Soluble Fiber and Omega-3 Fatty Acids Reduce Levels of Advanced Glycation End Products and Uremic Toxins in Senior Dogs by Modulating the Gut Microbiome
Article Information
Eden Ephraim1*, Matthew I Jackson1, Maha Yerramilli2, Dennis E Jewell3
1Hill’s Pet Nutrition, Topeka, Kansas, United States of America
2IDEXX Laboratories Inc., Westbrook, Maine, United States of America
3Kansas State University, Manhattan, Kansas, United States of America
*Corresponding Author: Eden Ephraim, 1035 NE 43rd St. Topeka, Kansas, 66617, USA
Received: 18 January 2020; Accepted: 07 February 2020; Published: 14 February 2020
Citation:
Eden Ephraim, Matthew I Jackson, Maha Yerramilli, Dennis E Jewell. Soluble Fiber and Omega-3 Fatty Acids Reduce Levels of Advanced Glycation End Products and Uremic Toxins in Senior Dogs by Modulating the Gut Microbiome. Journal of Food Science and Nutrition Research 3 (2020): 018-033.
Share at FacebookAbstract
Aging is associated with changes in the gut microbiome composition and levels of circulating metabolites. This study evaluates the effect of increased soluble fiber and omega-3 fatty acids on the gut microbial composition and levels of circulating and fecal metabolites in senior dogs, older than 7 years. Thirty six dogs between 8 and 13 years of age were maintained on a control food (control 1) containing 0.5% soluble fiber and 0.4 % omega-3 fatty acids, dry matter basis (DM). After 30 days, dogs were divided into two groups of 18 dogs. One of the groups received a test food containing increased soluble fiber (2.7%) and omega 3 fatty acids (0.92%) for 30 days while the other group received a control food (Control 2), containing 1.8% soluble fiber and 0.43% omega-3 fatty acids. After a washout period on the control 1 food for 30 days, a cross-over was performed to feed the test or the control 2 food for 30 days. Samples from feces and blood were collected after each 30 days period to analyze changes in gut microbial composition and metabolites. The consumption of the test food led to increased proportions of the bacterial genera Phascolarcobacteria, Faecalibacterium and Ruminococcus, and families Christensenellaceae and Ruminococcaceae. Dogs had lower abundance of Megamonas, Salmonella, Fusobacterium and Enterobacteriaceae after eating the test food. Pets had higher levels of glycerol and fatty acids and lower levels of pyrraline and mucin amino acids in feces. The test food reduced circulating levels of pyrraline, symmetric dimethylarginine and phenolic uremic toxins, including the microbial, 4-ethylphenyl sulfate, which as linked to brain damage. Christensenellaceae abundance was strongly associated with the observed health benefits. The study shows omega-3 fatty acids and soluble fiber enhance health in senior dogs by modulating the gut microbiome and metabolites associated with in aging.
Keywords
Soluble fiber, Omega-3 fatty acids, Dogs, Microbiome, Plasma, Feces, Metabolomics, Aging
Article Details
1. Introduction
Aging is associated with shifts in the composition of gut microbiota. An example of this is the increase in the number of facultative anaerobes and a decline in the proportion of beneficial bacteria associated with aging [1, 2]. This shift in the microbial composition leads to the accumulation of toxic microbial metabolites in the body causing inflammation, oxidative stress and contributing to various diseases prominent in the aging condition [3]. The reduction in the proportion of beneficial bacteria may lead to constipation, mal-absorption and longer colonic transit time. Decreased absorption of dietary protein in the upper intestine and longer colonic transit times encourage increased abundance of proteolytic bacteria, whose fermentation products deteriorate intestinal barrier integrity [4]. Foods containing fermentable fibers are known to benefit dogs by increasing nutrient absorption and reducing enteric infection [5]. In an in vitro study, Swanson et al. [6] confirmed the fermentability of fruits and vegetables by canine fecal microflora with the resulting production of short chain fatty acids. This study evaluates the effect of a test food containing components of citrus, carrot, spinach and tomato on the microbial composition as well as metabolites associated with aging, kidney, brain and gut health in senior dogs. Hall and Jewell [7] showed that fish oil, medium-triglycerides and L-carnitine improve age-related circulating metabolites in dogs. This study evaluates the effect of increased soluble fiber and omega 3 fatty acids on the gut microbial composition and age-related circulating and fecal metabolites in healthy senior dogs.
2. Materials and Methods
2.1 Dogs
All study protocols were reviewed and approved by the Institutional Animal Care and Use Committee, Hill’s Pet Nutrition, Inc., Topeka, KS, USA. Criteria for inclusion were healthy dogs above the age of 7 years. Dogs having chronic disease conditions such as inflammatory bowel disease, dermatitis, food allergy, cancer, tumor, kidney disease, liver disease and chronic urinary tract infections were excluded from the study. A total of 36 dogs between the ages of 8 and 13 years were grouped into a two groups of 18 each. Each group contained equal number of female and male dogs. All dogs were spayed or neutered. A summary of the description of the dogs included in this study is shown in Table 1.
2.2 Foods
The study used a test food and two control foods; all in dry form. All foods were produced by Hill’s Pet Nutrition, Inc. Topeka, KS and were essentially isocaloric with respect to metabolizable energy (control 1 = 3674 kcal/kg; control 2 = 3666 kcal/kg; test = 3684 kcal/kg). The foods were formulated to meet similar protein and carbohydrate profiles (Table 2) and contained grain sources such as rice, millet, oat groats, corn, wheat and/or barley. The test food had the highest soluble fiber level (2.7%) and contained added fiber sources from citrus, carrot, tomato and spinach in addition to the multiple grains. Unlike the test food, the first control (control 1) and the second control (control 2) foods did not have the unique fiber sources from fruit and vegetables. The first control food (control 1) had only 0.5% soluble fiber and used corn as major source of grain fiber and did not have multiple grain sources as the test or the control 2 food. The test food had increased omega 3 fatty acids (0.92%) compared to both Control 1 (0.4%) and Control 2 (0.43%) foods. The composition of the foods expressed as percentage of food as fed is shown in Table 2. Food analytical measurements were determined by Eurofins Scientific Inc. (Des Moines, IA) using Association of Analytical Communities (AOAC) methods.
2.3 Study design and sample collections
All dogs were maintained on control 1 food for 30 days and were divided into two groups. At the beginning of the test food feeding period, one of the groups received the test food while the other group received control 2 food for 30 days. Both groups were then fed the control 1 food for the next 30 days after which a cross-over was performed so that the test or the control 2 food were fed for 30 days to dogs which did not eat them during the first assignment to test foods. Water was available ad libitum. All dogs were meal fed from electronic feeders, where fresh food was offered daily with amounts calculated to maintain body weight. Exposure to food was allowed for up to 30 minutes to complete diet consumption. Daily food intake (g/d) was recorded for each dog. Body weights were measured weekly. Blood and fecal samples were collected at the end of each 30 days period to compare the effect of food on the abundance of various bacterial genera and various metabolites (Table 3).
|
Species |
Dogs |
|
Age |
Group 1: 10.6 ± 1.3; Group 2: 10.2 ± 1.1 |
|
Sex |
Control: 9M, 9F, Test: 9M, 9F |
|
Breed |
Beagles |
|
Initial body weight |
Control: 11.2 ± 2.1 Kg, Test: 11.5 ± 1.8 Kg |
|
Reproductive status |
All dogs were spayed or neutered |
|
Health status |
Healthy |
Table 1: Description of dogs used in the study.
|
Nutrient |
Control 1 |
Control 2 |
Test Food |
|
Moisture |
7.6 |
8.91 |
9 |
|
Ash |
4.52 |
4.8 |
4.41 |
|
Crude Fiber |
1.2 |
2 |
2.5 |
|
Crude Protein |
19.6 |
17.77 |
19.89 |
|
Carbohydrates* |
54.11 |
53.64 |
48.92 |
|
Soluble Fiber |
0.5 |
1.8 |
2.7 |
|
Insoluble Fiber |
6.6 |
7.2 |
5.8 |
|
Crude Fat |
12.67 |
12.98 |
15.18 |
|
C18:2 Omega 6 (Linoleic) |
3.43 |
3.29 |
3.32 |
|
C18:3 omega 3 (alpha-Linolenic) |
0.36 |
0.41 |
0.75 |
|
C20:4 Omega 6 |
0.05 |
0.05 |
0.09 |
|
C20:5 EPA Omega 3 |
0.01 |
0.01 |
0.08 |
|
C22:6 DHA Omega 3 |
0.01 |
0.01 |
0.06 |
|
Omega 3 Sum |
0.4 |
0.43 |
0.92 |
|
Omega 6 Sum |
3.54 |
3.41 |
3.49 |
|
C16:1 Palmitoleic |
0.28 |
0.26 |
0.26 |
|
C18:0 Stearic |
0.93 |
0.91 |
0.91 |
|
Lysine |
0.92 |
0.84 |
1.27 |
|
Threonine |
0.7 |
0.62 |
0.76 |
|
Tryptophan |
0.24 |
0.19 |
0.29 |
*Carbohydrate (Nitrogen-free extract) =100% - (%Protein + %Fat + %Fiber + %Ash + %Moisture)
Table 2: Comparison of the three different foods as fed (g/100g).
|
Sample/measurement |
Analysis |
Phase |
Days |
|
Blood |
Blood chemistry, SDMA, inflammatory cytokines, metabolomics |
Pre-feed |
25 |
|
Treatment |
25, 55 |
||
|
Feces |
Microbiome sequencing, metabolomics |
Pre-feed |
23, 24 |
|
Treatment |
23, 24, 53, 54 |
Table 3: Sample analyses and measurement.
2.4 Sequencing of the 16S rRNA Gene
Fecal samples were collected within 30 minutes of defecation and stored at -80oC until processed. Approximately 25mg of frozen stool homogenate was used for DNA isolation with MoBio PowerFecal® Kit (MoBio, Carlsbad, CA). Instructions provided by the manufacturer were followed except that a sonication step was added before vortexing the bead tubes with feces samples for 15 minutes. The DNA extracts were stored at -20oC until further processed. One microliter of each DNA sample was used to amplify the V3V4 region of the 16S rRNA gene using primers 347F and 803R containing Illumina adapters [8]. Amplification was performed on BioRad C1000 Touch Thermal Cycler under the following conditions: 25 cycles of denaturation at 95°C for 30 seconds, annealing at 55°C for 30 seconds extension at 72°C for 45 seconds, and a final elongation step at 72°C for 5 minutes. An internal normalized mock community DNA and PCR-grade water were used as positive and negative controls, respectively. The mock community was formed by mixing genomic DNA of 28 bacterial species representing 25 genera obtained from the American Type Culture Collection (ATCC, Rockville, MD). The mock community represented equal copy numbers of the 16S rRNA gene of each species as described by Diaz et al. [9].
PCR amplicons (25µl) were purified by using Agencourt AmPure XP beads (Beckman Coulter) and concentrations were measured by using Qubit fluorometer 3.0 (Life Technologies). The quality of the amplicon was assessed by using Agilent 2100 Bioanalyzer. Index PCR, library quantification, normalization and pooling were performed following the Illumina’s 16S metagenomic sequencing library preparation protocol (Part # 15044223 Rev. A, Illumina, CA). Libraries were mixed with Illumina generated PhiX control library and denatured using fresh NaOH. Final sequencing libraries were then loaded onto the Illumina Miseq v3 reagent cartridge and 251-base paired-end reads were generated using Miseq Control Software (MCS) 2.4., RTA 1.18.54 and Miseq Reporter 2.4. For every Miseq run, a mock community sample and water were run as a positive and a negative control, respectively.
The reads were de-multiplexed using Miseq built-in workflow to obtain FASTQ files processed using Mothur, version 1.32 [10]. Sequences were retained based on criteria such as having reads between 431 and 458 base pairs, maximum ambiguous bases of 0 and maximum homopolymer length of 6. The remaining sequences were chimera detected using the UCHIME algorithm implemented in MOTHUR and excluded from further processing [11]. All retained sequences were aligned to the GreenGenes 16S rRNA gene reference database of (gg.13.5.99). The database was used for taxonomical assignment of operational taxonomic units (OTUs) at an 80% confidence threshold by using the naïve Baysian algorithm [12] implemented in MOTHUR.
2.5 Blood and fecal metabolites
Metabolomic profiles of blood and fecal samples were determined by Metabolon (Durham, NC). The methods utilized a Waters ACQUITY ultra-performance liquid chromatography (UPLC) and a Thermo Scientific Q-Exactive high resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. Different aliquots of sample extracts were analyzed under different chromatographic conditions optimized for hydrophilic or hydrophobic compounds [7]. Standards present in each aliquot were used to ensure injection and chromatography consistency. Peaks were identified and processed using proprietary hardware and software. The relative quantification of the metabolites was performed by using area-under-the curve. Symmetric dimethylarginine (SDMA) concentrations in blood samples were determined using liquid chromatography-mass spectroscopy (LC-MS) as described before (13,14).
2.6 Statistical analysis
Matched-pair analyses were performed with JMP version 12 (SAS Institute, Carry, NC) to compare differences between means of the microbial abundances and relative levels of metabolites on samples collected from the same dog after the consumption of the test or the control 2 food. P values were calculated for differences between means and false discovery rate (FDR) corrections were made on each group of markers. FDR-P values less than 0.05 were considered significant. A bivariate regression analysis was performed to evaluate correlations between the changes in the microbial abundance and fecal metabolites.
3. Results
3.1 Food intake and body weight
All dogs completed the study successfully and there was no adverse health report. There was a trend (P=0.06) in the body weights of the dogs consuming the test food (10.96 Kg) to increase when compared to the control 2 food (10.74 Kg). There was very little difference in intakes of the test (113.41 Kcal/Body weight^0.75, SE=3.89) or the control 2 food (113.9 Kcal/Body weight^0.75, SE=4.15).
3.2 Changes in the Gut Microbial Composition
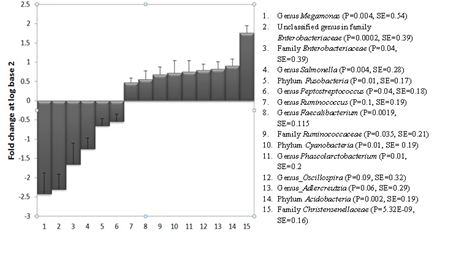
The test food led to significant changes in the proportions of bacteria at various taxa levels. Figure 1 summarizes the log (base 2) fold changes of the taxa after the consumption of the test food compared to the control 2 food. At the phylum level, Acidobacteria and Cyanobacteria increased by 0.91 and 0.72 log fold changes, respectively. This was accompanied by a -0.65 log fold reduction in the phylum Fusobacteria. This was equivalent to 87% and 65% increase in Acidobacteria and Cyanobacteria, respectively, and a 66% reduction in Fusobacteria. At the family level, Christensenellaceae and Ruminococcaceae increased by 1.76 and 0.68 log fold changes, respectively. These were equivalent to a 138% and 60% increase in the proportions of Christensenellaceae and Ruminococcaceae, respectively, compared to their levels on the Control 2 food. On the contrary, the test food led to 1.65 log fold reduction (68%) in Enterobacteriaceae. Bacteria in the genus Phascolarctobacterium increased by 68%.
3.3 Levels of fatty acids and glycerol
The levels of fecal and circulating unsaturated fatty acids increased after the consumption of the test food (Table 4). In plasma, levels of docosahexaenoate (DHA; 24:6n3), docosapentaenoate (DPA; 22:5n6), eicosapentaenoate (EPA; 22:5n3), linolenate (18:3n3) and stearidonate (18:4n3) were increased. In feces, in addition to these fatty acids, docosapentaenoate (DPA; 22:5n3) and palmitoleate (16:1n7) were increased on the test food. Despite the similar levels of circulating glycerol on both foods, fecal levels of glycerol increased by 24% when the pets consumed the test food compared to the control 2 food (P=0.001) (Table 4).
3.4 Fecal levels of mucin amino acids
The relative fecal levels of amino acids that make up the mucin layer, such as aspartate, proline, serine and threonine were significantly affected by the type of food consumed by the senior dogs. Compared to the control 2 food, the consumption of the test food led to a 28-61% reduction in levels of these amino acids in the feces (Table 5).
3.5 Advanced Glycation End Products (AGE)
High levels of circulating advanced glycation end products (AGE) are associated with aging and various age-related diseases. The test food led to about 70% reductions in both circulating (P=1.15E-07 and fecal (P=5.29E-13) levels of one of the AGE, pyrraline. The circulating level of another AGE, N6-carboxymethyllysine (CML), was not affected by the different diets; but the fecal levels were higher on the test food. The third AGE, N6-carboxyethyllysine (CEL), was detected only in feces and did not change during the consumption of the different diets (Table 6).
3.6 Changes in circulating uremic toxins
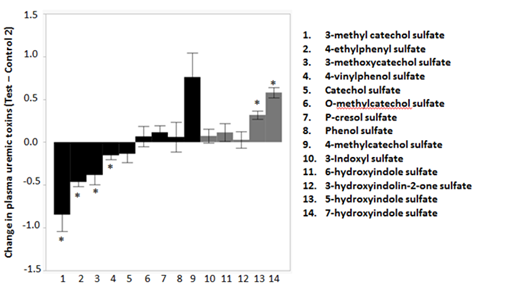
Uremic toxins are among the major toxic metabolites that lead to renal and associated diseases in aging. Some uremic toxins originate from protein fermentation in the colon by proteolytic bacteria. Products of the putrefaction process are absorbed and converted to toxic derivatives causing an increased burden on kidney function. We detected a total of 14 phenolic and indolic uremic toxins in plasma (Figure 2). The phenolic uremic toxins, 3-methyl catechol sulfate (P=0.0015, SE=0.19), 4-ethylphenyl sulfate (P=2.38E-09, SE=0.05), 3-methoxycatechol sulfate (P=0.02, SE=0.11) and 4-vinylphenol sulfate (P=0.05, SE=0.05) declined by 175%, 73%, 67% and 23%, respectively after the consumption of the test food. On the contrary, the indolic uremic toxins 5-hydroxyindole sulfate (P=2.23E-06, SE=0.05) and 7-hydroxyindole sulfate (P=2.97E-10, SE=0.06) increased by 29% and 43%, respectively, after the consumption of the test food. None of the other uremic toxins were significantly influenced by the different foods.
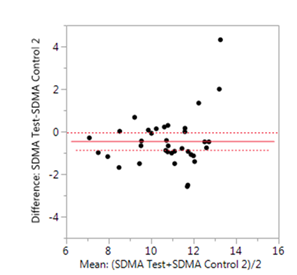
Symmetric dimethylarginine (SDMA) is a uremic toxin originating from the host metabolism and methylation of arginine (15, 16). The test food resulted in a significant reduction in blood concentrations of SDMA (P=0.035, SE=0.2) in the senior dogs compared to the control 2 food (Figure 3).
3.7 Correlations of microbial taxa with changes in metabolites
The genus Adlercreutzi and the family Christensenellaceae were strong positive predictors of glycerol levels in feces (Table 7). Faecalibacterium
prausnitzii, family Ruminococcaceae, genus Phascolarctobacterium and phylum Actinobacteria also correlated positively with fecal levels of glycerol. On the contrary, phylum Fusobacterium and genus Salmonella negatively correlated with glycerol levels in feces (Table 7). The genera Oscillospira and Adlercreutzia also had a negative correlation with levels of pyrraline in feces. The phylum Fusobacterium correlated positively with fecal levels of pyrraline and threonine. Salmonella also had a positive correlation with pyrraline levels in feces (Table 7).
The test food led to a significant reduction in the proportion of bacteria belonging to the genus Megamonas, an unclassified genus in family Enterobacteriacea, Salmonella and Peptostreptococcus. The consumption of the test food significantly increased the proportions of the genera Phascolarcobacterium, Faecalibacterium and Ruminococcus. At the family level, the test food led to a significant increase in Christensenellaceae and Ruminococcaceae and a significant reduction in Enterobacteriaceae. At the phylum level, the test food increased the phyla Acidobacteria and Cyanobacteria and led to a significant reduction in Fusobacteria. Dogs had reduced proportions of the genera Salmonella, Megamonas, Peptostreptococcus and an unknown genus in the family Enterobacteriaceae (OTU_10001_Enterobacteriaceae_g) by 58%, 81%, 32%, 80%, respectively. Supplementary Table 1 (S1) provides the means and standard errors of the proportions of the above taxa on the test and the control 2 foods.
|
Metabolite in feces |
Feces |
Plasma |
||||||
|
% change on Test |
P Value (FDR) |
Mean difference (test-Control 2) |
SE |
% change on Test |
P Value (FDR) |
Mean diference (test-Control 2) |
SE |
|
|
arachidonate (20:4n6) |
-16.24 |
1.49 |
-0.2 |
0.12 |
10.51 |
2.46 |
0.11 |
.08 |
|
docosahexaenoate (DHA; 22:6n3) |
256.47 |
3.52E-11 |
2.17 |
0.21 |
177.33 |
1.02E-10 |
1.44 |
0.14 |
|
docosapentaenoate (DPA; 22:5n3) |
43.85 |
0.02 |
0.45 |
0.13 |
35.53 |
0.11 |
0.36 |
0.12 |
|
docosapentaenoate (n6 DPA; 22:5n6) |
120.98 |
1.84E-07 |
1.1 |
0.15 |
57.13 |
0.0003 |
0.57 |
0.11 |
|
eicosapentaenoate (EPA; 20:5n3) |
434.21 |
1.22E-13 |
3.97 |
0.3 |
191.50 |
4.83E-08 |
1.61 |
0.21 |
|
glycerol |
31.70 |
0.01 |
0.36 |
0.1 |
3.33 |
1.0* |
0.04 |
0.09 |
|
laurate (12:0) |
-4.52 |
1.0* |
-0.05 |
0.06 |
2.45 |
1.0* |
0.03 |
0.08 |
|
linoleate (18:2n6) |
-4.84 |
1.0* |
-0.05 |
0.07 |
7.86 |
1.0* |
.081 |
0.08 |
|
linolenate (18:3n3 or 3n6) |
45.47 |
0.004 |
0.44 |
0.1 |
40.36 |
0.02 |
0.42 |
0.12 |
|
myristate (14:0) |
10.73 |
1.0* |
0.11 |
0.07 |
5.10 |
1.0* |
0.06 |
0.1 |
|
oleate/vaccenate (18:1) |
8.80 |
1.0* |
0.1 |
0.08 |
9.49 |
1.0* |
.096 |
0.07 |
|
palmitate (16:0) |
1.01 |
1.0* |
0.01 |
0.09 |
7.64 |
1.0* |
0.08 |
0.06 |
|
palmitoleate (16:1n7) |
121.28 |
2.01E-09 |
1.23 |
0.1 |
26.21 |
0.51 |
0.28 |
0.13 |
|
stearate (18:0) |
-22.59 |
0.08 |
-0.26 |
0.09 |
3.03 |
1.0* |
0.03 |
0.06 |
|
stearidonate (18:4n3) |
776.56 |
1.33E-14 |
8.1 |
0.6 |
132.47 |
7.68E-06 |
1.29 |
0.21 |
Changes that were statistically significant (P<0.05) are marked grey. 1.0*: FDR P values greater than 1 are referred as 1.
Table 4: Change in levels of fatty acids and glycerol in feces and plasma of the senior dogs during the consumption of the Test versus the Control 2 food.
|
Amino acid in feces |
% change on Test |
P Value (FDR) |
|
Asparagine |
-61.03 |
0.007 |
|
Proline |
-28.5 |
0.007 |
|
Serine |
-36.2 |
0.004 |
|
Threonine |
-35.6 |
0.001 |
Table 5: Relative levels of mucin amino acids in feces.
|
Matrix |
AGE |
% change on Test |
P Value (FDR) |
Mean difference (Test-Control 2) |
SE |
|
Feces |
Pyrraline |
-69.65 |
5.29E-13 |
-1.74 |
0.15 |
|
Plasma |
Pyrraline |
-68.82 |
1.15E-07 |
-1.56 |
.23 |
|
Feces |
N6-carboxymethyllysine |
48.86 |
0.0006 |
0.44 |
0.11 |
|
Plasma |
N6-carboxymethyllysine |
4.25 |
0.89 |
0.05 |
.07 |
|
Feces |
N6-carboxyethyllysine |
9.15 |
1.0 |
0.08 |
.09 |
Changes that were statistically significant are marked grey.
Table 6: Changes in levels of advanced glycation end products (AGE).
The test food led to significant (*: False discovery rate corrected (FDR) P-value <0.05) reductions in levels of phenolic uremic toxins such as 3-methyl catechol sulfate, 4-ethylphenyl sulfate, 3-methoxycatechol sulfate and 4-vinylphenol sulfate. Two of the indolic uremic toxins, namely 5-hydroxyindole sulfate and 7-hydroxyindole sulfate, increased after the consumption of the test food. There were no significant changes in the typical uremic toxins such as 3-indoxyl sulfate or P-cresol sulfate.
Matched pair analyses of each dog on the test food versus the control 2 food showed significant reduction in plasma concentrations of symmetric dimethylarginine (SDMA) (P=0.035, SE=0.2) after the consumption of the test food.
|
Correlations with glycerol in feces |
FDR P-value |
R |
|
Genus Adlercreutzi |
3.95E-09 |
0.51 |
|
Family Christensenellaceae |
0.0001 |
0.42 |
|
Faecalibacterium prausnitzii |
0.02 |
0.39 |
|
Family Ruminococcaceae |
0.02 |
0.39 |
|
Genus Phascolarctobacterium |
0.003 |
0.33 |
|
Phylum Actinobacteria |
0.0005 |
0.32 |
|
Phylum Fusobacterium |
0.002 |
-0.4 |
|
Genus Salmonella |
0.005 |
-0.25 |
|
Correlations with pyrraline in feces |
||
|
Family Christensenellaceae |
1.05E-12 |
-0.52 |
|
Genus Oscillospira |
0.002 |
-0.48 |
|
Genus Adlercreutzia |
1.52E-14 |
-0.44 |
|
Genus Salmonella |
0.026 |
0.48 |
|
Phylum Fusobacterium |
0.017 |
0.41 |
|
Correlations with mucin amino acids in feces |
||
|
Family Christensenellaceae and threonine |
0.0004 |
-0.32 |
|
Family Christensenellaceae and serine |
0.0007 |
-0.27 |
|
Phylum Fusobacterium and threonine |
0.00015 |
0.33 |
|
Phylum Fusobacterium and serine |
0.02 |
0.25 |
Table 7: Correlations of different taxa with fecal levels of glycerol, pyrraline and mucin amino acids.
4. Discussion
This study showed senior dogs may benefit from increased soluble fiber and omega 3 fatty acids that lead to reduced circulating levels of metabolites associated with aging.. These changes were associated with changes in the gut microbial composition. The test food increased the relative abundance of health-promoting bacteria belonging to the genera Phascolarctobacterium. The abundance of the short-chain fatty acids producers Phascolarctobacterium was reported to have a positive association with positive mood and their number declines in elderly humans [17]. Some bacteria including Phascolarctobacterium have the capacity to metabolize isoflavones to equol, which has been implicated to have antioxidative properties and prevent various age-related diseases including diabetes and obesity (18-20). Equol is also associated with a decreased risk of certain types of cancer; therefore increasing the abundance of equol-producing gut microbiota has been recommended to reduce this risk [21]. In this study, although it did not reach statistical significance (P=0.17), the fecal level of equol increased by 59.6% after the consumption of the test food. Polyphenols bound to fruits and vegetables present in the test food may have led to the increased abundance of these bacteria in the senior dogs.
The consumption of the test food led to an increase in the proportion of the butyrate producer Faecalibacterium prausnitizii. F. prausnitzii has been reported to have anti-inflammatory effects [22] and the abundance of Faecalibacterium species declines during active inflammatory bowel disease [23]. The family Christensenellacea also increased after the consumption of the test food. Our correlation analysis showed Christensenellacea abundance was a strong positive predictor of fecal levels of glycerol. In a study that compared the microbiome of 416 twin-pairs, Christensenellacea were in a greater abundance in lean individuals compared to obese [24]. Christensenellacea have also been reported to have the capability to produce short-chain fatty acids (SCF) [25]. SCF are known to improve the intestinal barrier integrity, which is in line with the result of our correlation analysis showing a negative association between fecal levels of mucin amino acids and the proportion of Christensenellacea. Their increased abundance may have reduced the degradation of the mucin layer, which in turn would decrease inflammation attributed to the translocation of bacteria and their secretions through the gut barrier.
In a study that compared the microbial composition of Japanese people ranging from infants to the elderly [26], the relative proportions of bacteria in the genera Fusobacterium and Megamonas increased with age. The positive correlation of Fusobacterium with fecal levels of mucin amino acids supports a previous report that showed the capacity of Fusobacterium species to degrade mucin [27]. Odamaki et al. [26] showed a negative correlation between Enterobacteriaceae and a cluster of butyrate producing bacteria including Faecalibacterium. The decline in the proportions of Enterobacteriaceae in senior dogs after the consumption of the test food may have benefited the senior dogs as some of these bacteria are endotoxin producers, which compromise intestinal barrier integrity leading to inflammation. Salmonella belonging to Enterobacteriaceae also declined due to the test food consumption. Some species of Salmonella are major public health concerns causing salmonellosis. Although dogs are subclinical carriers of Salmonella, the intimate relationship between dogs and humans may lead to the risk of human exposure to Salmonella. The test food led to a significant relative reduction in Salmonella shedding by increasing the proportion of other bacteria that may have an anti-pathogenic effect.
Despite dietary levels of threonine being higher in the test food, fecal threonine declined when the dogs consumed the test food. This suggests that the increased fecal excretion of threonine is associated with the degradation of the mucin layer as reported by Weir et al. [28]. The composition of the gut microbiota is a key factor in maintaining intestinal barrier integrity. The reduction in the proportion of beneficial bacteria may lead to constipation, mal-absorption and longer colonic transit time. This encourages increased presence of proteolytic bacteria, whose products of fermentation deteriorate the intestinal barrier.
The consumption of the test food reduced levels of the advanced glycation end product, pyrraline. Advanced glycation end products (AGE) are derived from the non-enzymatic glycation of proteins, lipids, and nucleic acids. They can also be acquired from food; thus restriction of foods with high levels of AGE has been recommended to decrease circulating AGE in the body [29]. AGE are known to accelerate the process of aging and they are linked to a number of age-related diseases such as diabetes, vascular and renal diseases mainly by inducing inflammation and oxidative stress [30, 31]. Fecal microorganisms have been shown to be capable of degrading various AGE including pyrraline [32]. The negative correlation of pyrraline with Christensenellaceaemay suggests the capability of these bacteria to degrade pyrraline or prevent its formation. The consumption of diets rich in AGE has been shown to shift the microbiota towards a more detrimental composition [33]. This is in line with our correlation analyses that showed a positive association of pyrraline with Salmonella and Fusobacterium. After the consumption of the test food, the level of pyrraline in blood declined by almost the same amount (70%) as in feces. This implies induced microbial degradation of pyrraline or its precursors by the above microbes as a more likely mechanism than the test food influencing absorption of pyrraline from the GI tract. Interestingly, one of the AGE, N6-carboxymethyllysin (CML), increased in feces after the consumption of the test food. However, the level of CML in the blood did not change. This may suggest that fecal levels of CML may not be biologically significant.
The level of glycerol in the feces of the senior dogs was higher when they were fed the test food. Glycerol is known to increase water retention in the colon and thus it is used to treat constipation [34]. Prolonged transit times are risks to develop various diseases due to the exposure to toxic products accumulating due to putrefaction [35]. A shorter transit time leads to a limited accumulation of such products that may cause various diseases [35]. People with functional constipation have been shown to contain bacteria with more abundant genes to degrade glycerol [36]. The strong negative correlation between fecal levels of glycerol versus Fusobacteria and Salmonella may suggest the capacity of these bacteria to degrade glycerol. The reduction in the proportions of these bacteria after the consumption of the test food may have led to the increased levels of glycerol in feces. On the other hand, other taxa such as Adlercreutzi, Christensenellaceae, Faecalibacterium prausnitzii, Ruminococcaceae, Phascolarctobacterium and Actinobacteria correlated positively with glycerol. Weir et al. [28] found a positive association of a Rumonococcus species with fecal levels of glycerol and free fatty acids. To our knowledge, this study is the first to show the associations of the other taxa with fecal glycerol concentration. This may suggest the test food altered the microbial composition towards a population with higher lipase activity. Along with the increased level of glycerol in feces, the level of both fecal and plasma levels of omega fatty acids, DHA, EPA, DPA also increased in feces after the consumption of the test food due to added fish oil. Omega-3 fatty acids have health benefits throughout life by improving cardiovascular, immune, cognitive and other functions [37]. Similarly, the increased level of linoleate after consuming the test food is due to the higher dietary concentrations in the test food. Despite the similar levels of C:16:1 and C:18:0 fatty acids measured in the foods (Table 2), the levels of these fatty acids in feces increased after the consumption of the test food. This supports the possible increased microbial lipase activity attributed to the change in the microbial composition.
Uremic toxins are among the major metabolites that cause age-related complications. There was a marked improvement in markers of kidney health after the consumption of the test food. Circulating symmetric dimethylarginine (SDMA) has been shown to be a good biomarker of kidney function in dogs as it detects reduction in glomerular filtration rate (GFR) much earlier than serum creatinine [13, 16]. The reduction in circulating concentration of SDMA in the senior dogs after the consumption of the test food may thus indicate an increased GFR and improved kidney function. Furthermore, the test food reduced several phenolic uremic toxins originating from microbial fermentation of protein [38]. One of these metabolites was 4-ethylphenyl sulfate (4-EPS), which is also known to have a negative impact on brain health by causing anxiety-like symptoms [39, 40]. The role of foods containing fish oil, medium triglycerides and L-carnitine in improving age-related circulating markershas been reported [7, 41]. The significant reduction in such metabolites in the senior dogs after the consumption of the test food may be due to the changes in the microbial composition. The two indolic uremic toxins, 5-hydroxyindole sulfate and 7-hydroxyindole sulfate, increased after the consumption of the test food. Indolic uremic toxins originate from colonic fermentation of the amino acid tryptophan [42]. The test food was formulated to contain 53% higher tryptophan compared to the control 2 food. The presence of more substrate may have led to increased levels of the two indolic metabolites. However, the typical indolic uremic toxin, 3-indoxyl sulfate, was not affected by the consumption of the different diets.
5. Conclusion
The main finding of this study was that senior dogs benefit from increased content of soluble fiber and omega-3 fatty acids in their food. Microbiome data suggests that consumption of such food leads to increased abundance of various bacterial taxa associated with health benefits. Metabolomics data indicates decreased proteolysis in the gut after dogs ate the food with high soluble fiber and omega-3 fatty acids. The levels of age-related metabolites such as advanced glycation end products and uremic toxins in the plasma were significantly reduced. Some of these changes correlated with changes in the microbial composition. These observations should be further characterized with future work.
Acknowledgments
E. and D. E. J designed and conducted research; M. I. J. and E.E. analyzed data; M.Y. analyzed SDMA, E.E. wrote the paper and had primary responsibility for final content. All authors read and approved the final manuscript. The work was funded by and performed at the Pet Nutrition Center, Hill’s Pet Nutrition, Topeka, Kansas.
References
- Woodmansey EJ. Intestinal bacteria and ageing. Journal of Applied Microbiology 102 (2007): 1178-1186.
- Conley MN, Wong CP, Duyck KM, et al. Aging and serum MCP-1 are associated with gut microbiome composition in a murine model. Peer Journal 4 (2016): e1854.
- Rehman T. Role of the gut microbiota in age-related chronic inflammation. Endocrine, Metabolic and Immune Disords - Drug Targets 12 (2012): 361-367.
- De Santis S, Cavalcanti E, Mastronardi M, et al. Nutritional Keys for Intestinal Barrier Modulation. Frontiers in Immunology 6 (2015): 612.
- Buddington RK, Buddington KK and Sunvold GD. Influence of fermentable fiber on small intestinal dimensions and transport of glucose and proline in dogs. American Journal of Veterinary Research 60 (1999): 354-358.
- Swanson KS, Grieshop CM, Clapper GM, et al. Fruit and vegetable fiber fermentation by gut microflora from canines. Journal of Animal Science 79 (2001): 919-926.
- Hall JA and Jewell DE. Feeding healthy beagles medium-chain triglycerides, fish oil, and carnitine offsets age-related changes in serum fatty acids and carnitine metabolites. Plos One 7 (2012): e49510.
- Nossa CW, Oberdorf WE, Yang L, et al. Design of 16S rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World Journal of Gastroenterology 16 (2013): 4135-4144.
- Diaz PI, Dupuy AK, Abusleme L, et al. Using high throughput sequencing to explore the biodiversity in oral bacterial communities. Molecular Oral Microbiology 27 (2012): 182-201.
- Schloss PD, Westcott S, Ryabin T, et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Applied Environtal Microbiology 75 (2009): 7537-7541.
- Edgar RC, Haas BJ, Clemente JC, et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27 (2011): 2194-2200.
- Wang Q, Garrity G.M, Tiedje JM, et al. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appied Environtal Microbiology 73 (2007): 5261-5267.
- Hall JA, Yerramilli M, Obare E, et al. Serum concentrations of symmetric dimethylarginine and creatinine in dogs with naturally occurring chronic kidney disease. Journal of Veterinary Internal Medicine 30 (2016): 794-802.
- Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. Journal of Veterinary Internal Medicine 28 (2014): 1676-1683.
- Schepers E, Barreto DV, Liabeuf S, et al. Symmetric dimethylarginine as a proinflammatory agent in chronic kidney disease. Clinical Journal of American Society of Nephrology 6 (2011): 2374-83.
- Hall JA, MacLeay J, Yerramilli M, et al. Positive Impact of Nutritional Interventions on Serum Symmetric Dimethylarginine and Creatinine Concentrations in Client-Owned Geriatric Dogs. Plos One 11 (2016): e0153653.
- Li L, Su Q, Xie B, et al. Gut microbes in correlation with mood: case study in a closed experimental human life support system. Neurogastroenterology and Motility 28 (2016):1233-1240.
- Maruo T, Sakamoto M, Ito C, et al. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. International Journal of Systematic and Evolutionary Microbiology 58 (2008): 1221-1227.
- Matthies A, Clavel T, Guetschow M, et al. Conversion of daidzein and genistein by an anaerobic bacterium newly isolated from the mouse intestine. Applied and Environmental Microbiology 74 (2008): 4847-4852.
- Cross TWL, Zidon TM, Welly RJ, et al. Soy improves cardiometabolic health and cecal microbiota in female low-fit rats. Scientific Reports 7 (2017): 9261.
- Sugiyama Y, Masumori N, Fukuta F, et al. Influence of isoflavone intake and equol-producing intestinal flora on prostate cancer risk. Asian Pacific Journal of Cancer Prevention 14 (2013): 1-4.
- Martin R, Miquel S, Benevides L, et al. Functional Characterization of Novel Faecalibacterium prausnitziiStrains Isolated from Healthy Volunteers: A Step Forward in the Use of F. prausnitzii as a Next-Generation Probiotic. Frontiers in Microbiology 8 (2017): 1226.
- Suchodolski JS, Markel ME, Garcia-Mazcorro JF, et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. Plos One 7 (2012): e51907.
- Goodrich JK, Waters JL, Poole AC, et al. Human genetics shape the gut microbiome. Cell 159 (2014): 789-799.
- Zhang C and Zhao L. Strain-level dissection of the contribution of the gut microbiome to human metabolic disease. Genome Medicine 8 (2016): 41.
- Odamaki T, Kato K, Sugahara H, et al. Age-related changes in gut microbiota from newborn to centenarian: a cross –sectional study. BMC Microbiology 16 (2016): 90.
- Flynn JM, Niccum D, Dunitz JM, et al. Evidence and role for bacterial mucin degradation in cyctic fibrosis airway disease. Plos Pathogens 12 (2016): e1005846.
- Weir TL, Manter DK, Sheflin AM, et al. Stool Microbiome and Metabolome Differences between Colorectal Cancer Patients and Healthy Adults. Plos One 8 (2013): e70803.
- Semba RD, Nicklett EJ and Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? Journal of Gerontology. Series A, Biological Sciences and Medical Sciences 65 (2010): 963-975.
- Luevano-Contreras C and Chapman-Novakofski K. Foodary advanced glycation end products and aging. Nutrients 2 (2010): 1247-1265.
- Younessi P and Yoonessi A. Advanced Glycation End-Products and Their Receptor-Mediated Roles: Inflammation and Oxidative Stress. International Journal of Molecular Sciences 36 (2011): 154-166.
- Hellwig M, Bunzel D, Huch M, et al. Stability of Individual Maillard Reaction Products in the Presence of the Human Colonic Microbiota. Journal of Agricultural and Food Chemistry 63 (2015): 6723-6730.
- Mills DJ, Tuohy KM, Booth J, et al. Dietary glycated protein modulates the colonic microbiota towards a more detrimental composition in ulcerative colitis patients and non-ulcerative colitis subjects. Journal of Applied Microbiology 105 (2008): 706-714.
- Portalatin M and Winstead N. Medical management of constipation. Clinics in Colon and Rectal Surgery 25 (2012): 12-19.
- Lewis SJ and Heaton KW. The metabolic consequences of slow colonic transit. American Journal of Gastroenterology 94 (1999): 2010-2016.
- Mancabelli L, Milani C, Lugli GA, et al. Unveiling the gut microbiota composition and functionality associated with constipation through metagenomic analyses. Scientific reports 7 (2017): 9879.
- Swanson D, Block R and Mousa AA. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. America Society for Nutrition. Advances in Nutrition 3 (2012): 1-7.
- Tanaka H, Sirich TL, Plummer NS, et al. An enlarged profile of uremic solutes. Plos One 10 (2015): e0135657.
- Hsiao EY, McBride SW, Hsien S, et al. The microbiota modulates gut physiology and behavioral abnormalities associated with autism. Cell 155 (2013): 1451-1463.
- Dorrestein PC, Mazmanian SK and Knight R. Finding the missing links among metabolites, microbes, and the host. Immunity 40 (2014): 824-832.
- Goraya N, Simoni J, Jo CH, et al. A Comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clinical Journal of American Society of Nephrology 8 (2013): 371-381.
- Aronov PA, Luo FJG, Plummer NS, et al. Colonic Contribution to Uremic Solutes. Journal of American Society of Nephrology 22 (2011): 1-8.