Role of Sodium-Glucose Cotransporter Inhibitors in the Management of Heart Failure: A Position Statement
Article Information
PP Mohanan1, Vijay Kumar Chopra2, Rakesh Yadav3, Jamshed Dalal4, Mrinal Kanti Das5, Saumitra Ray6, Harikrishnan P7, Prafulla Kerkar8*
1West Fort Hi-Tech Hospital, Thrissur, Kerala, India
2Max Super Speciality Hospital, No. 1, 2, Press Enclave Road, Mandir Marg, Saket Institutional Area, Saket, New Delhi, Delhi, India
3Department of Cardiology, Cardiac Thoracic Sciences Center, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India
4Kokilaben Dhirubhai Ambani Hospital and Medical Research Institute, Rao Saheb, Achutrao Patwardhan Marg, Four Bungalows, Andheri West, Mumbai, Maharashtra, India
5C.K. Birla Hospitals, 1,1, National Library Ave, Alipore, Kolkata, West Bengal, India
6Heart Clinic. 99/5/C, Ballygunge Place, Kolkata, West Bengal, India
7Apollo Speciality Hospital, Plot no 64, Vanagaram, Ambattur Road, Ayanambakkam, Chennai, Tamil Nadu, India
8Asian Heart Institute G/N Block, Bandra-Kurla Complex, Bandra(E), Mumbai, Maharashtra, India
*Corresponding author: Dr. Prafulla Kerkar, Asian Heart Institute, G/N Block, Bandra-Kurla Complex, Bandra(E), Mumbai, Maharashtra, India.
Received: 17 February 2023; Accepted: 22 May 2023; Published: 20 June 2023
Citation: PP Mohanan, Vijay Kumar Chopra, Rakesh Yadav, Jamshed Dalal, Mrinal Kanti Das, Saumitra Ray, Harikrishnan P, Prafulla Kerkar. Role of Sodium-Glucose Cotransporter Inhibitors in the Management of Heart Failure: A Position Statement. Cardiology and Cardiovascular Medicine. 7 (2023): 188-197.
Share at FacebookAbstract
Heart Failure (HF) is a chronic debilitating condition, which affects millions globally. India has recorded the second-highest increase in the number of HF cases worldwide, along with the second-highest 1-year mortality outcome for HF. Despite significant progress in the management of HF, the mortality and morbidity rates remain unacceptably high, which indicates unmet needs in HF management. Accumulating evidence has established consistent cardiovascular and renoprotective effects of sodium–glucose cotransporter 2 inhibitors (SGLT2i), irrespective of diabetes status. The overall safety and benefits of SGLT2i in the context of HF hospitalization and mortality have been acknowledged by recent guidelines. The benefits of SGLT2i have been established for HF persons with reduced ejection fraction (HFrEF). Consequently, SGLT2i including dapagliflozin and empagliflozin has been positioned among the first-line therapy in the treatment algorithm for HFrEF by the European Society of Cardiology 2021 guidelines. Recent findings indicate similar benefits of SGLT2i in HF persons with preserved ejection fraction (HFpEF). Moreover, SGLT2i fulfills the criteria for a potential therapeutic option for cardiorenal syndrome. Therefore, SGLT2i is a strong candidate to be positioned as a potent disease-modifying therapy for HF with wide acceptance by clinicians.
Keywords
SGLT2 inhibitors; Heart failure; HFrEF; Cardiovascular outcomes; Cardiorenal syndrome
SGLT2 inhibitors articles; Heart failure articles; HFrEF articles; Cardiovascular outcomes articles; Cardiorenal syndrome articles
Article Details
Acknowledgment:
The authors would like to thank AstraZeneca Pharma India Ltd for providing medical writing assistance in the development of this manuscript, in collaboration with BioQuest Solutions in accordance with GPP3 guidelines (http://www.ismpp.org/gpp3).
Conflict of Interest:
There are no conflicts of interest.
Funding:
The Medical Writing work was supported by AstraZeneca Pharma India Ltd.
Abbreviations:
ACC: American College of Cardiology; ACEi: angiotensin-converting enzyme inhibitors; ADA: American Diabetes Association; ARB: Angiotensin II receptor blockers; ARNI: angiotensin receptor neprilysin inhibitor; CANVAS: Canagliflozin Cardiovascular Assessment Study; CREDENCE: Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy; CKD: chronic kidney disease; CRT-D: Cardiac resynchronization therapy with a defibrillator; CRT-P: Cardiac resynchronization therapy with a pacemaker; CV: cardiovascular; CVOTs: cardiovascular outcome trials; DAPA-CKD: Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease; DAPA-HF: Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; DEFINE-HF: Dapagliflozin Effect on Symptoms and Biomarkers in Patients With Heart Failure; DECLARE-TIMI 58: Dapagliflozin Effect on Cardiovascular Events; EF: ejection fraction; eGFR: estimated glomerular filtration rate; EMPA-REG OUTCOME: Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; EMPEROR-Reduced: Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction; ESC: European Society of Cardiology; GDMT: guideline-directed medical therapy; HF: Heart failure; HHF: Hospitalization for heart failure; HFimpEF: HF with improved EF; HFrEF: HF patients with reduced ejection fraction; HFpEF: in HF patients with preserved ejection fraction; HR: hazard ratio; ICD: Implantable cardioverter-defibrillator; LVEF: Left ventricular ejection fraction; MRA: mineralocorticoid receptor antagonists; MACE: Major adverse cardiovascular events; NYHA: New York Heart Association; QRS: Q, R, and S waves (on a 12-lead electrocardiogram); SCORED: Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk; SOLOIST-WHF: Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure; SGLT2i: sodium-glucose cotransporter 2 inhibitors; SR: Sinus rhythm; T1DM: Type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus; UTIs: urinary tract infections; VERTIS-CV: Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial; c : Nominal p value
Introduction
Chronic heart failure (HF) is a complex syndrome that is characterized by the compromised circulatory function of the heart resulting from functional and/or structural alterations [1] The prevalence and health loss burden associated with HF are increasing worldwide, especially in low-to–middle sociodemographic index regions and in the older population [2]. At present, the global prevalence of HF is 64.34 million [2].
Burden of Heart Failure in India
In India, the prevalence of HF is approximately 1%, and the mortality rate associated with HF is 0.1–0.16 million cases per year [3]. Annually, 1.8 million hospitalizations are attributable to HF in India with HF being one of the commonest reasons for hospitalization among the older people [4]. India has the second-highest 1-year mortality outcome for HF (23%) [5] Persons with HF are categorized based on their symptoms and ejection fraction (EF); HF with preserved EF (HFpEF; EF ≥50%), HF with midrange EF (HFmrEF; EF in the range of 40%–49%; the recent recommendation is to denote HFmrEF as mildly reduced rather than midrange), and HF with reduced EF (HFrEF; EF <40%) [6]. A study from India has shown that in persons with HFrEF, the 1-year all-cause mortality rate is substantially high (17.6%) [7]. Chronic HF, particularly HFrEF, is associated with worse prognosis and high hospitalization and mortality rates, even in developing countries like India [8]. A new category has been introduced by the recent position paper by the European Society of Cardiology (ESC), HF with improved EF (HFimpEF), defined as an initial EF of 40% or below with an improvement by 10 points with a final EF above 40% [9]
Unmet Needs of HF Management
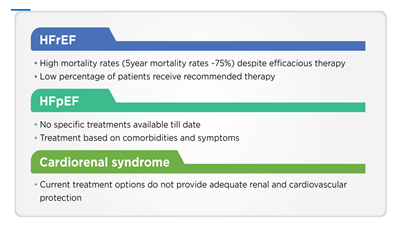
Although over the past three decades significant progress has been made in HF management, it continues to be a major cause of morbidity and mortality even in developed countries causing a significant impact on the economic burden of the healthcare system [10]. The therapeutic management of HF includes beta-blockers, angiotensin-converting enzyme inhibitors (ACEi), and mineralocorticoid receptor antagonists (MRA) [11]. Despite such interventions, the high rates of HF-related hospitalization and mortality imply that there are some unmet needs in the current HF management [11]. One of the unmet needs is the requirement of new therapies for HF subtypes wherein current therapies potentially relieve symptoms but fall short of addressing the disease [12]. Although the current interventions are associated with reduced mortality in persons with HFrEF, the prognosis remains poor [6]. Further, a large portion of persons with HFrEF do not receive the guideline-directed medical therapy (GDMT) even if there are no contraindications; in cases where they receive it, doses may not be as recommended by guidelines [6]. Therefore, there is an unmet need for disease-modifying therapies in persons with HFrEF, which can positively affect the person’s well-being without having dose-limiting side effects [6]. The current unmet needs in HF, including HFrEF, HFpEF, and cardiorenal syndrome, are presented in Figure 1.
HFpEF: Heart failure with preserved ejection fraction; HFrEF: Heart failure with reduced ejection fraction.
SGLT2i in HF: Transition From Diabetes to HF Realm
The glucose-lowering drug, sodium–glucose cotransporter 2 inhibitors (SGLT2i) acts by blocking the SGLT2 protein in the proximal convoluted tubule, which reabsorbs nearly 90% of filtered glucose [13.] Recent findings indicate that SGLT2i have substantial cardiovascular (CV) risk reduction potential [13]. This notion emanated from the recent landmark clinical trials, wherein besides improving glycemic control, SGLT2i also lowered CV events and hospitalization for HF (HHF) in persons with type 2 diabetes mellitus (T2DM). Notably, the CV clinical benefits were also consistently observed in persons with HF even in the absence of T2DM [13]. With such multifaceted effects, SGLT2i have made a promising transition from the realm of antidiabetes therapy into a new role as a therapeutic intervention for HFrEF[14].
Initial Evidence of Benefits of SGLT2i in HF: Findings From CVOTs in Persons With T2DM
SGLT2i have demonstrated lowering effects on CV events in multiple cardiovascular outcome trials (CVOTs) [13]. In terms of CV benefits, SGLT2i have demonstrated consistently improved outcomes related to HF. Particularly, the reduction in HHF within months of randomization indicated a separate cardioprotective mechanism of SGLT2i compared to those of other antidiabetic medications, where such CV benefits take years to manifest [13]. The first CVOT to demonstrate CV benefits of SGLT2i was the EMPA-REG OUTCOME study wherein empagliflozin lowered hospitalization for worsening HF among persons with T2DM who were at a high risk of CV events [14,15]. In subsequent CVOTs involving other SGLT2i, that is, canagliflozin in the CANVAS study and dapagliflozin in the DECLARE-TIMI 58 study, consistent reduction in HHF was observed among persons with T2DM managed with SGLT2i [14,16,17].
SGLT2i in HF: Possible Mechanisms of Action
The probable mechanisms of action of SGLT2i have been enlisted below:
- Diuretic and antihypertensive effects: SGLT2i exert antihypertensive effects by lowering plasma volume and improving endothelial function, which leads to the reduction in blood pressure and afterload [13]. However, these effects of SGLT2i are nonsignificant and possibly are not related to their CV benefits [18]. Endocardial blood flow is improved by the reduction of cardiac afterload [19,20]. Furthermore, through its diuretic effects, SGLT2i cause a reduction in ventricular preload, which improves ventricular loading conditions [13]. As opposed to conventional diuretics that selectively reduce the intravascular volume, SGLT2i reduce extravascular volume preferentially, thus minimizing the chances of postural hypotension or prerenal failure.
- Weight reduction and improved glycemic control: SGLT2i cause weight loss through increased glucagon:insulin ratio that increases lipid mobilization and is accountable for lowered HF mortality effect associated with SGLT2i [13,20-22].
- Increase in hematocrit: SGLT2i have been associated with an increase in red blood cell mass, renal erythropoietin production, and hematocrit, which lead to improved CV outcomes [13,23]. Besides directly increasing myocardial tissue oxygen delivery, an increase in erythropoietin production favorably influences cardiomyocyte cell proliferation, mitochondrial function, angiogenesis, and inflammation [18].
- Direct cardiac effects: In HF, inflammation, fibrosis, and cardiac hypertrophy are the causes of adverse cardiac remodeling that increases the severity of the condition. It has been proposed that SGLT2i have a role in reversing the adverse cardiac remodeling, thereby improving HF outcomes [13,22].
- Improved cardiac metabolism and myocardial energetics: Due to increased urinary glucose excretion, there is increased production of ketone bodies, which provide a fuel source alternative to glucose and fatty acids, leading to increased ATP production and improved cardiac efficiency and output [13,21,23,24].
- Improved myocardial ionic homeostasis: SGLT2i improve cardiac contractility by reversing calcium overload through inhibition of SGLT1 and sodium hydrogen exchanger 1 expression, which lowers intracytosolic sodium content [13].
- Autophagy: Autophagy induction has favorable effects on HF. It has been postulated that SGLT2i induces autophagy through periods of increased catabolism resulting from constant glucosuria [13].
- Altered adipokine regulation: Altered adipokine regulation results in an epicardial fat deposition that leads to the development of HF. SGLT2i imparts cardioprotective effects through increased serum adiponectin and lowered leptin concentrations [13]. SGLT2i restores the balance between inflammatory and anti-inflammatory adipokines, thereby regulating cardiac inflammation and fibrosis [24].
- Left ventricular remodeling: Left ventricular (LV) remodeling is a key contributor to HF. SGLT2i may have beneficial effects on multiple pathways involved in LV remodeling, such as inflammation, hypertrophy, cardiomyocyte cell death, and increased production of extracellular matrix [19,20].
Benefits of SGLT2i in HFrEF
The recent CVOTs demonstrated that SGLT2i contributed to improved CV outcomes in persons with T2DM and reduced the risk of HHF by 30%–35% [13,25]. Subsequently, the DAPA-HF, the SOLOIST-WHF, and the EMPEROR-Reduced trials were conducted in persons who were primarily diagnosed with HF, either with or without T2DM [26–28]. Consistent CV outcomes were noted in EMPEROR-Reduced and DAPA-HF studies, which involved persons with HFrEF [26,28]. A meta-analysis of these two trials demonstrated that SGLT2i were associated with a 25% decrease in the composite of recurrent HHF or CV death (p<0·0001), and a reduction in the combined risk of first HHF or CV related death by 26% (p<0·0001) [29]. According to a post hoc analysis of the DAPA-HF trial, when added to conventional therapies, such as diuretics, ACEi, angiotensin receptor-neprilysin inhibitors (ARNI), ARB, beta-blockers, or MRA, dapagliflozin lowered the risk of cardiac arrest, sudden death, or any serious ventricular arrhythmia in persons with HFrEF [30]. A meta-analysis on the efficacy and safety of SGLT2i in 16,820 persons with HF (including three trials related to HF-SOLOIST-WHF, EMPEROR-Reduced, and DAPA-HF, —and four CVOTs—CANVAS, DECLARE-TIMI 58, EMPA-REG OUTCOME, and the VERTIS-CV) revealed that SGLT2i significantly reduced the risk of HF hospitalization in persons with HF [15–17,26–28,31,32]. Further, a beneficial trend was observed in persons with HFpEF for the composite of CV death and HHF [31]. The findings remained consistent even in persons with HFrEF, both with or without the presence of T2DM [31]. These findings implied that all persons with HF might benefit from SGLT2i, irrespective of diabetes status [31]. In another meta-analysis involving seven studies with 14,113 persons with HF (including 58.9% persons with co-existing T2DM), a subgroup analysis of persons with HF without T2DM revealed favorable CV and HHF outcomes of SGLT2i as compared to placebo. These findings affirmed that the HF benefits of SGLT2i were independent of its antidiabetic effects [25]. The key trials of SGLT2i are summarized in Table 1.
|
Study name |
No. of participants |
Study design |
Persons with HF |
Mean eGFR (mL/min/ 1.73 m2) |
Follow-up (mean/median) |
Study outcomes |
|
EMPA-REG (Empagliflozin) [15] |
7020 |
Empagliflozin 10 mg, 25 mg vs. placebo |
HF (unspecified): 706 (10.1%) |
74 |
3.1 years |
Higher reduction in CV mortality, nonfatal MI, or nonfatal stroke compared to placebo (10.5% vs. 12.1%) |
|
CANVAS (Canagliflozin) [16] |
10142 |
Canagliflozin 100 mg, 300 mg vs. placebo |
HF (unspecified): 1461 (14.4%) |
76.5 |
188 weeks |
Lower risk of CV events compared to placebo |
|
DECLARE-TIMI (Dapagliflozin) [17] |
17160 |
Dapagliflozin 10 mg vs. placebo |
HFrEF: 671 (3.9%) |
85.2 |
4.2 years |
Decreased risk of MACE (8.8% vs. 9.4%), CV death and HHF (4.9% vs. 5.8%) compared to placebo |
|
VERTIS-CV (Ertugliflozin) [32] |
8246 |
Ertugliflozin 5 mg, 15 mg vs. placebo |
HF (unspecified): 23.4% |
76 |
3.5 years |
Noninferior to placebo in terms of MACE |
|
DAPA-HF (Dapagliflozin) [26] |
4744 |
Dapagliflozin 10 mg vs. placebo |
HFrEF: 4744 |
65.8 |
18.2 months |
Reduced risk of worsening HF or death from CV causes; outcomes similar in persons with or without T2DM |
|
EMPEROR-Reduced (Empagliflozin) [28] |
3730 |
Empagliflozin 10 mg vs. placebo |
HFrEF: 3730 |
62 |
16 months |
Reduced composite of CV death or HHF; irrespective of diabetes status |
|
SOLOIST-WHF (Sotagliflozin) [27] |
1222 |
Sotagliflozin 200 mg (increased up to 400 mg) vs. placebo |
1222 (100%) |
49.7 (median) |
9 months |
Reduced rate of death from CV causes (10.6 vs. 12.5) and rate of death from any cause (13.5 vs 16.3) compared to placebo |
|
CV: Cardiovascular; HHF: Hospitalization for heart failure; HF: Heart failure; HFpEF: Heart failure with preserved ejection fraction; HFrEF: Heart failure with reduced ejection fraction; MACE: Major adverse cardiovascular events; T2DM: Type 2 diabetes mellitus; |
||||||
Table 1: Summary of key trials of SGLT2i33
Does SGLT2i Provide Mortality Benefit?
The CVOT data for SGLT2i demonstrated an unanticipated improvement in CV endpoints. Subsequently, the DAPA-HF trial showed that SGLT2i reduced all-cause mortality in patients with HFrEF (dapagliflozin vs. placebo: 11.6% vs. 13.9% hazard ratio [HR]: 0.83; 95% confidence interval [CI]: 0.71–0.97), which is comparable to that of the standard guideline-recommended HFrEF medical therapies [26,34]. Notably, death from CV causes was significantly lower in dapagliflozin group compared to placebo (9.6% vs 11.5%; HR: 0.82; 95% CI, 0.69 to 0.98; p<0.001) [26]. The DAPA-HF data showed significant cardiovascular mortality benefit with dapagliflozin (18% reduction; HR: 0.82; 95% CI, 0.69 to 0.98), compared to the findings of the EMPEROR-Reduced trial with empagliflozin (8% reduction; HR: 0.92; 95% CI, 0.75 to 1.12; statistically not significant) [28]. Further, a 17% reduction in all-cause mortality (7.9 versus 9.5 events per 100 patient-years compared to placebo; HR:0.83; 95% CI, 0.71 to 0.97; p<0.022c) was also reported in the DAPA-HF trial [34]. In the DAPA-CKD trial, there was a 31% absolute risk reduction in all-cause mortality with dapagliflozin vs. placebo (HR: 0.69; 95% CI: 0.53–0.88; p=0.004) [35].
Sequencing of Therapies in HFrEF
Based on the encouraging findings of SGLT2i for persons with HFrEF as seen in the DAPA-HF and EMPEROR-Reduced trials, there has been mounting enthusiasm regarding the incorporation of SGLT2i into the guideline-recommended management therapy for HFrEF [36]. While assessing the benefits of SGLT2i in persons with HFrEF, it is important to note that in the HF trials, DAPA-HF and EMPEROR-Reduced, participants had been receiving guideline-recommended therapy, which included ACEi/ARB, ARNI, beta-blockers, and MRA. The HF benefits of SGLT2i were seen regardless of the use of such therapies [36]. Further, the benefits of SGLT2i on CV outcomes are comparable to those of ARNI therapy, and the combination of both therapies leads to even better CV protective effects [36,37]. These data imply that SGLT2i, such as dapagliflozin or empagliflozin, can be reasonably used in combination with ARNI, ACEi, or ARB therapy in persons with HFrEF [36]. Therefore, it becomes imperative to appropriately position or sequence SGLT2i for therapy in this group of persons with HF. Currently, physicians are asked to initiate therapy with ACEi or ARB, followed by beta-blockers, MRA, ARNI, and finally SGLT2i. However, this approach is associated with some important limitations. Each class of therapy is supposed to be titrated to reach the target dose, before proceeding to the next class of drugs in the therapy sequence, which might require 6 months to prescribe all recommended therapies. Thus, such a treatment algorithm requires adjustment of treatment doses at majority of the visits, which is rarely met in routine clinical practice. Therefore, a significant number of individuals do not receive the targeted doses of all recommended therapies [38]. It has been proposed that initiation of quadruple therapy (either simultaneous or rapid sequencing) involving ARNI, MRA, beta-blockers, and SGLT2i may lower the risk of death by 73% over 2 years and may improve adherence, tolerance, and persistence to therapy. The tolerability of each of these drugs could be enhanced by initiating therapy at lower doses. Thus, the proposed quadruple therapy with simultaneous or rapid sequencing intends to overcome clinical inertia and treat persons with HFrEF with the required urgency [39].The timeline, strategy, and clinical benefits related to simultaneous initiation of quadruple therapy in persons with HFrEF are presented in Table 2 [39]. Regarding sequencing of therapy, a new evidence-based treatment algorithm has been proposed for persons with HFrEF (Table 2). This algorithm should be individualized for special circumstances, such as persons hospitalized with decompensated HF, or individuals hospitalized on intravenous therapy. This algorithm can help achieve treatment with all four classes of foundational HF treatments within 4 weeks. Thereafter, the uptitration of target doses should be done. This sequencing algorithm has been proposed to help achieve highly effective therapy along with rapid prevention of HHF and deaths, with improved tolerability of concurrent or subsequently prescribed therapies [38].
|
Day(s) |
ARNI |
Beta-blocker |
MRA |
SGLT2i |
|
|
1 |
Initiate at low dose |
Initiate |
|||
|
45121 |
Continue |
Titrate as tolerated |
Continue |
||
|
14-28 |
Titrate as tolerated |
Continue |
|||
|
21-42 |
Titrate as tolerated |
Continue |
|||
|
After Day 42 |
• Maintenance or additional titration as necessary |
||||
|
Sequencing |
Step 1 |
Step 2 |
Step 3 |
Step 4 |
Step 5 |
|
Conventional (uptitration at each step requires ≥6 months) |
ACEi/ARB |
Beta-blocker |
MRA |
ARNI |
SGLT2i |
|
Proposed (achieve all 3 steps within 4 weeks; then uptitrate to target dose) |
Beta-blocker + SGLT2i |
ARNI |
MRA |
||
|
ACEi: Angiotensin-converting enzyme inhibitors; ARB: Angiotensin II receptor blockers; ARNI: angiotensin receptor neprilysin inhibitor; MRA: Mineralocorticoid receptor agonist; SGLT2i: Sodium-glucose cotransporter 2 inhibitor. |
|||||
Table 2: Simultaneous or rapid sequence initiation of quadruple therapy in persons with HFrEF (upper panel); conventional and new sequencing of therapy for persons with HFrEF (lower panel) (Adapted from [38,39])
A recent consensus by the Heart Failure Association of the ESC has suggested a personalized approach to help achieve a better and more comprehensive therapy as compared to the traditional, forced titration of every class of drug before treatment initiation with the next. Based on parameters such as heart rate (<60 bpm or ≥70 bpm), estimated glomerular filtration rate (<30 mL/min/1.73 m2 or >30 mL/min/1.73 m2), symptomatic low blood pressure, presence of atrial fibrillation, or hyperkalemia, persons with HF were categorized into nine profiles relevant for persons with HFrEF. Since persons with HF may have varying presentations in terms of kidney function, hemodynamic status, and congestion, prioritizing or adjusting drugs as per the individual profile might be a reasonable way to provide the individualized benefit of GDMT to every individual [40].
Cardiorenal Syndrome in HF
Renal dysfunction is one of the important comorbidities in persons with HF. While decreased estimated glomerular filtration rate (eGFR) is a potential predictor of CV complications and mortality, worsening HF can hasten the worsening of renal function [41]. This implies that disorders occurring in any of the two systems mostly involve the other system. Thus, both CV and renal disorders often progress in a continuous manner, which is termed the cardiorenal continuum. Usage of any therapeutic approach that disrupts any step of this vicious cascade can ensure CV and/or renal protection [42]. However, the outcomes of persons with cardiorenal syndrome have remained poor due to the lack of therapies capable of providing both CV and renal protection [6]. The REVEAL-CKD study was a multinational initiative for evaluating undiagnosed CKD. The findings of the study showed that 62.4% (95% CI: 62.2–62.6) of persons with stage 3 CKD in the United States were undiagnosed, thereby suggesting an unmet need for a more proactive CKD diagnosis at early stages and monitoring [43].
Implications of Renal Impairment in the Management of HFrEF
Studies have shown that the prevalence of chronic kidney disease (CKD) in persons with HF ranges from 20% to 67% [6]. The risk of mortality in these individuals is 25%–30% higher compared to individuals with HF alone [6]. The pathophysiology of cardiorenal syndrome is complex. Low cardiac output and increased venous pressure in persons with HF can lead to CKD; renal dysfunction can also worsen HF through accelerated atherosclerosis, inflammation, neurohormonal activation, increased sodium and fluid retention, uremic toxins, and anemia [6]. The management of HF in persons with renal dysfunction can also be challenging owing to drug-induced creatinine and electrolyte changes, device therapy-induced infections, and resistance to diuretics [6, 44]. Besides ARNI and ivabradine, recently, SGLT2i have shown promising results in improving HHF and CV-related deaths among persons with HFrEF and CKD stages 1, 2, and 3 [44].
SGLT2i in the Management of Cardiorenal Syndrome
Although the initial design of the CVOTs was to assess the CV safety of SGLT2i, the secondary endpoints and prespecified renal outcomes of these trials provided extensive data on the renal effects of SGLT2i [42]. The subanalysis of these trials established the renal benefits of SGLT2i in persons with T2DM at high CV risk or established atherosclerotic CV disease [45-47]. Data from new trials, including the CREDENCE, DAPA-HF, EMPEROR-Reduced, the DAPA-CKD, and the SCORED trials, have affirmed the consistent CV protective and renoprotective benefits of SGLT2i [26,28,35,48,49]. In the DAPA-CKD study, the HR for the composite of HHF or death from CV causes was 0.71 (95% CI: 0.55–0.92; p=0.009) [35]. Therefore, accumulating evidence has shown that SGLT2i lowers HHF across different stages of the CV continuum, independent of baseline kidney function or dysfunction stage (Table 3). In parallel, SGLT2i delays the progress of renal dysfunction, end-stage kidney disease, and albuminuria across various stages of the renal continuum. Thus, the cardioprotective and renoprotective effects of SGLT2i are two sides of the same coin, and SGLT2i can potentially disrupt the series of pathophysiological events that lead to cardiorenal syndrome, irrespective of diabetes status [42].
|
Study name |
HHF outcomes compared to placebo |
Renal outcomes compared to placebo |
|
EMPA-REG OUTCOME15 |
−35% |
−46% |
|
CANVAS16 |
−33% |
−40% |
|
DECLARE-TIMI 5817 |
−27% |
−47% |
|
VERTIS-CV32 |
−30% |
−46% |
|
CREDENCE48 |
−39% |
−34% |
|
DAPA-HF26 |
−30% |
−50% |
|
EMPEROR-Reduced28 |
−31% |
−50% |
|
DAPA-CKD35* |
−29% |
−44% |
|
*DAPA-CKD presented a combined endpoint of HHF + CV mortality. |
||
Table 3: Comparing the effects of SGLT2i on HHF and renal outcomes (Adapted from [42])
The recent position paper from the ESC acknowledges a lower risk of HHF or CV death with dapagliflozin and empagliflozin therapy in persons with HFrEF. Further, the effect of sotagliflozin in lowering the risk of HHF and CV death in persons with T2DM recently hospitalized for HF has also been highlighted [12].
Safety of SGLT2i
Findings from several trials of SGLT2i in persons with T2DM suggest that overall, this pharmacological class is well tolerated. During early treatment exposure, incidences of genital mycotic infections and urinary tract infections (UTIs) are common. Although UTIs with SGLT2i are mild to moderate and easily manageable, genital mycotic infections are relatively more frequent. Genital infections associated with SGLT2i are generally mild and can be easily managed [50]. SGLT2i has also been associated with diabetic ketoacidosis, although such incidences are very rare and are mainly observed in persons on insulin therapy or in persons associated with acute serious clinical conditions, such as surgery or septicemia [50]. However, it is recommended to discontinue SGLT2i treatment if symptoms of diabetic ketoacidosis appear [6]. In the CANVAS trial, the risk of lower limb amputation was higher with canagliflozin as compared to placebo. However, no such increase in the incidence of amputation was observed in the EMPA-REG OUTCOME and DECLARE-TIMI 58 trials [51]. Therefore, the influence of SGLT2i on the risk of amputations and fractures needs to be evaluated further [6]. A recent meta-analysis assessed the major safety outcomes associated with SGLT2i treatment in persons with HF based on data from seven randomized controlled trials [25,52]. The study reported that SGLT2i was generally safe with a lower incidence of serious adverse events. In the subgroup of persons with HF without diabetes, SGLT2i did not significantly increase the risk of UTI, hypoglycemia, amputation, bone fracture, or volume depletion. Notably, volume depletion risk was higher in the overall persons with HF treated with SGLT2i. Therefore, a combination of SGLT2i with diuretics should be used with caution. Overall, based on the safety profile, SGLT2i can be used as a potential therapeutic agent in persons with HF [25].
Guidance on the Use of SGLT2i
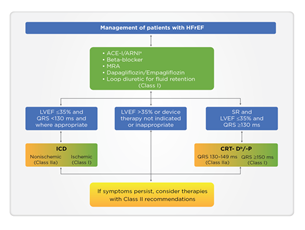
The 2021 guidelines of the ESC have included SGLT2i in the treatment algorithm for HFrEF among other conventional medications with class I indication for mortality reduction, either CV or all-cause mortality. Dapagliflozin and empagliflozin have received the strongest recommendation and highest evidence (class IA) and have been positioned as the first-line therapy in HFrEF (Figure 2). The guideline emphasizes the early administration of the four major classes of drugs: ACE/ARNI, beta-blockers, MRA, and SGLT2i. For the first time, dapagliflozin and empagliflozin have been recommended for managing persons with HFrEF to lower HHF and mortality (class IA). For persons with T2DM at high risk of CV events, empagliflozin, dapagliflozin, canagliflozin, ertugliflozin, and sotagliflozin are recommended, whereas, for persons with T2DM with HFrEF, empagliflozin, dapagliflozin, and sotagliflozin are recommended to reduce the risk of HHF and CV death [12]. In persons with T2DM with HFrEF, SGLT2i replaces metformin as the first-line therapy.
ACE-I: Angiotensin-converting enzyme inhibitor; ARNI: Angiotensin receptor-neprilysin inhibitor; CRT-D: Cardiac resynchronization therapy with a defibrillator; CRT-P: Cardiac resynchronization therapy with a pacemaker; HFrEF: Heart failure with reduced ejection fraction; ICD: Implantable cardioverter-defibrillator; LVEF: Left ventricular ejection fraction; MRA: Mineralocorticoid receptor antagonists; QRS: Q, R, and S waves (on a 12-lead electrocardiogram); SR: Sinus rhythm. aAs a replacement for ACE-I. bWhere appropriate. Class I = Green. Class IIa = Yellow.
The 2021 American College of Cardiology (ACC) consensus on HF treatment recommends the use of SGLT2i in persons with HFrEF with New York Heart Association (NYHA) class III–IV HF, irrespective of diabetes status. However, SGLT2i should be administered in conjunction with a guideline-directed medical therapy for HF [53]. The ACC 2021 guidelines on HF recommend ARNI as the preferential renin–angiotensin–aldosterone system blockade to initiate therapy. The 2021 guidelines of the American Diabetes Association (ADA) recommend the use of SGLT2i with demonstrated CV benefit in persons with T2DM at high CV risk, or with established CV disease [54]. The 2021 Canadian Cardiovascular Society Heart Failure guidelines have placed a higher value on combination therapy involving evidence-based therapeutic classes, which includes SGLT2i. Dapagliflozin and empagliflozin are strongly recommended for persons with HFrEF to lower HHF and/or CV mortality and to improve symptoms and quality of life. Further, for eligible individuals, it is recommended to start early therapy with SGLT2i [55].
Practical Guidance on the Use of SGLT2i: The Dos and Don’ts
Practical guidance on the clinical use of SGLT2i in persons with HFrEF, summarized based on recent evidence and guideline updates has been enumerated in Table 4.
|
Dos |
Don’ts |
|
Early initiation of SGLT2i in eligible individuals |
Use of SGLT2i in persons with low eGFR (<20 mL/min/1.73 m2 for empagliflozin and <30 mL/min/1.73 m2 for dapagliflozin due to limited data) |
|
Should be considered for first-line therapy along with ARNI (or ACEi/ARB), beta-blockers, and MRA |
Contraindications: persons with critical limb ischemia, allergy, or intolerance to SGLT2i; persons with T1DM; and pregnant or lactating women |
|
Dapagliflozin or empagliflozin should be used in persons with HFrEF for lowering the risk of CV mortality and/or HHF, irrespective of diabetes status |
Caution: volume depletion, active genital mycotic infections, hypotension (systolic blood pressure <95 mmHg), prior critical limb ischemia, diabetic ketoacidosis, history of severe hypoglycemia (for persons with diabetes) |
|
Dapagliflozin is to be used in persons with albuminuric renal disease, irrespective of diabetes status, to reduce HHF and progression of renal disease |
Genetic mycotic infections can be managed with antifungal drugs; hence, do not discontinue therapy with SGLT2i in case of such infections |
|
Careful monitoring of volume status is required while using SGLT2i |
ACEi: Angiotensin-converting enzyme inhibitors; ARB: Angiotensin II receptor blockers; ARNI: Angiotensin receptor neprilysin inhibitor; CV: Cardiovascular; eGFR: Estimated glomerular filtration rate; HHF: Hospitalization for heart failure; HFrEF: Heart failure with reduced ejection fraction; MRA: Mineralocorticoid receptor antagonists; SGLT2i: Sodium–glucose cotransporter-2 inhibitors; T1DM: Type 1 diabetes mellitus.
Table 4: Practical guidance on the use of SGLT2i [55–57].
Future Directions
There is substantial evidence supporting the benefit of SGLT2i in HFrEF, irrespective of diabetes status. High-level outcome data showing HF treatment effects of SGLT2i comparable to those of current GDMT agents for HFrEF are available for dapagliflozin from the DAPA-HF trial. Recent data from the EMPEROR-Preserved trial have elucidated that SGLT2i can reduce the combined risk of HHF or CV death in persons with HFpEF, irrespective of diabetes status (HR: 0.79; 95% CI: 0.69–0.90; p<0.001). Further, the risk and severity of a broad range of worsening HF events were also reduced [58]. According to the outcomes noted in the EMBRACE-HF trial, empagliflozin therapy in persons with HFpEF or HFrEF was associated with a rapid reduction in pulmonary artery pressure [59]. Dapagliflozin therapy was associated with improvements in natriuretic peptide levels and HF-related health status among persons with HFrEF in the DEFINE-HF trial; these benefits were also noted in persons without T2DM [52]. Further, the PRESERVED-HF trial outcomes suggest that dapagliflozin improves the quality of life in persons with HFpEF [60]. Emerging evidence from the ongoing trials will further reinforce the candidature of SGLT2i as a potent disease-modifying therapy in persons with HFrEF [34].
Conclusion
Incorporating SGLT2i as first-line therapy in the HFrEF treatment algorithm as per the ESC 2021 guidelines is likely to improve adherence to guideline-recommended dosage and sequencing. In addition, SGLT2i might potentially address a few of the current unmet needs in HF management, including persons with HFpEF. Nevertheless, further understanding of the underlying mechanism of HF benefits of SGLT2i might open new avenues to aid early diagnosis of HF, identify biomarkers for early identification, and develop novel targeted therapies for this group of vulnerable individuals.
References
- Mishra S, Mohan JC, Nair T, et al. Management protocols for chronic heart failure in India. Indian Heart J 70 (2018): 105-27
- Lippi G, Sanchis-Gomar F. Global epidemiology and future trends of heart failure. AME Med J 5 (2020): 15.
- Chaturvedi V, Parakh N, Seth S, et al. Heart failure in India: The INDUS (INDiaUkieri Study) study. J Pract Cardiovasc Sci 2 (2016): 28-35.
- Singh, A, Chauhan S, Devasia T, et al. Financial burden of heart failure in a developing country: cost analysis from Manipal Heart Failure Registry, India. J Public Health (Berl.) 29 (2021): 585-94.
- Bragazzi NL, Zhong W, Shu J, et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J PrevCardiol (2021): 147.
- Lam CSP, Chandramouli C, Ahooja V, et al. SGLT-2 Inhibitors in Heart Failure: Current Management, Unmet Needs, and Therapeutic Prospects. J Am Heart Assoc 8 (2019):e013389.
- Chopra VK, Mittal S, Bansal M, et al. Clinical profile and one-year survival of patients with heart failure with reduced ejection fraction: The largest report from India. Indian Heart J 71 (2019): 242-248.
- Shukkoor AA, George NE, Radhakrishnan S, et al. Clinical characteristics and outcomes of patients admitted with acute heart failure: insights from a single-center heart failure registry in South India. Egypt Heart J 73 (2021): 38.
- Bozkurt B, Coats AJ, Tsutsui H, et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail (2021): S1071-9164.
- Westphal JG, Bekfani T, Schulze PC. What’s new in heart failure therapy 2018? Interact Cardio Vasc Thorac Surg 27 (2018): 921-930.
- Pereira-Barretto AC. Addressing Major Unmet Needs in Patients with Systolic Heart Failure: The Role of Ivabradine. Am J Cardiovasc Drugs 16 (2016): 93-101.
- McDonagh TA, Metra M, Adamo M, et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42 (2021): 3599-3726
- Joshi SS, Singh T, Newby DE. Sodium-glucose co-transporter 2 inhibitor therapy: mechanisms of action in heart failure. Heart 107 (2021): 1032-1038.
- Gormley L. Poll Results: SGLT2 Inhibitors for Patients with Heart Failure with Reduced Ejection Fraction: Who Should Prescribe Them? American College of Cardiology (2020).
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 373 (2015): 2117-2128
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 377 (2017): 644-657.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 380 (2019): 347-357.
- Lopaschuk GD, Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci 5 (2020): 632-644.
- Verma S. Potential Mechanisms of Sodium-Glucose Co-Transporter 2 Inhibitor-Related Cardiovascular Benefits. Am J Cardiol 124 (2019): S36-44.
- Xiang B, Zhao X, Zhou X. Cardiovascular benefits of sodium-glucose cotransporter 2 inhibitors in diabetic and nondiabetic patients. Cardiovasc Diabetol 20 (2021): 78
- Rabizadeh S, Nakhjavani M, Esteghamati A. Cardiovascular and Renal Benefits of SGLT2 Inhibitors: A Narrative Review. Int J Endocrinol Metab 17 (2019): e84353.
- Staels B. Cardiovascular Protection by Sodium Glucose Cotransporter 2 Inhibitors: Potential Mechanisms. Am J Cardiol 120 (2017): S28-36.
- Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 17 (2020): 761-772.
- Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 61 (2018): 2108-2117.
- Li X, Zhang Q, Zhu L, et al. Effects of SGLT2 inhibitors on cardiovascular, renal, and major safety outcomes in heart failure: A meta-analysis of randomized controlled trials. Int J Cardiol 332 (2021): 119-126.
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 381 (2019): 1995-2008.
- Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med 384 (2021): 117-128.
- Packer M, Anker SD, Butler J. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med 383 (2020): 1413-1424.
- Zannad F, Ferreira JP, Pocock SJ. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 396 (2000): 819-829
- Curtain JP, Docherty KF, Jhund PS, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J 27 (2021): ehab560.
- Butler J, Usman MS, Khan MS, et al.Efficacy and safety of SGLT2 inhibitors in heart failure: systematic review and meta-analysis. ESC Heart Fail 7 (2020): 3298-3309.
- Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N Engl J Med 383 (2020): 1425-1435.
- Palmiero G, Cesaro A, Vetrano E, et al. Impact of SGLT2 Inhibitors on Heart Failure: From Pathophysiology to Clinical Effects. Int J Mol Sci 22 (2021): 5863.
- Genuardi MV, Mather PJ. The dawn of the four-drug era? SGLT2 inhibition in heart failure with reduced ejection fraction. Ther Adv Cardiovasc Dis 15 (2021): 17539447211002678.
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med 383 (2021): 1436-1446.
- Starr JA, Pinner NA, Lisenby KM, et al. Impact of SGLT2 inhibitors on cardiovascular outcomes in patients with heart failure with reduced ejection fraction. Pharmacotherapy 41 (2021): 526-536.
- Yan Y, Liu B, Du J, et al. SGLT2i versus ARNI in heart failure with reduced ejection fraction: a systematic review and meta-analysis. ESC Heart Fail 8 (2021): 2210-2219.
- McMurray JJV, Packer M. How Should We Sequence the Treatments for Heart Failure and a Reduced Ejection Fraction?: A Redefinition of Evidence-Based Medicine. Circulation 143 (2021): 875-877.
- Greene SJ, Butler J, Fonarow GC. Simultaneous or Rapid Sequence Initiation of Quadruple Medical Therapy for Heart Failure-Optimizing Therapy With the Need for Speed. JAMA Cardiol 6 (2021): 743-744.
- Rosano GMC, Moura B, Metra M, et al. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 23 (2021): 872-881.
- Liu PP. Cardiorenal syndrome in heart failure: a cardiologist's perspective. Can J Cardiol 24 (2008): 25B–9B.
- Fontes-Carvalho R, Santos-Ferreira D, Raz I, et al. Protective effects of SGLT-2 inhibitors across the cardiorenal continuum: two faces of the same coin. Eur J PrevCardiol (2021).
- Sultan AB, Barone S, Kumar S. REVEAL-CKD: Prevalence of and Patient Characteristics Associated with Undiagnosed Stage 3 Chronic Kidney Disease. Diabetes 70 (2021).
- Banerjee D, Wang AY. Personalising heart failure management in CKD patients. Nephrol Dial Transplant 2021.
- Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med 375 (2016): 1801-1802.
- Perkovic V, Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol 6 (2018): 691-704.
- Mosenzon O, Wiviott SD, Cahn A. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol 7 (2019): 606-617
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 380 (2019): 2295-2306.
- Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med 384 (2021): 129-139.
- Scheen AJ. An update on the safety of SGLT2 inhibitors. Expert Opin Drug Saf 18 (2019): 295-311.
- Zelniker TA, Wiviott SD, Raz Im K, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393 (2019): 31-39.
- Nassif ME. Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients With Heart Failure With Reduced Ejection Fraction The DEFINE-HF Trial. Circulation 140 (2019): 1463-1476.
- Maddox TM, Januzzi JL, Allen LA, et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol 77 (2021): 772-810.
- American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care 44 (2021): S111-24
- McDonald M, Virani S, Chan M, et al. CCS/CHFS Heart Failure Guidelines Update: Defining a New Pharmacologic Standard of Care for Heart Failure With Reduced Ejection Fraction. Can J Cardiol 37 (2021): 531-546.
- Practical approach to SGLT2 inhibitors for treatment of cardiovascular disease. Canadian Heart Failure Society (2021).
- Lam D, Shaikh A. Real-life prescribing of SGLT2 inhibitors: How to handle other medications, including glucose-lowering drugs and diuretics. Kidney360. 2 (2021).
- Packer M, Butler J, Zannad F, et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients with Heart Failure and a Preserved Ejection Fraction: The EMPEROR-Preserved Trial. Circulation (2021).
- Nassif ME, Qintar M, Windsor SL, et al. Empagliflozin Effects on Pulmonary Artery Pressure in Patients With Heart Failure: Results From the EMBRACE-HF Trial. Circulation 143 (2021): 1673-1686.
- Nassif ME, Windsor SL, Borlaug BA, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med 27 (2021): 1954-1960.