SARS-CoV-2 Induced CTPA Proven Pulmonary Embolism: A Case Report
Article Information
Muhammad Hanif1*, Abdul Wali Khan1, Muhammad Ishaq2, FNU Sundas1, Masroor Anwar1, Sidra Naz3
1Department of Internal Medicine, Hayatabad Medical Complex, Peshawar, Pakistan
2Department of Internal Medicine, Khyber Teaching Hospital, Peshawar, Pakistan
3Department of Internal Medicine, University of Health & Sciences, Lahore, Pakistan
*Corresponding author: Muhammad Hanif, Department of Internal Medicine, Hayatabad Medical Complex, Peshawar, Pakistan
Received: 01 November 2020; Accepted: 10 November 2020; Published: 20 November 2020
Citation:
Muhammad Hanif, Abdul Wali Khan, Muhammad Ishaq, FNU Sundas, Masroor Anwar, Sidra Naz. SARS-CoV-2 Induced CTPA Proven Pulmonary Embolism: A Case Report. Archives of Internal Medicine Research 3 (2020): 230-234.
Share at FacebookAbstract
SARS-CoV-2 causing coronavirus disease 2019 emerged in Wuhan, China in December 2019 as a cluster of atypical pneumonia and now it has been declared a pandemic by the World Health Organization and has caused the death of approximately one million people. Coagulopathy has been found in association with COVID-19 due to inflammation, endothelial damage, hypoxia, and diffuse intravascular coagulation (DIC). Here, we discussed a 60-year-diabetic, hypertensive female who was initially presented as a case COVID-19 and was later on diagnosed as pulmonary embolism secondary to COVID-19.
Keywords
COVID-19, SARS-CoV-2, Pulmonary Embolism, Coagulopathy
Article Details
1. Introduction
COVID-19 causing by SARS-CoV-2 first emerged in Wuhan, China as a cluster of atypical pneumonia and now it has caused approximately one million death around the globe. COVID-19 patients commonly present as fever, headache, dry cough, and fatigue but various neurological, myocardial, renal, and gastrointestinal complications have been reported in association with it [1, 2]. Coagulopathy is a common abnormality in COVID-19 patients due to inflammation, immobility, hypoxia, endothelial damage, and DIC [3, 4]. Pulmonary embolism has been reported in the literature in association with SARS-CoV-2 [5, 6]. Similarly, we have presented a case of a 60-year-female who was initially diagnosed as a case of COVID-19 and was later on found to have pulmonary embolism secondary to SARS-CoV-2 infection.
2. Case Report
A 60-year-female diabetic, the hypertensive patient was brought into the Emergency Department with one day history of fever, fatigue, and dry cough. On examination, her blood pressure was 110/70, her pulse rate was 110/min, her temperature was 101F, and oxygen saturation was 84 % on room air. Chest examination showed B/L crepitation. Baseline investigations and nasopharyngeal swab tests on quantitative reverse-transcriptase-polymerase-chain reaction (qRT-PCR) for SARS-CoV-2 infection were sent for analysis. She was admitted to the isolation unit as a suspected case of COVID-19 and was started on injectable azithromycin, ceftriaxone, oxygen inhalation, and 40mg subcutaneous enoxaparin 40mg once a day. Baseline investigations showed a raised inflammatory marker along with a high level of total leukocyte count (Table 1).
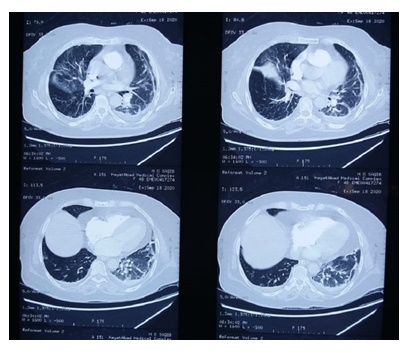
The nasopharyngeal swab test for COVID-19 resulted positive on quantitative reverse-transcriptase-polymerase-chain reaction (qRT-PCR) assay. A chest x-ray was done which showed mild interstitial infiltrate and features suggested of pulmonary embolism. In order to confirm chest x-ray findings, computed tomography pulmonary angiography (CTPA) was done which showed filling defects in the left main pulmonary artery (Figure 1). The dose of enoxaparin was increased from 40mg once a day to 60mg twice a day. Thrombophilia screen and anti-neutrophilic antibodies were sent for analysis, the result of which were negative (Table 2). Also, there were no signs of deep venous thrombosis (DVT) on doppler ultrasound. She was diagnosed as case of pulmonary embolism secondary to SARS-CoV-2 infection. Her condition got improved and she was discharged on home medications after one week and was advised follow up after 2 weeks.
|
Test |
Result |
|
Hemoglobin |
12.1 g/dL |
|
Total lymphocyte count |
14.2(x109/l) |
|
RBC |
4.6 (x1012/l) |
|
Platelets |
433x109/l |
|
Prothrombin time |
18 seconds (12 seconds control) |
|
Activated partial thromboplastin time |
37 seconds (28 seconds control) |
|
Blood urea |
47mg/dL |
|
Creatinine |
1.1 |
|
Sodium |
134 mEq/l |
|
Potassium |
4.2 mEq/l |
|
D-dimer |
2740 ng/FEUmL (reference value: upto 500 ng/FEUmL) |
|
C-Reactive Protein |
67mg/dl |
|
Lactate dehydrogenase (LDH) |
825 U/L |
|
Serum Ferritin level |
1835.3 µg/L |
Table 1: Laboratory Findings.
|
Factors |
Patient Value |
Normal Ranges |
|
Factor V leiden Screening |
1.05 ratio |
0.8-1.1 ratio |
|
Anti-thrombin 3 level |
108% |
75-125% |
|
Protein C |
130 % |
69-140 % |
|
Free Protein S |
92% |
67-140% |
|
ANA |
Negative (titer <1:80) |
Titer <1:80 |
Table 2: Thrombophilia screen & ANA results.
3. Discussion
As of October 1, 2020, there are now more than 30 million people affected by COVID-19, with more than one million deaths, as announced by the world health organization [7]. Multiple risk factors have been associated with poor prognosis and increase mortality, such as previous cardiovascular illnesses, immunodeficiencies, obesity, aged population, diabetes, and other metabolic conditions [8]. Initially, it was thought that COVID-19 infection is solely the disease of the respiratory system, but with time it is now clear that this virus can affect multiple organ systems [8]. The most common manifestation is fever (88.7%), followed by other signs and symptoms such as dry cough (67.8%), nausea & vomiting (5%), and diarrhea (3.8%) [9]. In approximately 15% cases, the COVID-19 is complicated by severe pneumonia leading to acute respiratory distress syndrome, acute kidney injury, DIC, and even death [9]. One of the most important manifestations of COVID-19 is thrombi formation, ranging from small vessel involvement to major vessel involvement such as the femoral artery and cerebral artery. Multiple manifestations resulting from thrombi formation have been reported, such as acute limb ischemia, massive ischemic strokes, mesenteric artery thrombosis, and acute myocardial infarction [10]. One study demonstrated the prevalence of pulmonary embolism in inpatient COVID-19 patients to be 8.3%, but most of the thrombi were small in size [11]. Only a few cases of CTPA proven massive pulmonary embolism have been reported so far.
To our understanding, this is one of the few cases of CTPA proven pulmonary embolism. In this patient, a 60-year-old diabetic female with no known major risk factors for thrombophilia, her sentinel event was preceded by signs and symptoms of SARS-CoV-2 infection; such as fever, dry cough, and fatigue. She also had a decreased level of consciousness but there was no sign of DVT and echocardiography showed no evidence of any cardiac source of thrombi. Thus, indicating that the primary site of thrombosis was the pulmonary artery. In this patient, pulmonary embolism was diagnosed within 48 hours of admission which is in line with other studies performed on different populations [11]. According to one study, the elevated levels of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), which are inflammatory markers might be associated with hyper-coagulation leading to vascular thrombosis. In our patient, the level of CRP, D-dimers, and ESR was markedly raised thus indicating some role in the pathogenesis of vascular thrombosis. The mortality rate from pulmonary embolism depends on the size of the embolus and it varies from one clinical setup to another. A study conducted in France reported that the mortality rate associated with pulmonary embolism was 12.2% [11]. In our case, the patient improved after receiving standard therapy and she is currently in follow-up.
4. Conclusions
Pulmonary embolism is a common clinical entity and it presents with various manifestations ranging from asymptomatic condition to shock. SARS-CoV-2 being notorious for coagulopathy can results in this entity. The physicians should keep in mind about the possible coagulopathy complications because early diagnosis and intervention can prevent hazard outcomes.
References
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (2020): 497-506.
- Chen L, Liu HG, Liu W, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 43 (2020): 203-208.
- Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 58 (2020): 1116-1120.
- Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368 (2020): m1091.
- Grillet F, Behr J, Calame P, et al. Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected with Pulmonary CT Angiography. Radiology 296 (2020): E186-E188.
- Ullah W, Saeed R, Sarwar U, et al. COVID-19 Complicated by Acute Pulmonary Embolism and Right-Sided Heart Failure. JACC Case Rep 2 (2020): 1379-1382.
- Coronavirus Update (Live): 34,323,750 Cases and 1,021,165 Deaths from COVID-19 Virus Pandemic - Worldometer [Internet]. Worldometers.info (2020).
- Singh SP, Pritam M, Pandey B, et al. Microstructure, pathophysiology, and potential therapeutics of COVID-19: A comprehensive review. J Med Virol (2020).
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382 (2020): 1708-1720.
- Ahmed S, Zimba O, Gasparyan AY. Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow's triad. Clin Rheumatol 39 (2020): 2529-2543.
- Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J 41 (2020): 3058-3068.