansed with HCl and HF, coated with gold, and observed using a MAIA 3 TESCAN SEM (scanning electron microscope) housed at the Nanjing Institute of Geology and Palaeontology (NIGPAS), Nanjing, China. One in situ seed in a fruit (lateral appendage) was dislocated and prepared for Micro-CT observation. Micro-CT was performed using a Zeiss Xradia 520 versa X-ray microscope at the Nanjing Institute of Geology and Palaeontology, Nanjing, China. The 3D reconstruction and virtual sections were generated using a VG Studio MAX 3.0. All photographs were saved in TIFF format and organized together for publication using Photoshop 7.0.
Results
Taiyuanostachya gen. nov
Diagnosis
Fertile shoot consisting of a peduncle and terminal reproductive organs. Peduncle slender, straight. Leaves on peduncle ensiform, spirally arranged. Reproductive organ cylindrical, single or in twins, including an axis and multiple lateral appendages. Lateral appendages in whorls, vertically attached to the organ axis, with up-turning distals parallel to organ axis, with or without filamentous tips. Ovules/seeds spheroidal, attached to the organ axis, enclosed in lateral appendages. Central canal in lateral appendages connecting ovule/seed with exterior, filled with spongy materials, with an opening on the surface of lateral appendages. Ovule orthotropous, unitegmic, with a micropyle. Seed with layered seed coat.
Remarks
The specimens studied here were previously described as Tingostachya tetralocularis (Konno) Gao and Thomas [10]. As stated in the paper [10], the specimens should be permanently deposited in the China Institute of Mining, Beijing, China. However, actually the specimens were left in UK after Gao finished his study in the University College, Cardiff, UK. Dr. Christopher Hill sent the specimens back to the Nanjing Institute of Geology and Palaeontology (NIGPAS) after he worked as an Einstein guest professor at NIGPAS in 2010. Now the specimens reported here (except one, B461), are deposited in NIGPAS. Konno’s diagnosis of Tingostachya was “Fertile shoot (in the geno-type, T. tetralocularis) consists of two parts: a long (?) slender axis and cylindrical terminal cones. Axis slender, forked dichotomously at apex, longitudinally ribbed with small uniform leafy sporophylls in four vertical series. Sporophylls uniform, more or less elongated, but only slightly modified from the foliage leaf in Tingia. A large tetralocular synangium, hemispherical, placed directly on the upper surface of each bract, apart from the cone axis, receiving one group of bundles from the axis which runs along the median zone of the bract” [10] and Konno’s spore was “often well preserved, usually of obovate contour, with the larger diameter from 150 μm to 130 μm” [10]. However, Gao and Thomas’ rediagnosis of the same genus was “Fertile shoot consisting of a peduncle, with spirally arranged leaves and a terminal cone once dichotomized at its base. Leaves on peduncle ensiform, spirally arranged. Cone axis slender. Sporophylls spirally arranged, pedicels arising at right angles, with upwardly extending lateral margins (alations). Laminae lanceolate, parallel to cone axis. Sporangia spheroidal, attached to proximal part of the adaxial surface of pedicel. Sporangia with small spores” [10].
|
Konno (1929) |
Gao and Thomas [10] |
Present study |
|
|
Spore |
150 μm to 130 μm in diameter |
20 μm (no figure) |
no |
|
Synangium |
On the upper surface of bract, away from cone axis |
Proximal adaxial surface of sporophyll pedicel, 1–1.5 mm |
no |
|
Tetralocular synangium |
yes |
no |
no |
|
Sporophyll arrangement |
in 4 vertical series |
spiral |
whorled |
|
Bract |
yes |
no |
no |
|
Scale |
absent |
no |
no |
|
Ovule |
no |
no |
yes |
|
Seed |
no |
no |
yes |
Table 1: Differences among the present and previous interpretations.
As seen in Table 1, Konno’s and Gao and Thomas’ interpretations of the assumed same taxon are quite different. This implies that they were facing quite different taxa of fossil plants. As Gao and Thomas [10] admitted, they had “a rather different morphological interpretation to that proposed by Konno (1929).” For example, their “sporophylls” were not in whorls of four, and their specimens had “certain undescribed features of sporophyll construction”. The assumed “adaxial surface” position of the spheroidal sporangia on the sporophyll pedicels could not be ascertained by Gao and Thomas’ figures, as they are actually enclosed in the lateral appendages and attached to the organ axis, as shown in our Figures 1d, 2a-d, 3h, S2a-b, d, S3f, and S4a-b as well as in Gao and Thomas’ Text-Figure 3b and Plate 90, Figure 6. These differences indicated that the specimens Gao and Thomas faced did not belong to Tingiostachya tetralocularis Konno (1929). In addition, although Gao and Thomas claimed “putative spores” in sporangia of their specimens, they failed to support their claim with any figure of spores [10]. The “tetralocular synangium” characteristic of Tingostachya tetralocularis Konno actually did not exist [10]. Gao and Thomas admitted that they did not know their fossils were a pteridophyte, a gymnosperm, or a progymnosperm. Considering the above unsupported claims by Gao and Thomas, we consider it decent to separate the specimens studied by Gao and Thomas [10] (also the ones re-studied here) from Tingostachya Konno 1929 and recognize a new taxon, Taiyuanostachya, based on the following new information. Although agreeing each other in calling the reproductive organs of Tingostachya as “cone”, the interpretations of Konno and Gao and Thomas [10] were not supported as there were no bracts and scales required for ovulate cones in conifers. Ovule with micropyle and seed with layered seed coat annihilate the presence of sporangia in Taiyuanostachya. Although megaspores may be similar to Taiyuanostachya seeds/ovules in size, megaspores fall off from the mother plants when mature and develop independently [17] and do not have sclerotesta-like wall while our seeds remain within the fruits permanently and have sclerotesta with sculpture (Figures 2a-f). If our so-called ovule/seed were actually sporangia (as previously interpreted), the integument and micropyle (Figures 3a-b) and layered seed coat (Figures 3c-f) would defy interpretations. On the contrary, these structures would be completely expected for seeds and ovules. For example, the layers of tissue shown in Figures. 3d-e are hard to find counterparts in any sporangium wall, while they are readily compared with sarcotesta and sclerotesta commonly seen in seed coat. The presence of spongy materials in the central canal in lateral appendages is newly recognized in Taiyuanostachya gen. nov (Figures 1e, 3i, S3f-g, S4a-b). However, this feature is not completely novel discovery, as in the documentation of Tingiostachya tetralocularis (Konno) Gao and Thomas, “a layer of light coloured sediment” was seen “between two layers of compression material (Pl. 90, Figure 5; text- Figure 3C-E) [10]. Our SEM revealed more details of such materials and have convinced us that it may be related to the pollination of Taiyuanostachya, as documented in some basal angiosperms [18]. Considering the above differences from original diagnosis of Tingostachya tetralocularis Konno, lack of spores in the specimens, occurrence of enclosed ovules/seeds, and central canal filled with spongy materials in the specimens, we prefer to separate these specimens from Tingostachya tetralocularis Konno and establish a taxon, although Gao and Thomas tried to designate one of their specimens (GP0094) as neotype of Tingostachya.
Etymology: Taiyuan- refers to the fossil locality, Taiyuan City, Shanxi, China; -stachya refers to the ear-like organ.
Type species: Taiyuanostachya ovuilifera gen. et sp. nov
Horizon: The Lower Shihhotse Formation, Permian.
Age: The Early Permian (>272 Ma ago).
Taiyuanostachya ovuilifera gen. et sp. nov
(Figures. 1-4, S1-S4)
Synonym:
Tingostachya tetralocularis (Konno) Gao and Thomas, Gao and Thomas 1987, page 818, Plate 89, Figure 10, Plate 90, Figures 1-7
Species diagnosis: The same as that of the genus.
Description: The specimens are coalified compressions embedded in yellowish or greenish siltstones (Figures 1a-d, S1a-c, S3a-d). The peduncle is up to 29 mm long and 2 mm in diameter (Figures 1b-c). The organs are cone-like structures, paired or single on a peduncle, cylindrical in form, curved or straight in form, 33 to 43 mm long, 10 to 11 mm in diameter, with 25 to 40 whorls of lateral appendages (Figures 1a-d, 2a, 5a-c, S1a-c, S3a-d). Numerous lateral appendages are whorled, attached to the organ axis vertically, with up-turning distal parts that may have filamentous tips (Figures 1a-f, 2a, 3h, 5a-c, S1a-c, S2a-c, S3a-d,f, S4a-b). Lateral appendages are 4-5 mm long, 1.2-1.7 mm thick, approximately 8-10 per whorl (Figures 1a-e, 2a, 5a-c, 3g-h, 4a, S1a-c, S2a-d). Lateral appendages in adjacent whorls alternate or opposite each other (Figures 1d, 2a, 3h, 4a, 5a-c, S1a-c, S2c, S3a-d,f). Inside each lateral appendage, there are a central canal running into the distal (Figures 1e-f, 5d-r, 3h-i, S4a-c, S3f-g, S4a-b) and an ovule directly attached to the axis (Figure 1a-d, 2a-d, 3g-h, 5c-d). The central canal is filled with spongy materials, has an opening in the outer surface (Figures. 1e-f, 3h-i, 4c-d, 5d-e, S3f-g, S4a-b). Filamentous tips on the up-turning parts range from 1 to 15 mm in length (Figure 1d, f, 5d-e, 3h, S3f). Ovules are round-triangular in form, 1.5-1.6 mm long and 1.2-1.3 mm in diameter, with a distal micropyle and one layer of integument approximately 0.2 mm thick, attached to the axis (Figures. 3a-b, 5f, S2a-b, S3e-f, S4a-b). A seed is invisible to naked eyes in an intact lateral appendage but visible to naked eyes when a lateral appendage is broken basally (Figures. 1d, 2a-d, 3a-h, S2a, b, d, S3f, S4a-b), discoid in form, 1.2-1.4 mm long, 0.9-1.4 mm wide, 0.4 mm thick, with a 0.1 mm–thick sarcotesta outside a 0.06- 0.14 mm–thick sclerotesta (Figures. 2a-f, 3c-h). Cells of the sclerotesta are rectangular-shaped, arranged in files, while those of the sarcotesta are more irregular and not arranged in regular files (Figure 3e).
Holotype: GP0094.
Further specimens: GP0093, GP0094-A, GP0095, B461.
Depository: GP0093, GP0094, GP0094-A, GP0095 are deposited in the Nanjing Institute of Geology and Palaeontology, Nanjing, China; B461 is deposited in the Beijing Museum of Natural History.
Remarks: The seed is in situ in the lateral appendage (Figures 2a-f), suggestive of a fruit nature of the lateral appendage. However, we prefer the term “lateral appendage” to “fruit” to remain neutral in our interpretation.
Etymology: ovuilifera refers to the ovules enclosed in the lateral appendages.
Locality: 5 km north-east of East Hill Mine, Taiyuan, Shanxi Province, northern China.
Horizon: the Lower Shihhotse Formation, Permian.
Age: the Early Permian (>272 Ma ago).

Figure 1: Taiyuanostachya ovuilifera gen. et sp. nov and the details of their lateral appendages. E-F, stereomicroscopy. A. Holotype of Taiyuanostachya gen. nov, on one of the two facing slabs. GP0094. Note another partially preserved organ (arrow). Scale bar = 1 cm. B. Twin organs on a single peduncle. GP0093. Scale bar = 1 cm. C. Whole organ with a long peduncle (arrow). GP0093. Scale bar = 1 cm. D. Partial organ. Note the filamentous tips (arrows) of lateral appendages. GP0095. Scale bar = 1 cm. E. Radial longitudinal section of a lateral appendage showing a central canal (arrows) running through the appendage. GP0094. Scale bar = 1 mm. F. Detailed view of the distal of a lateral appendage, showing the central canal (white arrows) and filamentous tip (black arrow). GP0094. Scale bar = 1 mm.
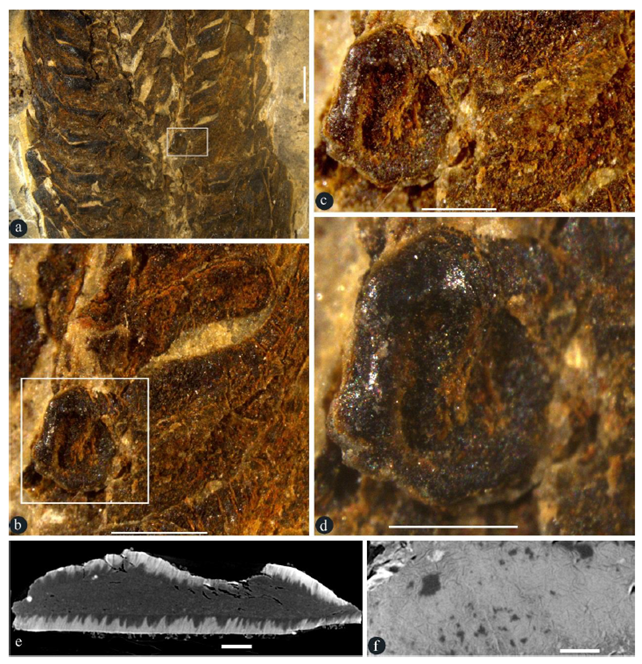
Figure 2: Details of an in-situ seed in a lateral appendage. A-D, stereomicroscopy; E-F, micro-CT virtual sections. GP0093. A. Partial view of the rectangle in Figure 1c. Scale bar = 2 mm. B. Detailed view of the rectangle in Figure 2a, showing a seed in base of a broken lateral appendage. Scale bar = 1 mm. C. Detailed view of the rectangle in Figure 2b, showing a basal in situ seed. Scale bar = 0.5 mm. D. Detailed view of the seed in Figure 2c, showing the shiny seed coat. Scale bar = 0.5 mm. E. Virtual section of the seed in Figure 2d, showing the seed coat (sclerotesta) surrounding the seed content. Scale bar = 0.1 mm. F. Surface view of the seed coat (sclerotesta) with sculpture. Scale bar = 0.1 mm.
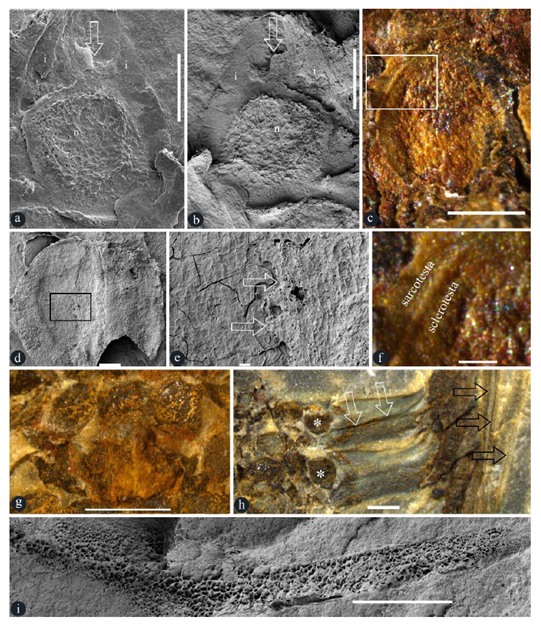
Figure 3: Details of in situ ovules, seeds, and others in Taiyuanostachya gen. nov. A, B, D, E and I are SEM, C, F-H are stereomicroscopy. A-F, I are of GP0094-A, G is of GP0094, H is of GP0095. A. An ovule with an upper-pointing micropyle (arrow), nucellus (n), and integument (i). Scale bar = 0.5 mm. B. An ovule with an upper-pointing micropyle (arrow), nucellus (n), and integument (i). Scale bar = 0.5 mm. C. A seed in situ in a lateral appendage. Scale bar = 0.5 mm. D. An in situ seed. Scale bar = 0.2 mm. E. Detailed view of the rectangle in Figure 3d, showing sarcotesta (arrow, right) over the sclerotesta (left). Scale bar = 20 μm. F. Detailed view of the rectangle in Figure 3c, showing sarcotesta outside the sclerotesta. Scale bar = 0.1 mm. G. Whorled arrangement of lateral appendage scars, suggestive of 8-10 lateral appendages (asterisks, on the same level) per cycle. Scale bar = 1 mm. H. Two lateral appendages and their in situ ovules (asterisks). Note the central canal (white arrows) and vertical filamentous tips (black arrows) of lateral appendages. Scale bar = 1 mm. I. Spongy filling in the central canal in a lateral appendage. Refer to Figure S4b. Scale bar = 1 mm.
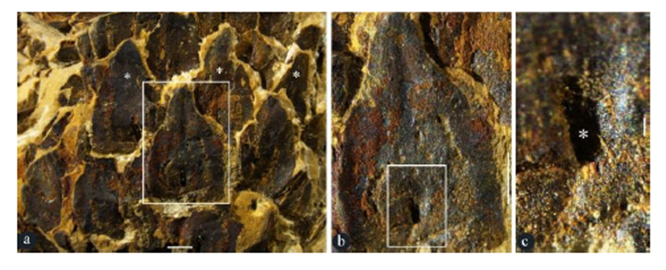
Figure 4: Surface views of lateral appendages of Taiyuanostachya gen. et sp. nov. Stereomicroscopy, GP0094. A. Alternate, whorled arrangement of lateral appendages (asterisks). Enlarged from Figure 1a. Scale bar = 1 mm. B. A lateral appendage in the rectangle in Figure 4a, showing surface view of the lateral appendages. Scale bar = 1 mm. C. Detailed view of rectangle in Figure 4b, showing the opening (asterisk) of the central canal. Scale bar = 0.1 mm.
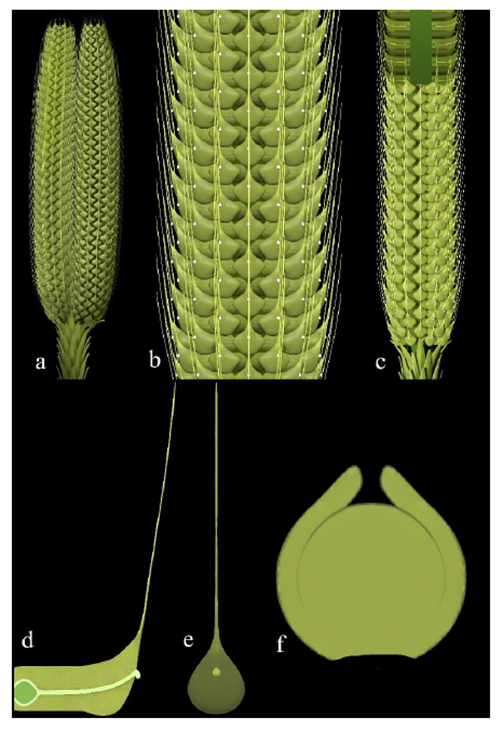
Figure 5: Reconstructions of Taiyuanostachya gen. et sp. nov. A. Twin organs. B. Detailed surface view of an organ. C. Partially dissected organ. D. A dissected lateral appendage, showing the sessile ovule, central canal connecting the ovule and pollination drop on the distal, and filamentous tip. E. A surface view of a lateral appendage, note the pollination drop and filamentous tip. F. A middle section of ovule.
Discussions
The core feature of angiosperms is “angiospermy”, which literally means enclosed seeds. However, as ODC (Offspring Development Conditioning) is a universal trend in all sexually reproduced organisms [19], it is not surprising that some conifers enclose and thus protect their seeds after pollination [9]. So enclosed seeds are not a patent of angiosperms any more [9]. Fortunately, enclosed ovules before pollination remains idiosyncratic of angiosperms [9]. This criterion is the foundation for our following reasoning and conclusion. The seed in Figures 2a-f was previously interpreted as sporangium by Gao and Thomas [10]. Since sporangia and ovule/seed may be of similar sizes, it is necessary to eliminate sporangium alternative before further discussion. Gao and Thomas [10] failed to support their interpretation with any figure of in situ spore or pollen. Our new observation indicates that there is a layered seed coat (including sarcotesta and sclerotesta) around the seed (Figures 3c-f), a structure never expected for any sporangium. This is consistent with the lack of spore/pollen in Gao and Thomas [10]. The position of “sporangia” in lateral appendages of Taiyuanostachya is disadvantageous for spore dispersal (important for the continuation of the phylogeny), therefore the internal position of Taiyuanostachya seed/ovule (Figures 2a-f) makes it unlikely to be a sporangium. The occurrence of sclerotesta of dense materials surrounding its content (Figures 2a-f) extinguishes its possibility of being a sporangium. This conclusion is further strengthened by the occurrence of micropyle and integument in the ovules of Taiyuanostachya (Figures 3a-b), both of which have never been seen in any sporangium but are characteristic of ovules. Finally, the spongy materials filling the central canal (Figures 1e-f, 3i, S3f-g, S4a-b) conjures to secretion secluding ovary in extant basal angiosperms [18]. Such an observation suggests that the ovules in Taiyuanostachya, although not physically secluded (due to the presence of a central canal), are isolated from the exterior space by the spongy materials, which may guide pollen tubes to micropyles during pollination. All these evidence converge to a conclusion that what seen in the lateral appendages of Taiyuanostachya is either an ovule or a seed. As it is shown in Figures 2a-f and 3c-f, the seeds are clearly enclosed in the lateral appendages of Taiyuanostachya, suggesting “angiospermy” in Taiyuanostachya. Since micropyle and integument are features characteristic of ovules, their occurrence in ovules enclosed in the lateral appendages of Taiyuanostachya (Figures 3a-b) indicates the occurrence of “angio-ovuly” in Taiyuanostachya. The occurrence of both “angiospermy” and “angio-ovuly” in Taiyuanostachya unequivocally pins down its angiospermous affinity. The Permian age, organ morphology, and organization of Taiyuanostachya all fall out of the expectations of existing angiosperm evolution theories. Such a discrepancy between theories and fossil facts prompts botanists to modify the existing botanical theories. Taiyuanostachya are coeval with Primocycas chinensis (B461 [11], GP0001, GP0027 [10]), the earliest confirmed fossil record of Cycads. All these plants belong the well-known Cathaysian Flora of the Lower Permian, which usually is characterized by the presences assemblage of Alethopteris-Emplectopteris-Tingia-Cathaysiopteris [15]. Tingiostachya and Tingia are frequently seen elements in the Cathaysian flora widely spread in Asia in the Early Permian [15]. It appears that angiosperms co-evolved and co-developed with cycads throughout their histories since the late Palaeozoic, although, as Stebbins [1] stated, angiosperms later “entered a dark tunnel of ignorance”. We wish to remind that not all specimens of Tingiostachya are Taiyuanostachya gen. nov and thus belong to angiosperms, but these taxa deserve further scrutinizing before any final conclusion is given. As an angiosperm commonly seen in the Permian, Taiyuanostachya represents the currently earliest record of angiosperms. The impact of this conclusion on the current plant systematics cannot be overstated. The current lack of knowledge of the whole plant of Taiyuanostachya calls for more efforts from palaeobotanists.
Conclusion
Taiyuanostachya gen. nov belongs to angiosperms as it demonstrates both enclosed seeds and enclosed ovules, the combination of which is a characteristic restricted to angiosperms hitherto. This discovery is abominable for botanists who believe, although groundlessly, that angiosperms do not exist before the Cretaceous. The Permian age of Taiyuanostachya gen. nov undermines the validity of many currently dominating botanical theories. It is apparent that botany as a science of plants should be updated and modified accordingly.
Acknowledgements
We thank Dr. Christopher Hill for his help with access to these valuable specimens, Ms. Yuyan Miao for her help accessing specimen B461 deposited in Beijing Museum of Natural History, Mr. Yemao Hou, Ms. Pengfei Yin, Ms. Suping Wu for their help with Micro-CT, Mr. Yan Fang for his help with photography using MAIA3 TESCAN SEM, and Mr. Dinghua Yang for his help with reconstructions. This research is supported by the National Natural Science Foundation of China (42288201, 41688103, 91514302) and the Strategic Priority Research Program (B) of Chinese Academy of Sciences (XDB26000000).
Author Contributions
X.W. designed the research plan. Q.F. and X. W. performed analysis, wrote the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Disclosure statement
This manuscript is a corrected version of the article posted on Research Square, https://www.researchsquare.com/article/rs-1768512/v1.
References
- Stebbins GL. Seeds, seedlings, and the origin of angiosperms. In: Beck C.B. (Ed.), Origin and early evolution of angiosperms. New York: Columbia University Press (1976): 300-311.
- Scott RA, ES Barghoorn, EB Leopold. How old are the angiosperms? American Journal of Science (1960): 258-A: 284-299.
- Herendeen PS, EM Friis, KR Pedersen, et al. Palaeobotanical redux: revisiting the age of the angiosperms. Nature Plants 3 (2017): 17015.
- Beck CB. Origin and early evolution of angiosperms: a perspective. In: Beck C.B. (Ed.), Origin and early evolution of angiosperms. New York: Columbia University Press (1976): 1-10.
- Wang X. Criterion is a touchstone in study of early angiosperms. Open Journal of Plant Science 6 (2021): 91-93.
- Friis EM, PR Crane, KR Pedersen. Hedyosmum-like fossils in the Early Cretaceous diversification of angiosperms. International Journal of Plant Sciences 180 (2019): 232-239.
- Wang X. Groundless research published on the International Journal of Plant Sciences. Voice of the Publisher 6 (2020): 167-169.
- Sun G, DL Dilcher, S Zheng, et al. In search of the first flower: a Jurassic angiosperm, Archaefructus, from Northeast China. Science 282 (1998): 1692-1695.
- Tomlinson PB, T Seed cone structure in conifers in relation to development and pollination: a biological approach. Canadian Journal of Botany 80 (2002): 1250-1273.
- Gao Z, BA Thomas. A re-evaluation of the plants Tingia and Tingiostachya from the Permian of Taiyuan, China. Palaeontology 30 (1987): 815-828.
- Zhu JN, XM Du. A new cycad -- Primocycas chinensis et sp. nov. discovered from the Lower Permian in Shanxi, China and its significance. Acta Botanica Sinica 23 (1981): 401-404.
- Gao Z, BA Thomas. A review of cycad megasporophylls with new evidence of Crossozamia Pomel and its associated leaves from the Lower Permian of Taiyuan, China. Review of Palaeobotany and Palynology 60 (1989): 205-223.
- Gao Z, BA Thomas. An enigmatic cone from the Lower Permian of Taiyuan, Review of Palaeobotany and Palynology 68 (1991): 197-201.
- Gao Z, BA Thomas. A new species of Discinites from the Lower Permian of China. Review of Palaeobotany and Palynology 81 (1994): 185-192.
- Li X, Z Yao. Carboniferous and Permian floral provinces in East Asia. In: Thomas D.H.W. Pfefferkorn (Eds.), Papers for the 9th International Congress of Carboniferous Stratigraphy and Geology. Urbana: South Illinois University Press (1985): 95-101.
- Zhu W. The use of the peeling method in palaeobotanical studies. Chinese Bulletin of Botany 1 (1983): 51-53.
- Herr JMJ. The origin of the ovule. American Journal of Botany 82 (1995): 547-564.
- Endress PK, A Igersheim. Gynoecium structure and evolution in basal angiosperms. International Journal of Plant Sciences 161 (2000): S211-S223.
- Fu Q, J Liu, X Wang. Offspring development conditioning (ODC): A universal evolutionary trend in sexual reproduction of organisms. Journal of Northwest University (Natural Science Edition) 51 (2021): 163-172.
