Seroprevalence of Hepatitis B Virus Infection and Associated Factors among Pregnant Women Attending Antenatal Care centers in Djbouti City, Republic of Djibouti
Article Information
Sahal Darar Dirir1, Ambroise Ahouidi2, Mohamed Osman Miguil3, Aboubacry Drame4, Assane Dieng5, Houssein Youssouf Darar6, Mohamed Houmed Aboubakar1, Makhtar Camara5, Coumba Toure-Kane2, Halimatou Diop Ndiaye5*
1Laboratoire de Diagnostic, Centre de Soins, Caisse Nationale de Sécurité Sociale (CNSS), Djibouti
2Institut de Recherche en Santé, de Surveillance Épidémiologique et de Formation, Sénégal
3Institut National de la Statistique de Djibouti
4Université Alioune Diop de Bambey, Sénégal
5Laboratoire Bactériologie-Virologie- Hôpital Aristide le Dantec, Sénégal and Université Cheik Anta Diop de Dakar, Sénégal
6Institut National de la Sante Publique, Djibouti
*Corresponding Author: Halimatou Diop-Ndiaye Université Cheikh Anta Diop, Dakar, and Laboratoire de Bactériologie-virologie, Hôpital Aristide Le Dantec, Dakar, Sénégal
Received: 31 May 2023; Accepted: 27 June 2023; Published: 14 July 2023
Citation: Sahal Darar Dirir, Ambroise Ahouidi, Mohamed Osman Miguil , Aboubacry Drame, Assane Dieng, Houssein Youssouf Darar, Mohamed Houmed Aboubakar, Makhtar Camara, Coumba Toure-Kane, Halimatou Diop Ndiaye. Seroprevalence of Hepatitis B Virus Infection and Associated Factors among Pregnant Women Attending Antenatal Care centers in Djbouti City, Republic of Djibouti. Archives of Clinical and Medical Case Reports. 7 (2023): 304-312.
Share at FacebookAbstract
Background: Hepatitis B virus (HBV) remains a major public health concern affecting millions of people worldwide. More than 90% of infections are acquired during infancy through perinatal transmission leading to chronicity and possibility to develop hepatocellular carcinoma.
Objective: This study was conducted to assess the seroprevalence of HBV infection and risk factors among pregnant women received at selected antenatal care centers in Djibouti city.
Methods: A total of 882 pregnant women were enrolled in the study using a systematic sampling technique. Data were collected using a questionnaire. Five milliliters of venous blood samples were collected and tested for HBV using the ELISA diagnostic test. The collected data were entered into CS Entry and exported to logiciel R for statistical analysis. Multivariate analysis was performed to determine predictor variables. Statistical significance was reported at p-value <0.05.
Results: The prevalence of infection was 9.3% (n=82), with CS2 and Einguela having the highest prevalence. Family history of HBV (OR=8; 95% CI 4.40-14.6), pregnant women with high education (OR=9.37; 95% CI 3.14-28.6), history of blood transfusion (OR=2.53; 95% CI 1.09-5.61), abortion (OR=2.08; 95% CI 1.03-4.12), and large multiparous wombs were predictive factors of HBV infection.
Conclusion: The results of our study indicate a highly endemic area, where family history of HBV, multiparous women, education, trimester and abortion were predictive factors for infection. It would therefore be important to increase awareness of the risks of transmission, early systematic screening in the first trimester of pregnancy, and extension of vaccination to household contacts of HBV-infected patients.
Keywords
Hepatitis B virus; Pregnancy; Seroprevalence; Risk factor; Djibouti city
Article Details
1. Introduction
Hepatitis B virus (HBV) infection is a global public health concern. The World Health Organization (WHO) estimated in 2020 that about one third of the world's population is infected with HBV, and 360 million people are living with chronic infection. In addition, 1 million people died from cirrhosis or hepatocellular carcinoma complications [1].
Worldwide, only less than 5% of people with chronic viral hepatitis know their status. The global prevalence of HBV infection is highly variable with areas of high prevalence (≥ 8%) in Africa, Asia, and the Western Pacific, moderate prevalence (2-7%) in Southern and Eastern Europe, and low prevalence (< 2%) in Western Europe, North America, and Australia [2, 3]. In sub-Saharan Africa, HBV prevalence among pregnant women is high, ranging from 2.4% in Ethiopia to 11.8% in Uganda [3, 4].
In these highly endemic areas, the main route of infection is mother-to-child transmission (MTCT) that represents a key link in maintaining the disease in the population [5].
Infants born to HBsAg and HBeAg-positive mothers have a 70-90% chance of acquiring perinatal HBV infection. Perinatally infected infants have an 85-90% chance of becoming chronic HBV carriers. It is estimated that more than 25% of these carriers will die of hepatocellular carcinoma (HCC) or cirrhosis of the liver [6].
Epidemiological studies of HBV infection have shown a significant association between the disease and sociological, obstetric, and behavioral characteristics of pregnant women [7].
In Djibouti, the epidemiology of HBV infection is poorly documented [8, 9]. The few studies conducted at various times (1986 and 2005) on specific populations (blood donors and adults attending hospitals in Djibouti) do not report any information on the risks associated with the disease. To our knowledge, no study on the prevalence of HBV in pregnant women has been reported. Knowing that unscreened pregnant women constitute a real reservoir of virus transmission [10], we conducted this work to determine the seroprevalence of hepatitis B virus infection in pregnant women in Djibouti, and to assess the risk factors associated with this infection.
2. Methods
2.1 Study population
This is a prospective, multicenter, open-label epidemiological study that was conducted from August 2020 to April 2021 in Djibouti, the capital of the country. Pregnant women were recruited as part of the antenatal check-up in 9 antenatal care centers (CSPN) located in 7 polyclinics, one community health center (CHC), and one private hospital. Eligible, consenting pregnant women whose pregnancy was confirmed by clinical and physical examination and/or obstetric ultrasound were enrolled in the study. A questionnaire on sociodemographic, obstetric, health care and risk behavior characteristics was administered. Pregnant women who were unable to provide appropriate information because of a severe disability or illness were excluded from the study. Our minimum sample size (n=379) was determined using Schartz's formula: ((N=t×p (1-p) ÷ mp=0.10 [9] [9], t=confidence level (the standard value of the 95% confidence level will be 1.96) hence t=1.96; m=margins of error set at 5%).
2.2 Sampling procedure
After the consent was obtained in pregnant women, a capillary blood sample was taken for HBsAg testing using the ACON® HBsAg Rapid Test (T&C Beheer BV, CHINA) according to the manufacturer's instructions. For each positive sample, a venous blood sample was taken on EDTA tube for confirmation at the laboratory of the Care Center 1 (CS1) of the National Social Security Fund (CNSS) using the VIDAS® HBs Ag Ultra test (BioMerieux, France). This confirmatory test was performed on serum according to the manufacturer's instructions.
2.3 Data collection methods
The entire questionnaire was reviewed for completeness, accuracy, and consistency. The validated questionnaire was then entered into CS Entry. The variable of interest was hepatitis B virus serostatus. The other variables were socio-demographic characteristics (age, marital status, education level, family income, occupation) and those related to pregnancy and parity.
Health care characteristics such as surgical procedures, mode of delivery, blood transfusions, trimester of pregnancy, and vaccination were considered health-related variables. Risk behaviors and practice characteristics such as body tattooing, nose piercing, abortion, sharing sharp equipment, and contact with an infected person were considered “risk behavior and practice” variables.
Data were analyzed on R software versions 4.0.1. For univariate analysis, descriptive statistics were performed to examine the frequency distribution, central tendency, variability, and overall distribution of the independent variables. A bivariate analysis was performed to select candidate variables for multivariate analysis.
Variables with P values <.0, 05 in the bivariate analysis were included in the multivariate logistic regression model. Multivariate logistic regression was performed to control possible confounding and identify the true effects of the selected predictors. Logistic regression was used with a 95% confidence interval (CI) and a p value < 0.05 was considered significant.
3. Results
3.1 Socio-demographic characteristics of the study participants
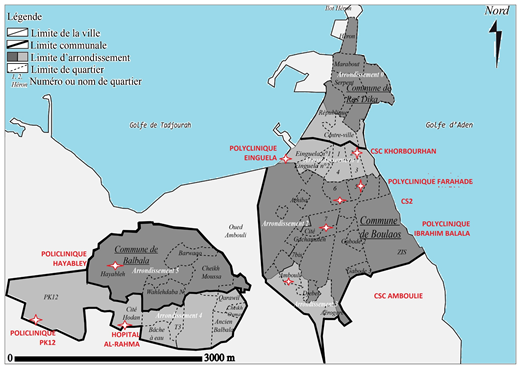
A total of 882 pregnant women were recruited between August 2020 and April 2021 from the 9 CSPN, as shown in Figure 1.
Table 1 shows the sociodemographic and obstetric characteristics of the pregnant women. The mean age was 28 years (ranged from 15 to 47 years). Most of the pregnant women had not attended school (n=556; 63%). All women were married (n=882; 100%) and 81.8% (n=721) were unemployed. Most of the pregnant women were followed by a health care giver (n=871; 98.8%) and 68% (n=607) of the participants were multiparous. In addition, 38.2% (n=337) and 33.9% (n=299) of participants were screened in the 2nd and 3rd trimester of pregnancy, respectively.
Among the pregnant women, 25% (n=662) had been hospitalized in a health facility, and 20% had undergone previous surgery (n=181). A family history of hepatitis B was found in 11% (n=98), and blood transfusions were noted in 7.3% (n=65) of the women. However, only 12 (1.4%) pregnant women had been vaccinated against HBV.
3.2 HBsAg seroprevalence in pregnant women
The serological analysis showed that 82 pregnant women were HBsAg positive, giving an overall HBsAg carriage rate of 9.3%. The Care center 2(CS2) and Einguella center had the highest positivity rates with 46% (n=38) and 13% (n=11), respectively, while the Hayebley center had the lowest positivity rate, with 3% (n=3).
|
Variables and categories |
Frequency |
(%) |
|
|
Age (years) |
|||
|
< 30 |
497 |
56.3 |
|
|
≥ 30 |
379 |
43.0 |
|
|
Not determined |
6 |
0.7 |
|
|
Educational status |
|||
|
Illiterate |
556 |
63.1 |
|
|
Primary |
96 |
10.9 |
|
|
Secondary |
190 |
21.5 |
|
|
University |
40 |
4.5 |
|
|
Health center for prenatal care |
|||
|
Al-Rahma |
86 |
9.8 |
|
|
Ambouli |
95 |
10.8 |
|
|
CS2 |
116 |
13.2 |
|
|
Einguella |
101 |
11.4 |
|
|
Farahade |
96 |
10.9 |
|
|
Hayabley |
98 |
11.1 |
|
|
Ibrahim balala |
94 |
10.7 |
|
|
Khorbourhan |
98 |
11.1 |
|
|
Pk12 |
98 |
11.1 |
|
|
Followed by a caregiver |
|||
|
No |
11 |
1.2 |
|
|
Yes |
871 |
98.8 |
|
|
Occupational status |
|||
|
Unemployed |
721 |
81.8 |
|
|
Employed |
161 |
18.2 |
|
|
Marital status |
|||
|
Married |
882 |
100 |
|
|
Age of pregnancy |
|||
|
1er trimester |
236 |
26.7 |
|
|
2ème trimester |
347 |
39.3 |
|
|
3rd trimester |
299 |
34 |
|
|
Parity |
|||
|
Nulli or Primiparous (0-1) |
278 |
30.6 |
|
|
Multiparous (2-3) |
334 |
38.8 |
|
|
Large muiltiparous( ≥ 4) |
270 |
30.6 |
|
|
Hospitalization with IntraVenous medication |
|||
|
No |
662 |
75.1 |
|
|
Yes |
220 |
24.9 |
|
|
Antecedent of surgical procedure |
|||
|
No |
701 |
79.5 |
|
|
Yes |
181 |
20.5 |
|
|
Abortion history |
|||
|
No |
743 |
84.2 |
|
|
Yes |
136 |
15.4 |
|
|
Undeterminated |
3 |
0.4 |
|
|
Family history of HBV |
|||
|
No |
784 |
88.9 |
|
|
Yes |
98 |
11.1 |
|
|
Blood transfusion |
|||
|
No |
817 |
92.6 |
|
|
Yes |
65 |
7.4 |
|
|
Vaccination |
|||
|
No |
870 |
98.6 |
|
|
yes |
12 |
1.4 |
|
Table 1: Socio-demographic and obstetrical characteristics of pregnant women attending the various CSPN in Djibouti.
|
Health center |
Ag HBs positif |
Ag HBs negatif |
|
Al-Rahma (n=86) |
4(4.6%) |
82(95.1%) |
|
Ambouli (n=95) |
5(5.3%) |
90(5.3%) |
|
CS2 (n=116) |
38(32.8%) |
78(67.2%) |
|
Einguela (n=101) |
11(10.9%) |
90(89.1%) |
|
Farahade (n=96) |
6(6.3%) |
90(93.7%) |
|
Hayabley (n=98) |
5(5.3%) |
95(96.9%) |
|
Ibrahim balala (n=94) |
6(6.1%) |
93(93.9%) |
|
Khorbourhan (n=99) |
6(6%) |
93(94%) |
|
Pk12 (n=97) |
4(4.1%) |
93(95.9%) |
|
Total |
82 |
880 |
Table 2: Distribution of HBsAg seroprevalence rate among women in the different health centers.
|
Characteristics |
Ag HBs négatif N=800 |
Ag HBs positif N=82 |
Total |
P-value |
|
Age (years) |
0,55 |
|||
|
< 30 |
458 (92.2%) |
39 (7.8%) |
497 (100%) |
|
|
≥ 30 |
339 (89.5%) |
40 (10.5%) |
379 (100%) |
|
|
Underminated |
3 (50%) |
3 (50%) |
6 (100%) |
|
|
Mode of delivery |
0,3 |
|||
|
Vaginal |
679 (91%) |
66 (8.9%) |
745 (100%) |
|
|
Cesarienne |
121 (88.3%) |
16 (11.7%) |
137 (100%) |
|
|
Occupational status |
<0.01 |
|||
|
Unemployed |
667 (92.5%) |
54 (7.5%) |
721(100%) |
|
|
Employed |
133 (82.6%) |
28 (17.4%) |
161 (100%) |
|
|
Parity |
<0,01 |
|||
|
Nulli or Primiparous 0-1 |
262(94.2%) |
16 (5.8%) |
270 (100%) |
|
|
Multiparous 2-3 |
306(91.6%) |
28(8.4%) |
262 (100%) |
|
|
Largemultiparous ≥4 |
232(85.9%) |
38(14.1%) |
270 (100%) |
|
|
Age of pregancy |
<0,01 |
|||
|
1er trimester |
222 (94.1%) |
14 (5.9%) |
236 (100%) |
|
|
2éme trimester |
320 (92.2%) |
27 (7,8%) |
347 (100%) |
|
|
3éme trimester |
258 (86.3%) |
41 (13.7%) |
299 (100%) |
|
|
Educational status |
<0.01 |
|||
|
university |
26 (65%) |
14 (35%) |
40 (100%) |
|
|
secondary |
167(87.9%) |
235(12.1%) |
190 (100%) |
|
|
primary |
88(91.6%) |
8(8.3%) |
96 (100%) |
|
|
Not educated |
519 (93.34%) |
37 (6.7%) |
556 (100%) |
|
|
Hospitalization with IntraVenous medication |
<0,01 |
|||
|
No |
609 (92%) |
53 (8%) |
662 (100%) |
|
|
Yes |
191 (86.8%) |
29 (13.2%) |
220 (100%) |
|
|
Surgical status |
0,02 |
|||
|
No |
644 (91.9%) |
57 (8.1%) |
701(100%) |
|
|
Yes |
156 (86.2%) |
25 (13.8%) |
181(100%) |
|
|
Abortion history |
<0.01 |
|||
|
No |
686 (92.7%) |
54 (7.3%) |
740 (100%) |
|
|
Yes |
114(82%) |
25(18%) |
139 (100%) |
|
|
Indeterminated |
0 |
3 |
3 |
|
|
Family history of HBV |
<0.01 |
|||
|
No |
739 (92.3%) |
45 (5.7%) |
784 (100%) |
|
|
Yes |
61 (62.2%) |
37 (37.8%) |
98 (100%) |
|
|
Blood transfusion |
<0.01 |
|||
|
No |
751 (91.9%) |
66 (8.1%) |
817 (100%) |
|
|
Yes |
49 (75.4%) |
16 (24.6%) |
65 (100%) |
|
|
Vaccination |
0,44 |
|||
|
No |
789 (90.69%) |
81 (9.31%) |
870 (100%) |
|
|
Yes |
11 (91.67%) |
1 (8.33%) |
12 (100%) |
|
|
P-value indicates association between infection in HBV and characteristic, p<0.05 was considered statistically significant (highlighted in bold) |
||||
Table 3: Bivariate analysis of factors associated with HBV infection among pregnant women consulting at the CSPN level in Djibouti city (N = 882).
|
Characteristics |
AOR |
95% CI |
p-value |
|
Educational status |
|||
|
not educated |
— |
— |
|
|
primary |
1.15 |
0.44-2.70 |
0.8 |
|
secondary |
2.37 |
1.17-4.72 |
0.01 |
|
university |
9.37 |
3.14-28.6 |
<0.01 |
|
Age of pregnancy |
|||
|
1re trimester |
— |
— |
|
|
2nd trimester |
1.66 |
0.77-3.75 |
0.2 |
|
3rd trimester |
3.29 |
1.57-7.38 |
<0.01 |
|
Parity |
|||
|
Nulli or Primiparous 0-1 |
— |
— |
|
|
Multiparous 2-3 |
1,87 |
0.824-4.23 |
0.13 |
|
Large multiparous ≥4 |
3.83 |
1.71-8.59 |
<0,01 |
|
Blood transfusion |
|||
|
no |
— |
— |
|
|
yes |
2.53 |
1.09-5.61 |
0.02 |
|
Family history of HBV |
|||
|
no |
— |
— |
|
|
yes |
8.00 |
4.40-14.6 |
<0.01 |
|
History of abortion |
|||
|
no |
— |
— |
|
|
yes |
2.08 |
1.03-4.12 |
0.03 |
|
AOR = Adjusted Odds Ratio, CI = Confidence Interval |
|||
Table 4: Multivariate analysis of factors associated with HBV infection among pregnant women attending the CSPN in Djibouti city (N = 882).
3.3 Bivariate analysis
Bivariate analysis of the association between seroprevalence and sociodemographic and obstetric characteristics showed that HBsAg seroprevalence was significantly associated with occupation (p<0.01), Parity (p<0.01), family history of HBV carriage (<0.001), blood transfusion (p<0.01), abortion (p<0.001), educational status (p<0,001), previous hospitalization (p<0.05), surgery (p<0.05) and pregnancy trimester (p<0.01). However, no significant association was observed with age group, vaccination, and mode of delivery. As stated in Table 4, candidates in the bivariate analysis were analyzed in the multivariate logistic regression.
3.4 Multivariate analysis
Logistic regression of the multivariate analysis showed that family history of HBV, level of education, 3rd trimester screening, parity, and abortion were predictive factors for HBV infection in pregnant women. Pregnant women with a family history of HBV were 8 times more likely to be infected (AOR=8; 95% CI: 4.40-14.6). Pregnant women with a higher level of education were also 9 times more likely to be infected (AOR=9.37; 95% CI: 3.14-28.6). Pregnant women with 3rd trimester screening and large multiparous are 3 times more likely to be infected. Finally, pregnant women with a history of blood transfusions (AOR=2.53; 95% CI: 1.09-5.61) and abortion (AOR=2.08; 95% CI: 1.03-4.12) had a 2-fold higher risk.
4. Discussion
Pregnant women infected with HBV but not screened constitute a real reservoir that facilitates the transmission of HBV, and the lack of epidemiological data is a real obstacle to the response. In Djibouti, the last publication on HBsAg carriage dates to 2005(9) , and to adapt and evaluate the hepatitis B control strategies in place, it was important to have recent data. The HBsAg seroprevalence among the participants in our study was 9.3%, with 82 positive patients out of 882 screened. This confirms that Djibouti remains an endemic area for HBV according to WHO criteria.
These seroprevalence results are similar to those obtained in blood donors at Peltier Hospital in Djibouti city, which was 10.4% (9). However, they are higher than those obtained by Abatte et al in 1986, with 7.4%. In countries bordering East Africa, HBV seroprevalence in pregnant women varies from 3 to 11% [11]. Lower results were found in Asmara 3.2% [12] and Kigali 4.3% [13]. However, prevalence much closer to our results was obtained in the Somali region of Ethiopia 8.03% [14], Dar el Salam 8.03% [15] and in Nairobi 9.3% [16]. Finally, a slightly higher prevalence than ours was noted in Juba 11% [17] and Uganda 11.8% [10]. Given the wide range of risk factors for HBV, these variations could be due to differences in cultural practices, sexual behaviors, sampling method, and/or laboratory testing methods used to detect HBsAg and the control strategies in place in different countries.
The seroprevalence was different between the CSPN, CS2 and Einguella had the highest prevalence. CS2 has a better technical platform and a respectable number of gynecologists who perform follow-up; prenatal checkups are free of charge and covered by the universal health insurance card which increases the frequency of consultations compared to the other sites. The Einguella center is in a neighborhood with a large Ethiopian and Yemeni refugee community, countries where HBV is highly endemic. Prenatal checkups are paid, around $20 for the 3 common tests (Complete Blood Count, blood sugar, HBV). Bivariate and multivariate analysis of risk factors associated with HBV, showed that the risk factors significantly associated with HBV among pregnant women attending CSPN in the city of Djibouti were parity, weeks of amenorrhea, blood transfusion, history of abortion, history of hepatitis B infection in the family, and level of education (being enrolled in school) are significantly associated with HBV infection.
However, sociodemographic and obstetric variables such as marital status, occupation, mode of delivery or age were not statistically associated with an elevated risk of HBV infection, which is in agreement with some studies conducted elsewhere [18]. However, other studies have reported that HBV infection is associated with older women [19]. This correlation is also a good indicator of vaccination when the date of vaccine introduction is known. In Djibouti, vaccination was introduced in 2008, so the first people to be vaccinated are still young and are not of childbearing age. Therefore, it would be essential to conduct another health survey (DHS) on HBV within 10 years.
Other obstetric variables, such as 3rd trimester and large-multiparous were significantly associated with the risk of HBV infection p<0.01). More than 34% (n=299) of pregnant women who presented for testing were in the third trimester of pregnancy. This result is similar to those obtained in Nigeria [20, 21], where pregnant women in the 2nd and 3rd trimesters of pregnancy had the highest HBV seroprevalence. It is, therefore, essential for midwives to advocate for screening during the first week of amenorrhea, which would facilitate management and limit the risk of perinatal transmission.
Higher education level was significantly associated with higher risk of HBV infection. This result contrasts with the many studies that found a significant association between low education and HBV infection [22, 23]. This discrepancy may reflect an evolution in the mode of transmission in society. It can suggested that the subject with a high level of education presents a suitable living environment, and at the same time, a more open sexual behavior (several sexual partners), which increases the risk of contamination [24].
The present study also shows that pregnant women with a history of blood transfusion were 2 times more likely to be infected (p=0.026). Numerous studies in Cameroon [25], Nigeria [26] Sudan [27] and Uganda [28] have reported high frequencies of infection in pregnant women with a history of blood transfusion. Blood transfusion being known as a potential risk factor for HBV transmission. The significant association between HBV infection and blood transfusion could be explained by a lack of HBV screening among blood donors in Djibouti. However, this interpretation is not appropriate in the context of Djibouti, where screening for HIV, HBV, HCV, and Syphilis has been systematic for all blood donors since 2002 but could be related to occult hepatitis.
In our study, we found that pregnant women with a history of abortion had a higher risk of infection compared to those who had never had an abortion. This result was consistent with the findings of other studies in Africa [29, 30]. These risks can be attributed to unprotected sex or clandestine abortions. Considering confidentiality, information on sexual practices was not included in this study. Finally, a family history of hepatitis B was statistically associated with a high risk of infection. Pregnant women with a family history of hepatitis B (father, mother, husband, brother, and sister) were 8 times more likely to be infected (p<0.001). These results corroborate previous studies in Egypt [31] and Ethiopia [32] that also found an association between infection and family history of HBV. These argued for horizontal intrafamilial or inter-child transmission during childhood and adolescence. Early screening of pregnant women and vaccination of all persons living in the same household as the infected individual and his/her spouse would be an excellent means of control.
5. Conclusion
Our study revealed a high rate of HBV seroprevalence among pregnant women attending CSPN in the city of Djibouti, which classifies it as a highly endemic area according to the WHO classification. Pregnant women with a history of HBV in their families, multiparous women, third trimesters, and abortion were associated with infection. Thus, it would be important to establish collaboration between the different stakeholders. For example, better awareness of the risks of transmission, early systematic screening in the first trimester of pregnancy, and extended vaccination to family contacts of HBV-infected patients. The main risk factors identified in this work should alert the relevant agencies to the need for a national HBV control program.
Ethical Considerations
This study was approved by the Djibouti “Comité Ethique National pour la Recherche en Santé” (Ethics Committee for Health Research) from the Ministry of Health of Djbouti with the number N=150/DG/INSPD/2023, and an informed consent form was completed and signed by each participant. The results of the HBsAg tests were communicated to each participant in the study.
References
- Hepatitis B [Internet] (2023).
- MacLachlan JH, Cowie BC. Hepatitis B Virus Epidemiology. Cold Spring Harbor Perspectives in Medicine. 1 mai 5 (2015): a021410-a021410.
- Nyamusi M, Marete O, Waweru W. Seroprevalence of hepatitis B among pregnant women in Kigali, Rwanda. Int J Community Med Public Health (2016): 3096-3101.
- Kebede KM, Abateneh DD, Belay AS. Hepatitis B virus infection among pregnant women in Ethiopia: a systematic review and Meta-analysis of prevalence studies. BMC Infect Dis 18 (2018): 322.
- Nguyen MH, Wong G, Gane E, et al. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin Microbiol Rev. 18 mars 33 (2020): e00046-e00019.
- Slowik MK, Jhaveri R. Hepatitis B and C Viruses in Infants and Young Children. Seminars in Pediatric Infectious Diseases 16 (2005): 296-305.
- Eyong EM, Yankam BM, Seraphine E, et al. The prevalence of HBsAg, knowledge and practice of hepatitis B prevention among pregnant women in the Limbe and Muyuka Health Districts of the South West region of Cameroon: a three-year retrospective study. Pan Afr Med J (2022): 32.
- Fox E, Abhalte EA, Said-Salah, et al. Viral hepatitis markers in Djibouti: an epidemioiogical survey. Transactions of the Royal Society of Tropical Medicine and Hygiene (1988): 750-752.
- Dray X, Dray-Spira R, Bronstein JA, et al. [Prevalences of HIV, hepatitis B and hepatitis C in blood donors in the Republic of Djibouti]. Med Trop (Mars) 65 (2005): 39-42.
- Bayo P, Ochola E, Oleo C, et al. High prevalence of hepatitis B virus infection among pregnant women attending antenatal care: a cross-sectional study in two hospitals in northern Uganda. BMJ Open (2014): e005889.
- Kassaw B, Abera N, Legesse T, et al. Sero-prevalence and associated factors of hepatitis B virus among pregnant women in Hawassa city public hospitals, Southern Ethiopia: Cross-sectional study design. SAGE Open Med (2022): 20503121221140776.
- Fessehaye N. Prevalence of Hepatitis B Virus Infection and Associated Seromarkers among Pregnant Women in Eritrea (2018).
- Muvunyi CM, Habtu M, Nyamusi MM, et al. Factors Associated with Hepatitis B Surface Antigen Seropositivity among Pregnant Women in Kigali, Rwanda: A Cross Sectional Study. J Comm Pub Health Nurs [Internet] (2022): 03.
- Roble AK, Roba KT, Mengistie B, et al. Seroprevalence of Hepatitis B Virus and Associated Factors Among Pregnant Women Attending Antenatal Care in Public Health Facilities in Jigjiga Town, Eastern Ethiopia. IJWH. janv 12 (2021): 1299-1310.
- Manyahi J, Msigwa Y, Mhimbira F, et al. High sero-prevalence of hepatitis B virus and human immunodeficiency virus infections among pregnant women attending antenatal clinic at Temeke municipal health facilities, Dar es Salaam, Tanzania: a cross sectional study. BMC Pregnancy Childbirth 17 (2017): 109.
- Okoth F, Mbuthia J, Gatheru Z, et al. Seroprevalence of Hepatitis B markers in pregnant women in Kenya. E Af Med Jrnl (2022): 83.
- Stephen Kirbak AL, Ng’ang’a Z, Omolo J, et al. Sero-prevalence for Hepatitis B virus among pregnant women attending antenatal clinic in Juba Teaching Hospital, Republic of South Sudan. Pan Afr Med J (2022): 26.
- Vázquez-Martínez JL, Coreño-Juárez MO, Montaño-Estrada LF, et al. Seroprevalence of hepatitis B in pregnant women in Mexico. Salud pública Méx. juin 45 (2003): 165-170.
- Awole M, Gebre-Selassie S. Seroprevalence of HBsAg and its risk factors amoung pregnant women in Jimma, Southwest Ethiopia. Ethiopian Journal of Health Development (2005): 45-50.
- Ndams IS, Joshua IA, Luka SA, et al. Epidemiology of Hepatitis B infection among pregnant women in Minna, Nigeria. Science World Journal (2010): 3.
- Yakasai I, Abubakar I, Ayyuba R, et al. Sero-prevalence of hepatitis B virus infection and its risk factors among pregnant women attending antenatal clinic at Aminu Kano teaching hospital, Kano, Nigeria. J Basic Clin Reprod Sci 1 (2012): 49.
- Eke AC, Eke UA, Okafor CI, et al. Prevalence, correlates and pattern of hepatitis B surface antigen in a low resource setting. Virol J 8 (2011): 12.
- Bayo P, Ochola E, Oleo C, et al. High prevalence of hepatitis B virus infection among pregnant women attending antenatal care: a cross-sectional study in two hospitals in northern Uganda. BMJ Open 4 (2014): e005889.
- Stroffolini T, Sagnelli E, Sagnelli C, et al. The burden of HBV infection in HCV patients in Italy and the risk of reactivation under DAA therapy. Digestive and Liver Disease 51 (2019): 434-437.
- Noubiap JJN, Nansseu JRN, Ndoula ST, et al. Prevalence, infectivity and correlates of hepatitis B virus infection among pregnant women in a rural district of the Far North Region of Cameroon. BMC Public Health 15 (2015): 454.
- Adewole OO, Anteyi E, Ajuwon Z, et al. Hepatitis B and C virus co-infection in Nigerian patients with HIV infection. J Infect Dev Ctries 3 (2009): 369-375.
- Mohammed Hammad Abuelgasim, Mohammed Basheer Koko Baraka. Prevalence of Hepatitis B Infection among Pregnant Women at Khartoum Teaching Hospital, Sudan. UCMS (2022): 12.
- Chiesa A, Ochola E, Oreni L, et al. Hepatitis B and HIV coinfection in Northern Uganda: Is a decline in HBV prevalence on the horizon? Blackard J, éditeur. PLoS ONE 15 (2020): e0242278.
- Yohanes T, Zerdo Z, Chufamo N. Seroprevalence and Predictors of Hepatitis B Virus Infection among Pregnant Women Attending Routine Antenatal Care in Arba Minch Hospital, South Ethiopia. Hepatitis Research and Treatment 2016 (2016): 1-7.
- Bafa TA, Egata AD. Seroepidemiological patterns and predictors of hepatitis B, C and HIV viruses among pregnant women attending antenatal care clinic of Atat Hospital, Southern Ethiopia. SAGE Open Medicine 8 (2020): 205031211990087.
- EL-Shabrawi M. Prevalence of Hepatitis B Virus Infection among Egyptian Pregnant Women - A Single Center Study. IJTDH 3 (2014): 157-168.
- Molla S, Munshea A, Nibret E. Seroprevalence of hepatitis B surface antigen and anti HCV antibody and its associated risk factors among pregnant women attending maternity ward of Felege Hiwot Referral Hospital, northwest Ethiopia: a cross-sectional study. Virol J 12 (2015): 204.