Right Sided Trouble: Persistent Shunting after Iatrogenic Atrial Septal Defect Closure with an Amplatzer PFO Occluder Treated Successfully With a Cardioform Septal Occluder
Article Information
Bradley Stauber1, Carleton Nibley2, Wayland Lim2, Jason H. Rogers1*
1University of California, Davis Medical Center, Department of Internal Medicine, Division of Cardiovascular Medicine, Sacramento, CA, USA
2John Muir Cardiovascular Group, Concord, CA, USA
*Corresponding author:Jason H. Rogers, Cardiovascular Medicine University of California, Davis Medical Center 4860 Y Street, Suite 2820 Sacramento, CA 95817, USA
Received: 18 August 2019; Accepted: 02 September 2019; Published: 04 September 2019
Citation: Bradley Stauber, Carleton Nibley, Wayland Lim, Jason H. Rogers. Right Sided Trouble: Persistent Shunting after Iatrogenic Atrial Septal Defect Closure with an Amplatzer PFO Occluder Treated Successfully With a Cardioform Septal Occluder. Cardiology and Cardiovascular Medicine 3 (2019): 313-321.
Share at FacebookAbstract
We describe a patient with severe, symptomatic platypnea-orthodeoxia after an atrial fibrillation ablation leading to an iatrogenic atrial septal defect. The shunt was initially closed with Amplatzer PFO occluder, however with continued symptoms and brisk flow through the device, an alternative solution had to be devised. After removal of the device and placement of a Gore Cardioform septal occluder sealed the defect, alleviating symptoms almost instantaneously. To our knowledge, this is the first such case reported.
Keywords
Platypnea-orthodeoxia syndrome; Amplatzer PFO occlude; Atrial septal defect; iatrogenic atrial septal defect; Paroxysmal atrial fibrillation
Article Details
Introduction
Platypnea-orthodeoxia syndrome (POS) is a clinical scenario characterized as dyspnea and hypoxia that is exacerbated when the patient is sitting or standing upright and improved when in the supine position. While rarely encountered in clinical practice, POS has been well described in the literature. In general, the cause of these symptoms can be separated into the three major groups: intra-cardiac shunting, intra-pulmonary shunting, and a mismatch of ventilation and perfusion [1]. Diagnosis and treatment of the syndrome requires recognition of the unusual clinical findings as well as understanding the underlying pathophysiology.
While the mechanisms causing POS have been postulated, the exact cause in many cases is still not completely understood. That said, it is believed that in order for this uncommon finding to exist, two conditions must be satisfied [2]. The first is an anatomic variant that leads to the ability of blood to bypass the normal pulmonary circulation, such as a patent foramen ovale (PFO) or atrial septal defect (ASD) [3]. The second is a functional component that enhances right to left shunting or ventilation perfusion mismatch while upright such as a prominent Eustachian ridge, constrictive pericarditis, COPD, or cirrhosis. Increased pressures on the right side of the heart, as can occur with pulmonary hypertension, or decreased right-sided chamber compliance can also cause blood to shunt from right atrium to left atrium.
In this case, we report a unique case of an iatrogenic atrial septal defect (iASD) after transseptal puncture causing severe POS that was initially treated with an Amplatzer PFO Occluder (APO). There was persistent right to left shunting through the mesh of the APO despite conservative measures, and this finding has not yet been reported in the literature. Due to continued POS symptoms and brisk flow through the device, an alternative solution had to be devised.
Case Summary
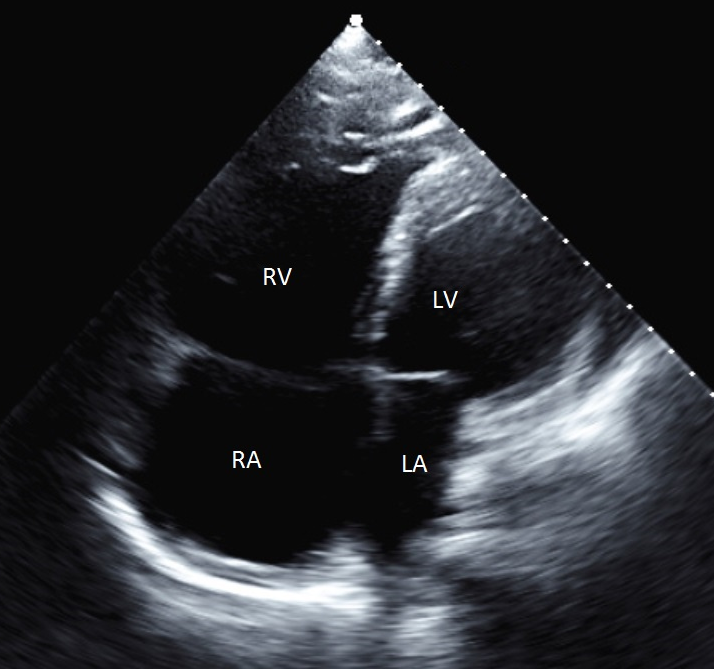
A 58-year-old man with a history of idiopathic right ventricular failure, ventricular tachycardia with ablation, marked right-sided chamber enlargement, and paroxysmal atrial fibrillation (PAF) on rivaroxban underwent elective ablation for PAF. Baseline transthoracic echocardiogram was performed, with notable features including extreme enlargement and hypokinesis of both the right atrium and right ventricle (Figure 1). Room air oxygen saturation at baseline was 99%. Rivaroxaban was not interrupted for the procedure.
Cavo-tricuspid isthmus dependent radiofrequency ablation was performed followed by transseptal puncture into the left atrium. A 12 Fr Cryoballoon (Medtronic, Minneapolis, MN, USA) was used for pulmonary vein isolation ablation. Upon withdrawal of the balloon back into the right atrium, the patient immediately developed severe hypoxia with oxygen saturations in the 80s requiring supplemental oxygen. An echocardiogram following the procedure showed right to left interatrial shunting through the iASD.
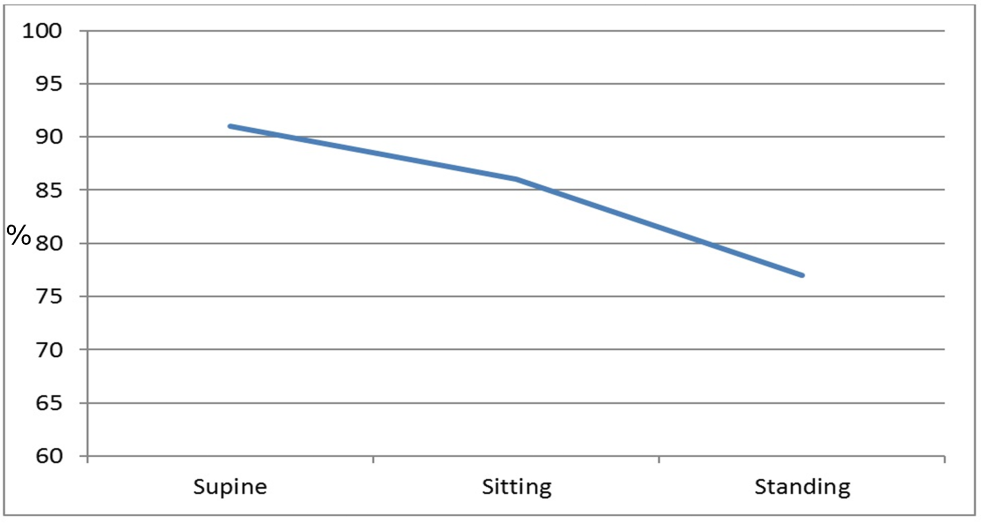
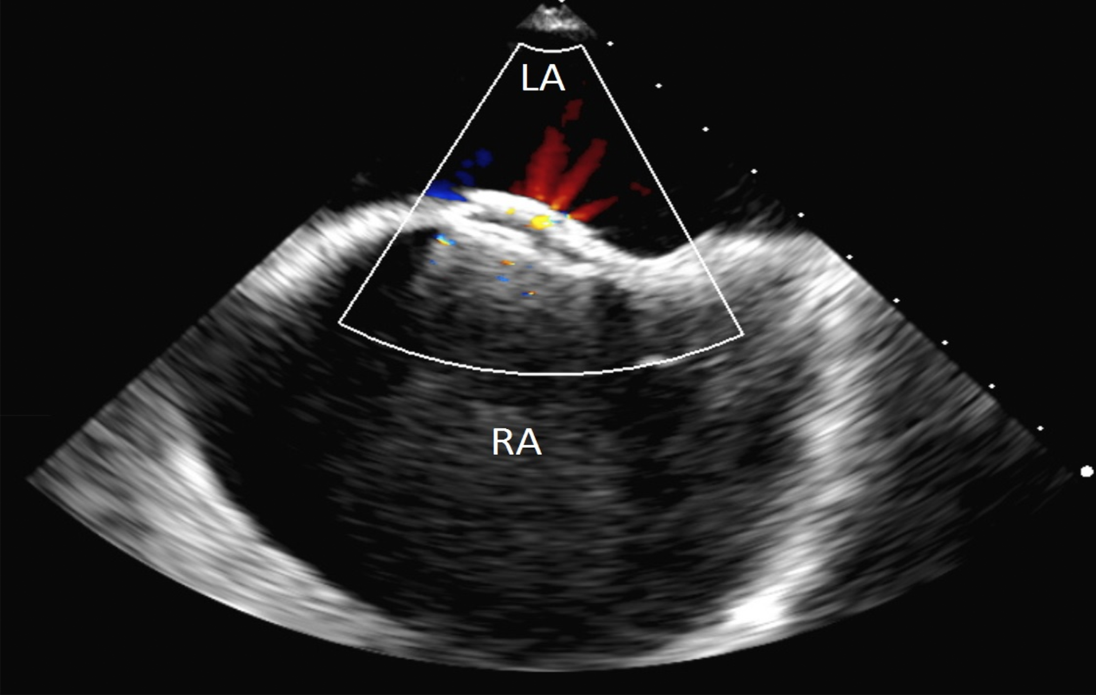
The patient remained markedly hypoxic, with shortness of breath and dyspnea which worsened with an upright position and improved when he was supine, consistent with platypnea-orthodeoxia (Figure 2). Additional work up including a transesophageal echocardiogram was performed to confirm the size and location of the iASD. This showed a moderate sized ASD 5- 10 mm in diameter with brisk right to left shunting. Given the patient’s lack of clinical improvement, the decision was made to close the iASD via transcatheter approach. Without interruption of rivaroxaban, femoral venous access was obtained and using intracardiac echocardiography (ICE) guidance, a 25/18 mm Amplatzer PFO Occluder (Abbott, Plymouth, MN, USA) was successfully placed. Rivaroxaban was continued and clopidogrel was added. Unfortunately, the patient continued to have significant positional desaturations. Repeat echocardiography showed the occluder in appropriate position without peri-device leak. However, there was brisk right to left flow through the central mesh of the device (Figure 3). The decision was made, after considerable debate in light of his recent extensive ablation, to hold the patient’s oral anticoagulant. Despite several days of stopping rivaroxaban (while continuing daily clopidogrel), a follow up bubble study remained grossly positive with all four chambers filling with bubbles within 1-2 beats (Figure 4). He was referred to our institution for snare removal of the previously placed septal occluder and placement of a new device.
Femoral venous access was obtained and baseline hemodynamics were: right atrium 10 mmHg, right ventricle (RV) 15/5, RV end diastolic pressure 9 mmHg, pulmonary artery (PA) 14/6 mean PA 10 mmHg, pulmonary capillary wedge pressure 3 mmHg, and cardiac output 2.95 L/min. A 14 Fr Cook TS sheath (Cook Medical, Bloomington, IN, USA) was advanced in the right atrium. In order to remove the previously placed Amplatzer device, an EnSnare (Merit Medical, South Jordan, UT, USA) 18-30 endovascular snare system was advanced and used to recapture and remove the Amplatzer device. Immediately after removal of the device, the patient was hypoxemic with oxygen saturation in the low 80s. Via intracardiac echocardiography, there was brisk shunting of blood from the right atrium to the left atrium.
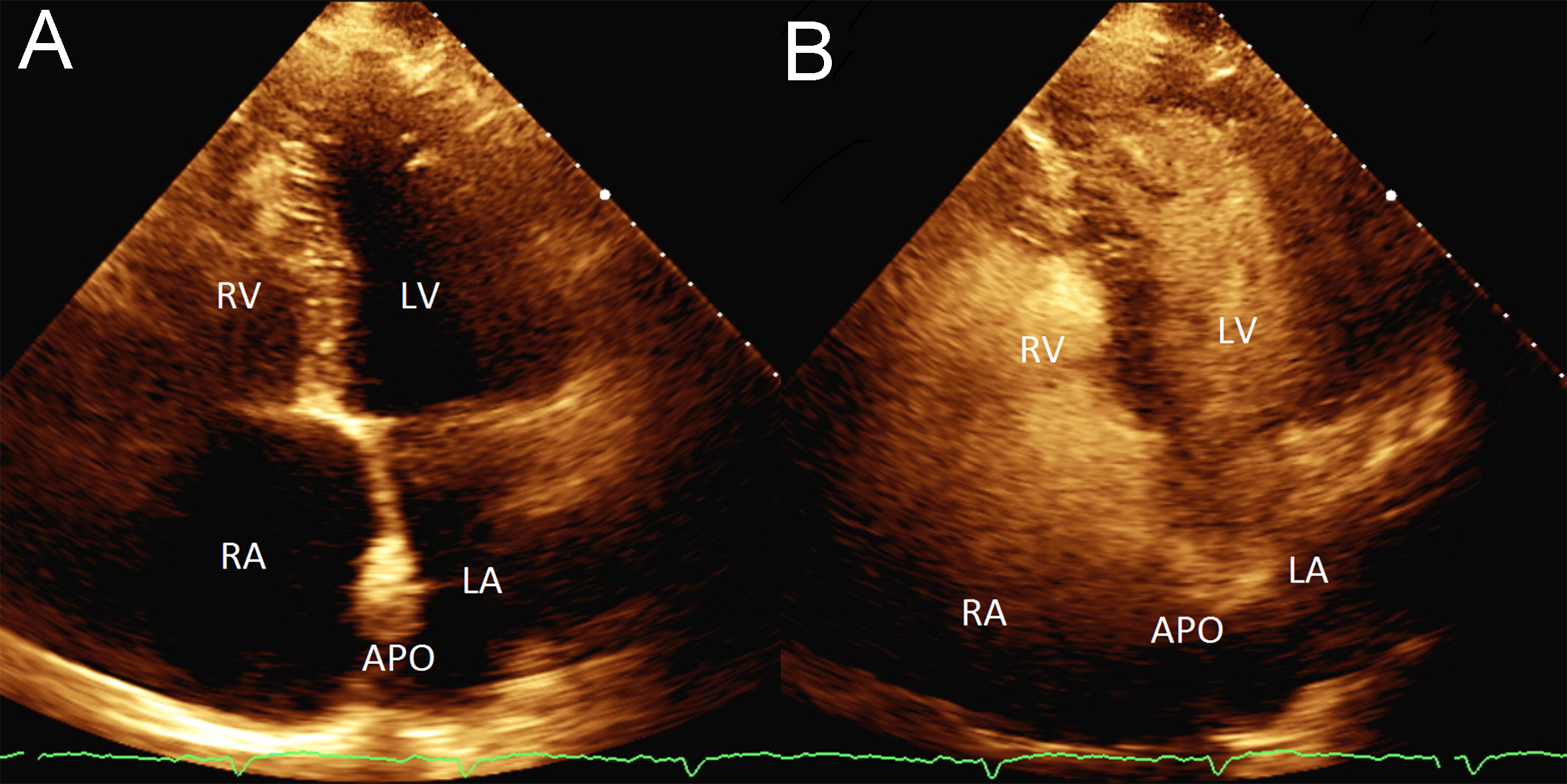
A 25 mm Cardioform septal occluder (Gore Medical, Flagstaff, AZ, USA) was then placed. There was immediate cessation of shunting across the interatrial septum, and the patient’s oxygen saturation immediately returned to 99% post device placement (Figure 5). Postprocedure echocardiography showed the device was shown well seated and a bubble study showed no evidence of bubbles crossing from the right to left (Supplemental Video 1). The patient was continued on rivaroxban indefinitely, and clopidogrel was continued for one month.
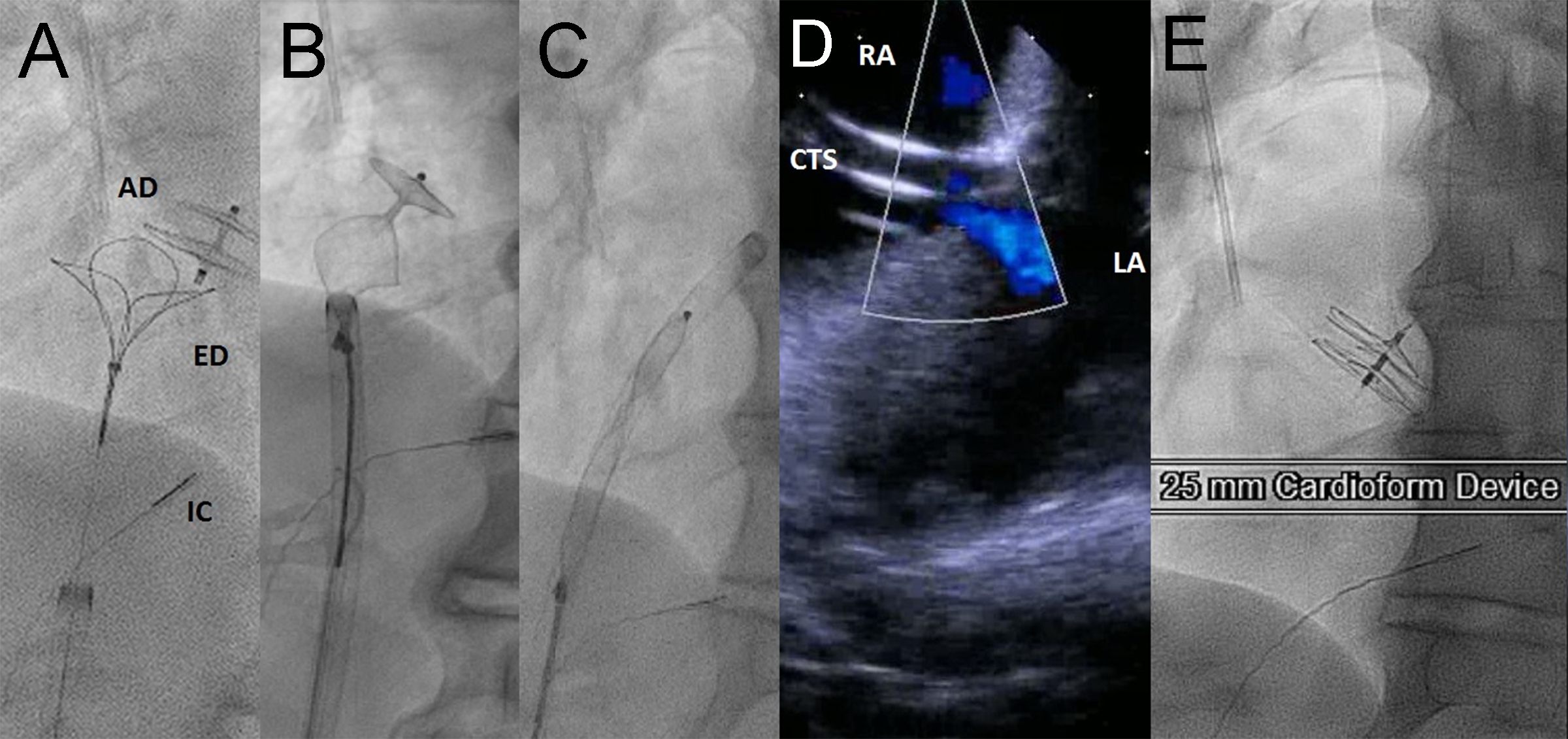
Figure 5: A-C: Snare retrieval of Amplatzer PFO Occluder. D: Residual right to left shunt through iatrogenic ASD with Cook transseptal sheath (CTS) in place as seen by right atrial intracardiac echocardiography (ICE) catheter. E: Final appearance of Cardioform device. Following Cardioform placement, there was immediate cessation of shunting across the interatrial septum, and the patient’s oxygen saturation immediately returned to 99% post device placement. AD: Amplatzer device; ED: EnSnare device; IC: intracardiac echocardiography catheter
At one month, the patient had a transthoracic echocardiogram with bubble study which was negative for any right to left flow. Given his previous history of ventricular tachycardia and diagnosis of “idiopathic” right ventricular failure, a cardiac MRI was performed to complete the work up. The MRI was consistent with arrhythmogenic right ventricular cardiomyopathy, and an implantable cardiac defibrillator was placed. The patient continues to do well in follow up.
Discussion
We have presented a unique case of iatrogenic ASD leading to severe platypnea-orthodeoxia syndrome. An Amplatzer PFO Occluder was placed given the patient’s symptoms and evidence of persistent right to left shunt after the iASD; however, it failed to completely seal the defect with continued brisk right to left flow through the central mesh and fabric of the device. This particular scenario with persistent leak through an Amplatzer PFO occluder in the interatrial septum has not been previously reported in the literature. We elected to correct this defect by snaring the Amplatzer device and putting in a Gore Cardioform occluder, which by nature of its construction with expanded polytetrafluoroethylene (ePTFE) was less permeable to blood flow and eliminated the shunt, leading to marked clinical improvement.
Platypnea-orthodeoxia syndrome, while infrequently diagnosed, is probably more prevalent than previously thought [4]. While rarely encountered in the literature previously, the last 20 years has seen it described with increasing frequency. Among the totality of case reports compiled by Rodrigues et al in 2009 with POS and an interatrial communication (N=110), 72% of patients had a PFO, 16% had an ASD, and 12% had an atrial septal aneurysm [5]. Different mechanisms have been postulated regarding the positional phenomenon of the syndrome. In our case, the patient’s POS was only possible due to both an anatomical and functional component which together allowed for continued right to left flow. Specifically, an iASD was the anatomical variant, with the patient’s severe right sided heart failure as the functional cause of the shunt. The pathophysiology of blood shunting from the right atrium to the left atrium in the setting of this anatomical defect is multifactorial. These include pressure gradients between the chambers throughout the cardiac cycle, relatively low left sided filling pressures (PCWP 3 mmHg) as compared to the only mildly elevated right sided atrial pressure (RA 10 mmg), blood being directed toward the defect via the IVC, and decreased right ventricular compliance leading blood to preferentially travel across the defect to the more compliant left atrium [6,7]. This patient had severe right ventricular chamber enlargement which would support the notion that decreased RV compliance was a significant contributor. This, in conjunction with a more compliant left atrium, led to blood shunting across the newly formed iASD and determined the patient’s clinical course.
While POS was successfully diagnosed and treatment was attempted, the Amplatzer PFO Occluder (APO) that was originally placed failed to correct the shunt. The APO is readily available in most cardiac catheterization labs and is widely used. It consists of two nitinol mesh discs and comes in a variety of sizes -- in this case a right atrial disc diameter of 25 mm and a left atrial diameter of 18 mm with a 4mm waist connecting the two discs [8]. The discs are filled with a similarly sized circular polyethylene terephthalate (PET or polyester) fabric. This polyester fabric is initially porous and is designed to enhance immediate closure through a coagulant mechanism, followed by rapid neoendothelial development and long-term tissue ingrowth [9]. The choice to initially use a PFO occluder in this case as opposed to an Amplatzer Septal Occluder was made by the treating physician. Unfortunately, the device failed to acutely occlude flow through the interatrial septum. We theorize this is due to the patient’s therapeutic anticoagulation, which likely suppressed hemostatic thrombus formation. Upon retrieval of the device, examination showed a marked absence of thrombus, and the polyester fabric was white consistent with lack of coagulation (Figure 6).
Known complications of the Amplatzer PFO Occluder device include device embolization, cardiac perforation, device erosion, and device thrombosis [10]. To our knowledge, failure of the device to occlude flow in the manner described in our case has yet to be reported. However, it is thought that in general, any residual shunt through the device should resolve with endothelialization of the device [11]. Despite almost two weeks of observation, the occluder failed to stop the shunting of blood from the right atrium to the left atrium. The mechanism is caused by a combination of the patient’s therapeutic anticoagulation in addition to his severe right heart dysfunction with a non-compliant right ventricle contributing to the continued right to left shunt. That said, it became critical to devise a treatment plan to address this issue.
The decision to remove the Amplatzer device involved snaring the device from the right atrium. This was accomplished by snaring the right atrial hub and pulling the device into the Cook transseptal sheath, which was then advanced over the device into the left atrium, alleviating the need to recross the iASD. Since the device had not been in place for long, it was easily removed. We did visualize the device with ICE prior removal and there was no visible thrombotic material on this device. This step is important since embolization of thrombus during device retrieval is a risk.
There was discussion as to the appropriate method to seal the defect after the initial occluder was retrieved. One option was to place an Amplatzer Septal Occluder sized to the defect. This device has a waist that has fabric sewn into it which could theoretically “fill” the defect more completely. However, given the failure of the PFO occluder (which also has 2 layers of polyester fabric) to seal the defect it was felt that an alternate strategy was required. Surgical closure was considered but the patient was at prohibitive risk for this approach. The Cardioform Septal Occluder comes in a variety of sizes, in this case both discs were 25 mm in diameter. The discs are constructed of a nitinol wire frame attached to an expanded PTFE fabric [12]. Also known as Gore-Tex, ePTFE is very hydrophobic [13]. The ePTFE was able to acutely completely stop flow across the patient’s septum, alleviating symptoms almost instantaneously.
Regarding the need for future transseptal puncture, there should be sufficient residual space on the interatrial septum to allow this, although the operator should anticipate the need to close any residual symptomatic iASD promptly.
Conclusion
To our knowledge, this is the first reported case of the failure of an Amplatzer PFO Occluder by way of blood shunting through the device placed on the interatrial septum and the failure of the PET mesh to seal. In this case, the combination of brisk right to left blood flow in the direction of the iatrogenic ASD was likely due poor right ventricular compliance, in the setting of therapeutic anticoagulation with a direct oral anticoagulant ultimately resulting in device failure. Fortunately, the device was retrievable and the iASD was successfully sealed with a different device.
Disclosures
Jason Rogers is a consultant for Gore and Abbott Structural Heart.
References
- Chen GP, Goldberg SL, Gill EA Jr. Patent foramen ovale and the platypnea-orthodeoxia syndrome. Cardiol Clin 23 (2005): 85-89.
- Cheng TO. Mechanisms of playtpnea-orthodeoxia: What causes water to flow uphill? Circulation 105 (2002): e47.
- Cheng TO. Platypnea-orthodeoxia syndrome: etiology, differential diagnosis, and management. Catheter Cardiovasc Interv 47 (1999): 64-66.
- Godart F, Rey C. Platypnea-orthodeoxia syndrome: a probably underestimated Sydnrome? Chest 119 (2001): 1624-1625.
- Rodrigues P, Palma P, Sousa-Pereira L. Platypnea-orthodeoxia syndrome in review: defining a New Disease? Cardiology 123 (2012): 15-23.
- Robin ED, McCauley RR. An analysis of platypnea-orthodeoxia syndrome including a “new” therapeutic approach. Chest 112 (1997): 1149-1451.
- Gomperts N, Fowler R, Horlick E, Mclaughlin P. A broken heart: right-to-left shunt in the setting of normal cardiac pressures. Can J Cardiol 24 (2008): 227-229.
- Amplatzer PFO Occluder Device specifications in Instructions for Use.
- Huang Y, Kong JF, Venkatraman SS. Biomaterials and design in occlusion devices for cardiac defects: a review. Acta 10 (2014): 1088-1101.
- Spence MS, Qureshi SA. Complications of transcatheter closure of atrial septal defects. Heart 91 (2005): 1512-1514.
- Taggart NW. 2014. Interventional echocardiography in congenital heart disease. In: Eidem B, O’leary P, Cetta F, editors. Echocardiography in Pediatric and Adult Congenital Heart Disease 2nd ed. Philadelphia: Wolters Kluwer Health Ch 36.
- De Hemptinne Q, Horlick EM, Osten MD, Millan X, Tadros VX, et al. Initial clinical experience with GORE®CARDIOFORM ASD occluder for transcatheter atrial septal defect closure. Cather Cardiovasc Interv 90 (2017): 495-503.
- Hijazi Z, Feldman T, Sievert H, Al-Qbandi A. 2010. Transcatheter Closure of ASDs and PFOs: A Comprehensive Assessment. Minneapolis: Cardiotext Publishing. 350p.