Synergistic Growth Inhibitory Effects of Lycium barbarum (Goji berry) Extract with Doxorubicin against Human Breast Cancer Cells
Article Information
Kaloyan D Georgiev1*, Iliya J Slavov2, Ivan A Iliev3
1Department of Pharmaceutical Technologies, Faculty of Pharmacy, Prof. Paraskev Stoianov Medical University, Varna, Bulgaria
2Department of Biology, Faculty of Pharmacy, Prof. Paraskev Stoianov Medical University, Varna, Bulgaria
3Institute of Experimental Morphology, Pathology and Anthropology with Museum, Bulgarian Academy of Sciences, Sofia, Bulgaria
*Corresponding Author: Kaloyan D Georgiev, Department of Pharmaceutical Technologies, Faculty of Pharmacy, Prof. Paraskev Stoianov Medical University, Varna, Bulgaria
Received: 23 June 2019; Accepted: 12 July 2019; Published: 11 September 2019
Citation: Kaloyan D Georgiev, Iliya J Slavov, Ivan A Iliev. Synergistic Growth Inhibitory Effects of Lycium barbarum (Goji berry) Extract with Doxorubicin against Human Breast Cancer Cells. J Pharm Pharmacol Res 3 (2019): 051-058.
Share at FacebookAbstract
Lycium barbarum L. known as Goji berry is a plant with wide variety of medicinal properties, including anticancer. The present study aimed to explore the cytotoxic effect of Lycium barbarum fruit extract (LBE), and its combination with doxorubicin on MCF-7 and MDA-MB-231 breast cancer cells. The result of MTT assay showed that the single treatment of doxorubicin performed significant cytotoxic effect on MCF-7 and MDA-MB-231 cell lines. The combination of LBE and doxorubicin on MCF-7 and MDA-MB-231 cells showed synergistic cytotoxic effect based on the fractional effect analysis (FA) and the calculated combination index (CI). Increasing the anticancer effects on the one hand and preventing the dose-limiting effect, like cardiotoxicity on the other hand, of the anthracycline antibiotics indicate L. barbarum fruits as chemosensitizing and chemoprotective at the same time.
Keywords
L.barbarum, Goji berry, Breast cancer, Pharmacodynamics drug interactions, Combination therapy
L.barbarum articles, Goji berry articles, Breast cancer articles, Pharmacodynamics drug interactions articles, Combination therapy articles
Article Details
Abbreviations:
LBE-L.barbarum extract; CI-combination index; FA-fractional effect analysis1. Introduction
Lycium barbarum L. (Solanaceae), known as Goji berry, is widely used as traditional medicinal plant or food supplement in China for many years. The part of the plant that is mainly used for medical purposes is the fruit. Constituents of the L. barbarum fruit include basically polysaccharides and proteoglycans (23% of dried mass), carotenoids (mainly zeaxanthin dipalmitate), vitamins (riboflavin, thiamin and ascorbic acid), fatty acids, free amino acids, flavonoids, phenolic acids and anthocyanins [1, 2]. The multiple potential health benefit effects of the consumption of L. barbarum fruits are described in the literature, the main ones are antioxidant and anticancer activities [3-6]. Doxorubicin is an anthracycline antibiotic widely used in the treatment of oncological diseases, including breast cancer. The main mechanism of action is described by intercalation in the structure of DNA and RNA, which leads to suppression of synthesis. Subsequently, chain breaks occur through inhibition of topoisomerase II and free radical formation [7]. Cardiotoxicity is a dose-limiting adverse effect of cancer treatment with doxorubicin. It can hinder the treatment and even lead to a fatal outcome [8]. The primary mechanism suggested to cause cardiotoxicity is increased oxidative stress [9]. The main purpose of this article is to evaluate the combined effects of doxorubicin with L. barbarum fruit extract (LBE) on two breast cancer cell lines.
2. Materials and Methods
2.1 Reagentsbarbarum fruits (Lot ?: L05042017) were provided by Paula Fruits Ltd-an official importer of Goji berries for Bulgaria with guaranteed Chinese origin. Preparation of total L. barbarum extract: 200 g of dried Goji berries were soaked in cold distilled water for 30 minutes and then homogenized in a laboratory blender. The obtained mixture was extracted for 1 hour at 60°C and shaking on thermostatic water bath (NUVE, Turkey). After that, extract was centrifuged (6000 g) and supernatant was denoted as L. barbarum total extract (LBE). Doxorubicin and all other used chemicals were of analytical grade and were purchased from the local representatives of Merck (Darmstadt, Germany) and Sigma (St. Louis, USA), unless otherwise indicated.
2.2 Cell cultures
The breast cancer cell lines MCF-7 (estrogen, progesterone receptors +, HER2-), and MDA-MB-231 (triple negative, ER-, PR-,HER-) cells were cultured in Dulbecco Modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (Gibco, Austria), 100 U/ml penicillin and 0.1 mg/ml streptomycin (Lonza, Belgium) under 5% CO2 and 95% air atmosphere at 37°C. Plastic flasks 25 cm2 supplied by Greiner, Germany, were used to grow the cells. For experiments the cells in exponential phase of growth after treatment with trypsin-EDTA (FlowLab, Australia) were seeded into 96-well plates (100 μl/well) at a density 1x104 cells/ml.
2.3 Cell viability assay
Cell viability was evaluated in MCF-7 and MDA-MB-231 cells by MTT dye reduction assay according to the standard protocol [10], with some modifications. MCF-7 and MDA-MB-231 cells were seeded into 96-well plates in exponentially growing manner. The cells were incubated with the used compounds, and cell viability was determined 72 h later. The treatment medium was replaced with DMEM containing 0.5 mg/mL MTT and the cells were incubated for three hours. The MTT solution was removed from the plates and the formazan crystals were dissolved in DMSO. The optical density of the samples was measured by a microplate reader (TECAN, Sunrise TM, Groedig/Salzburg, Austria) at 540 nm. Cell viability was expressed as per cent of untreated control.
2.4 Analysis of drug combinations
We used two methods for determining the pharmacodynamics drug interactions. The first of them is the most widely used and is based on the multiple drug effect method of Chou–Talalay [11], represented as the following equation:
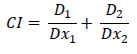
Where D1 and D2 are the doses of the two drugs in combination, that inhibit the proliferation of the cells by x% and Dx1 and Dx2 are the doses of the drugs alone, that are expected to be necessary to achieve the experimentally measured response by x%. In our study, the CI values were determined for each combination of LBE and doxorubicin using CalcuSyn® (Biosoft, Cambridge, UK) [12]. A CI value less than 0.9 indicates synergism, CI equal to 0.9-1.10 indicates additive interaction, and CI greater than 1.10 indicates antagonism. The other used method is fractional effect analysis (FA) [13] or Bliss independence [14]. The effect in this method is considered synergistic when the observed effect is greater than the product of the effects of each individual agent. The equation used for calculating the combining effect (CE) is:
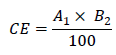
where A1 and B2 are percentage of inhibition with single agents, expressed as percentage of the untreated control. For each concentration we calculated the theoretical values, which we further compared with those we received actually: for CEmeasured = CEcalculated we conceded the effect as additive; for CEmeasured < CEcalculated as synergistic; and for CEmeasured > CEcalculated as antagonistic.
2.5 Statistical analysis
The statistical evaluation was performed using Graph Pad Prism 8.01 software, and significant differences between groups were analyzed using ANOVA. All results are expressed as arithmetic means ± standard deviation (SD) of the means of three separate experiments (each experiment was done with three parallels). A difference at P < 0.05 was considered statistically significant.
3. Results
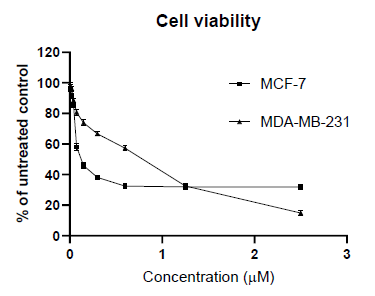
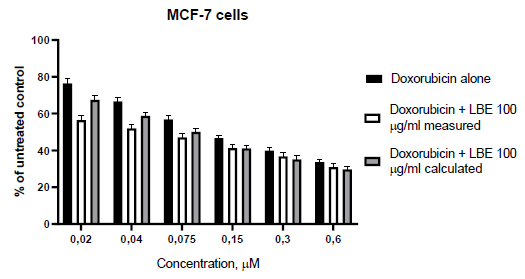
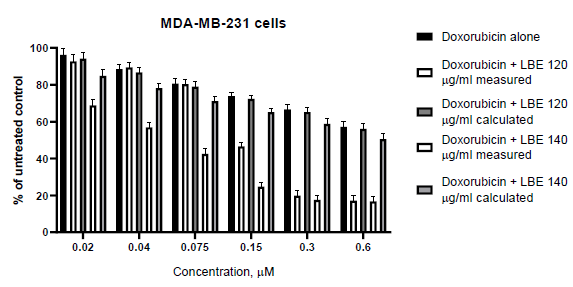
In the first set of our work, we defined the sensitivity of the used cell lines to doxorubicin. The both cell lines showed dose-dependent decreases in cell viability compared to the control (untreated cells). The IC50 value against MCF-7 cells were 0.07 µM, while against MDA-MB-231 were 0.79 µM. The obtained dose-response curves are given on Figure 1. As can be seen on the figure 1, MDA-MB-231 (triple negative) cells are significantly more resistant to the action of doxorubicin. To determine whether L. barbarum extract (LBE) could sensitizes MCF-7 and MDA-MB-231 to treatment with doxorubicin, we cultured cells with doxorubicin at concentration range of 0.02 to 0.6 μM, alone or in combination with fixed 100, 120 or 140 µg/ml LBE concentrations, for 72 hours. The cell proliferation was assessed using MTT assay. The results showed that cell viability was much lower with the combination treatment than with doxorubicin alone. The calculated and measured combination effects, using the Bliss method, are presented in Figure 2 and 3.
Using the same results and the CalcuSyn® software, we calculated combination index. The results are presented in Table 1 and Table 2.
|
Doxorubicin concentration (μM) |
LBE concentration (μg/ml) |
CI |
|
0.02 |
100.0 |
0.75862 |
|
0.04 |
100.0 |
0.83698 |
|
0.075 |
100.0 |
0.89938 |
|
0.15 |
100.0 |
0.97530 |
|
0.3 |
100.0 |
1.19463 |
|
0.6 |
100.0 |
1.32293 |
Table 1: Calculated combination index (CI) after treatment of MCF-7 cells with combination doxorubicin (0,02-0,6 µM) + LBE (100 µg/ml).
|
Doxorubicin concentration (μM) |
LBE concentration (μg/ml) |
CI |
LBE concentration (μg/ml) |
CI |
|
0.02 |
120.0 |
1.51415 |
140.0 |
0.92343 |
|
0.04 |
120.0 |
1.66865 |
140.0 |
0.89044 |
|
0.075 |
120.0 |
1.42208 |
140.0 |
0.82990 |
|
0.15 |
120.0 |
0.84412 |
140.0 |
0.74387 |
|
0.3 |
120.0 |
0.64732 |
140.0 |
0.72160 |
|
0.6 |
120.0 |
0.69030 |
140.0 |
0.76843 |
Table 2: Calculated combination index (CI) after treatment of MDA-MB-231 cells with combination doxorubicin (0.02-0.6 µM) + LBE (120 and 140 µg/ml).
4. Discussion
?he search for effective drug combinations and methods for their evaluation is the basis of in vitro pharmacodynamic drug interactions. Therefore, the aim of the present study was to evaluate in vitro combinations, which could be tested subsequently in in vivo conditions or in following clinical studies and to evaluate the most used methods for them. The most conventional chemotherapeutic agents, anthracyclines and taxanes are most commonly used in therapeutic protocols in breast cancer therapy. Doxorubicin, an antibiotic from anthracycline group, is one of the most effective antineoplastic drugs in the treatment of solid tumors (including breast cancer), but with many dose-limiting toxicities, such as bone marrow damage, cardiotoxicity etc. Therefore, we are looking for ways to preserve its high efficacy, or even in some cases to increase it, without increasing the possibility of adverse effects. The L. barbarum extract has been shown in numerous studies to have antitumor activity, expressed selectivity on tumor cells, organ protective effects (e.g. cardioprotective) and is suitable for combination with the chemotherapy [15-17].
In our previous study, we have proven that L. barbarum polyphenolic fraction has considerable antioxidant effects and this correlates with its cytotoxicity against breast cancer cell lines [4]. Also we have shown that polysaccharide fraction contributes to the antiproliferative effects of L. barbarum. Therefore, for the present study we used total water extract of L. barbarum fruits, which contains both polyphenols and polysaccharides. As could be seen from figure 1, MCF-7 cells show 10-fold more sensitivity to doxorubicin in comparison to MDA-MB-231 cells. When using the combination of doxorubicin (0.02-0.6 µM) with fixed 100 µg/ml LBE we observed synergistic effects at low concentrations (0.02-0.075 µM) and additive effects with the increase of doxorubicin concentrations. The CalcuSyn® software calculated combinational index (CI) values showed results that overlap significantly with fractional analysis (table 1). Only one difference is detected, the highest concentration of doxorubicin (0.6 µM) with LBE 100 µg/ml is assessed as antagonistic rather than additive.
Further, it should also be noted that the value of IC50 in the single use of doxorubicin (0.07 µM) and in the combination is not altered (0.069 µM). Triple-negative breast cancer cells, as MDA-MB-231, are a subject of intensive research as this type of cancer is one of the most aggressive of all breast cancers. In contrast to the previous set, where the doxorubicin concentrations used was in IC50 range; in the case with MDA-MB-231 cells they were below it. In the low used concentrations, doxorubicin (0.02-0.075 µM) and 120 µg/ml LBE showed rather antagonistic than additive effects, while at high concentrations (0.15-0.6 µ?), the observed combination responses were strong synergistic effects (Figure 3). The calculated combination index shows the same results-antagonistic in low concentrations and synergic effects in high concentrations (Table 2). We increased the concentration of LBE to 140 µg/ml, in order to see, if there is a change. The both methods used to assess the combination effects undoubtedly showed synergistic effects in all of the doxorubicin concentrations used.
Moreover, the addition of 120 or 140 µg/ml LBE resulted in a tenfold decrease in the IC50 values of doxorubicin on the MDA-MB-231 cell line-0.09369 µM and 0.07231 µM respectively. Similar synergistic effects of conventional chemotherapeutic agents, such as doxorubicin, with natural products are reported by other authors [18, 19]. In addition, our previous studies conducted in rats, have shown that L. barbarum isolated fractions are able to reduce doxorubicin-induced cardiotoxicity [20]. Biochemical cardiac markers of cardiotoxicity and histopathology have showed significantly less damage in the groups pretreated with L. barbarum isolated fractions, which have been supported by other publications [21, 22]. The both methods-Chou-Talalay Combination Index method (CTCI) and Fractional effect analysis (FA), are widely used to characterize pharmacodynamic drug interactions under in vitro conditions [23, 24]. In our case, the results obtained on both methods showed good correlation (r=0.764559) and no statistical difference between them (p=0.637). Therefore both methods could be used to evaluate pharmacodynamic interactions.
5. ConclusionLycium barbarum fruit (Goji berry) extract is a very good partner in the combination treatment with anthracycline antibiotics (e.g. doxorubicin). On the one hand, it is able to increase the antitumor activity (the therapeutic effect) and, on the other hand, to reduce the risk of dose-dependent cardiotoxicity (an adverse effect) in anthracycline therapeutic regimen against breast cancer.
Acknowledgements
Declared none.
Conflict of Interest
The authors declare no conflict of interest.
References
- Potterat O Goji. (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med 76 (2010): 7-19.
- Georgiev K, Jelev I, Georgieva S. Investigation of active ingredients of Goji berry (Lycium barbarum).Varna Medical Forum 3 (2013): 229-233.
- Hou YM, Wang J, Zhang XZ. Lycium barbarum polysaccharide exhibits cardioprotection in an experimental model of ischemia-reperfusion damage. Mol Med Rep 15 (2017): 2653-2658.
- Georgiev KD, Slavov IJ, Iliev IA. Antioxidant activity and antiproliferative effects of Lycium barbarum’s (Goji berry) fractions on breast cancer cell lines. Folia Med (Plovdiv) 61 (2019): 96-103.
- Gao Y, Wei Y, Wang Y, Gao F, Chen Z. Lycium Barbarum: A Traditional Chinese Herb and A Promising Anti-Aging Agent. Aging and disease 8 (2017): 778-791.
- Cheng J, Zhou ZW, Sheng HP, He LJ, Fan XW, He ZX, et al. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des Devel Ther 9 (2014): 33-78.
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56 (2004): 185-229.
- Dos Santos DS, dos Santos Goldenberg RC. Doxorubicin-Induced Cardiotoxicity: From Mechanisms to Development of Efficient Therapy, Cardiotoxicity, Wenyong Tan, IntechOpen (2018).
- Simunek T, Sterba M, Popelova O, Adamcova M, Hrdina R, Gersl V. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep 61 (2009): 154-171.
- Mossman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meths 65 (19836): 55-63.
- Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70 (2010): 440-446.
- Chou TC, Hayball MP. CalcuSyn for Windows: multiple-drug dose-effect analyzer and manual. Cambridge (UK): Biosoft (1997).
- Bliss CI. The toxicity of poisons applied jointly. Ann Appl Biol 26 (1939): 585-615.
- Webb JL. Enzyme and Metabolic Inhibitors. New York: Academic Press (1963): 55-79.
- Xin YF, Zhou GL, Deng ZY, Chen YX, Wu YG, Xu PS, et al. Protective effect of Lycium barbarum on doxorubicin-induced cardiotoxicity. Phytother Res 21 (2007): 1020-1024.
- Wawruszak A, Czerwonka A, Ok?a K, Rzeski W. Anticancer effect of ethanol Lycium barbarum (Goji berry) extract on human breast cancer T47D cell line. Nat Prod Res 30 (2016): 1993-1996.
- Deng X, Luo S, Luo X, Hu M, Ma F, Wang Y, et al. Fraction From Lycium barbarum Polysaccharides Reduces Immunotoxicity and Enhances Antitumor Activity of Doxorubicin in Mice. Integr Cancer Ther 17 (2018): 860-866.
- Wakharde AA, Awad AH, Bhagat A, Karuppayil SM. Synergistic Activation of Doxorubicin against Cancer: A Review. Am J Clin Microbiol Antimicrob 1 (2018): 1009.
- Guestini F, McNamara KM, Sasano H. The use of chemosensitizers to enhance the response to conventional therapy in triple-negative breast cancer patients. Breast Cancer Manag 6 (2017): 127-131.
- Radeva-Ilieva M, Zhelev I, Georgiev K. Cardioprotective effect of Lycium barbarum isolated fractions in doxorubicin-induced cardiotoxicity. Scripta Scientifica Pharmaceutica 5 (2018): 52.
- Xin YF, Zhou GL, Deng ZY, Chen YX, Wu YG, Xu PS, et al. Protective effect of Lycium barbarum on doxorubicin-induced cardiotoxicity. Phytother Res 21 (2007): 1020-1024.
- Xin YF, Wan LL, Peng JL, Guo C. Alleviation of the acute doxorubicin-induced cardiotoxicity by Lycium barbarum polysaccharides through the suppression of oxidative stress. Food Chem Toxicol 49 (2011): 259-264.
- Elwakeela A, Soudan H, Eldoksh A, Shalaby M, Eldemellawy M, Ghareeb D, et al. Implementation of the Chou-Talalay method for studying the in vitro pharmacodynamic interactions of binary and ternary drug combinations on MDA-MB-231 triple negative breast cancer cells. Synergy 8 (2019): 100047.
- Roell KR, Reif DM, Motsinger-Reif AA. An Introduction to Terminology and Methodology of Chemical Synergy-Perspectives from Across Disciplines. Front Pharmacol 8 (2017): 158.