Removal Of Metals By Sorption With Metal Concentration Colour Sensitive Azotized Polyethylene Terephthalate Adsorbent
Article Information
Isaac Mwangi1, Nicholas Cheruiyot1, Ruth Wanjau1 and Jane Catherine Ngila2
1Department of Chemistry, Kenyatta University, P.O. Box 43844-00100, Nairobi, Kenya
2Department of physical sciences, Kabianga University P.O.BOX 2030 - 20200 Kericho, Kenya.
3Department of Chemical Technology, University of Johannesburg, Doornfontein Campus, P.O Box 17011, Doornfontein 2028, Johannesburg, SA
*Corresponding Author:Isaac Mwangi, 1Department of Chemistry, Kenyatta University, P.O. Box 43844-00100, Nairobi, Kenya
Received: 14 March 2023; Accepted: 24 March 2023; Published: 03 April 2023
Citation: Isaac Mwangi, Nicholas Cheruiyot, Ruth Wanjau and Jane Catherine Ngila. Removal Of Metals By Sorption With Metal Concentration Colour Sensitive Azotized Polyethylene Terephthalate Adsorbent. Journal of Radiology and Clinical Imaging. 6 (2023): 62-77
Share at FacebookAbstract
This paper reports on the modification of polyethylene terephthalate (PET) by anchoring an azo group forming of a stable and colour sensitive absorbent capable of interacting with metal ions and removing them in aqueous media to alleviate heavy metal poisoning from its consumers. The modification was confirmed by FTIR (Fourier transform infrared) analysis and its metal adsorption property confirmed by FAAS (flame atomic absorption spectroscopic) analysis. The material exhibited colour variations upon interaction with copper, lead, cadmium and chromium ions which were the metal ions used in this study. This indicated when the material was exhausted thus signaling when to be regenerated. The modified material was then applied for their removal at their experimentally established parameters at a fixed temperature (25 °C). The optimum pH for the adsorption of copper, lead and chromium was 6.0 except for cadmium which was 5.5 as different pH values affects the stability of different complexes differently. The uptake of the metals was very fast as about 90% was adsorbed within the first 10 minutes of contact time. The sorption prescribed to second order kinetics thus bi molecular interaction of multilayer Langmuir adsorption. The adsorption capacities of copper, lead, cadmium and chromium were found to be 46.47, 33.65, 70.93 and 59.06 mg g-1 respectively. The study confirmed that adsorbent was regenerated by the use of 1.0 M nitric acid. This confirmed that azotized PET has potential application as a colour sensitive sorbent for the removal of heavy metal from water
Keywords
Heavy metal pollutions, azotized Polyethylene terephthalate;, pollution, Sorption.
Heavy metal pollutions articles; azotized Polyethylene terephthalate articles; pollutio articles, Sorption articles
Heavy metal pollutions articles Heavy metal pollutions Research articles Heavy metal pollutions review articles Heavy metal pollutions PubMed articles Heavy metal pollutions PubMed Central articles Heavy metal pollutions 2023 articles Heavy metal pollutions 2024 articles Heavy metal pollutions Scopus articles Heavy metal pollutions impact factor journals Heavy metal pollutions Scopus journals Heavy metal pollutions PubMed journals Heavy metal pollutions medical journals Heavy metal pollutions free journals Heavy metal pollutions best journals Heavy metal pollutions top journals Heavy metal pollutions free medical journals Heavy metal pollutions famous journals Heavy metal pollutions Google Scholar indexed journals azotized Polyethylene terephthalate articles azotized Polyethylene terephthalate Research articles azotized Polyethylene terephthalate review articles azotized Polyethylene terephthalate PubMed articles azotized Polyethylene terephthalate PubMed Central articles azotized Polyethylene terephthalate 2023 articles azotized Polyethylene terephthalate 2024 articles azotized Polyethylene terephthalate Scopus articles azotized Polyethylene terephthalate impact factor journals azotized Polyethylene terephthalate Scopus journals azotized Polyethylene terephthalate PubMed journals azotized Polyethylene terephthalate medical journals azotized Polyethylene terephthalate free journals azotized Polyethylene terephthalate best journals azotized Polyethylene terephthalate top journals azotized Polyethylene terephthalate free medical journals azotized Polyethylene terephthalate famous journals azotized Polyethylene terephthalate Google Scholar indexed journals pollution articles pollution Research articles pollution review articles pollution PubMed articles pollution PubMed Central articles pollution 2023 articles pollution 2024 articles pollution Scopus articles pollution impact factor journals pollution Scopus journals pollution PubMed journals pollution medical journals pollution free journals pollution best journals pollution top journals pollution free medical journals pollution famous journals pollution Google Scholar indexed journals Sorption articles Sorption Research articles Sorption review articles Sorption PubMed articles Sorption PubMed Central articles Sorption 2023 articles Sorption 2024 articles Sorption Scopus articles Sorption impact factor journals Sorption Scopus journals Sorption PubMed journals Sorption medical journals Sorption free journals Sorption best journals Sorption top journals Sorption free medical journals Sorption famous journals Sorption Google Scholar indexed journals polyethylene terephthalate articles polyethylene terephthalate Research articles polyethylene terephthalate review articles polyethylene terephthalate PubMed articles polyethylene terephthalate PubMed Central articles polyethylene terephthalate 2023 articles polyethylene terephthalate 2024 articles polyethylene terephthalate Scopus articles polyethylene terephthalate impact factor journals polyethylene terephthalate Scopus journals polyethylene terephthalate PubMed journals polyethylene terephthalate medical journals polyethylene terephthalate free journals polyethylene terephthalate best journals polyethylene terephthalate top journals polyethylene terephthalate free medical journals polyethylene terephthalate famous journals polyethylene terephthalate Google Scholar indexed journals Fourier transform infrared articles Fourier transform infrared Research articles Fourier transform infrared review articles Fourier transform infrared PubMed articles Fourier transform infrared PubMed Central articles Fourier transform infrared 2023 articles Fourier transform infrared 2024 articles Fourier transform infrared Scopus articles Fourier transform infrared impact factor journals Fourier transform infrared Scopus journals Fourier transform infrared PubMed journals Fourier transform infrared medical journals Fourier transform infrared free journals Fourier transform infrared best journals Fourier transform infrared top journals Fourier transform infrared free medical journals Fourier transform infrared famous journals Fourier transform infrared Google Scholar indexed journals flame atomic absorption spectroscopic articles flame atomic absorption spectroscopic Research articles flame atomic absorption spectroscopic review articles flame atomic absorption spectroscopic PubMed articles flame atomic absorption spectroscopic PubMed Central articles flame atomic absorption spectroscopic 2023 articles flame atomic absorption spectroscopic 2024 articles flame atomic absorption spectroscopic Scopus articles flame atomic absorption spectroscopic impact factor journals flame atomic absorption spectroscopic Scopus journals flame atomic absorption spectroscopic PubMed journals flame atomic absorption spectroscopic medical journals flame atomic absorption spectroscopic free journals flame atomic absorption spectroscopic best journals flame atomic absorption spectroscopic top journals flame atomic absorption spectroscopic free medical journals flame atomic absorption spectroscopic famous journals flame atomic absorption spectroscopic Google Scholar indexed journals flame atomic absorption spectrophotometer articles flame atomic absorption spectrophotometer Research articles flame atomic absorption spectrophotometer review articles flame atomic absorption spectrophotometer PubMed articles flame atomic absorption spectrophotometer PubMed Central articles flame atomic absorption spectrophotometer 2023 articles flame atomic absorption spectrophotometer 2024 articles flame atomic absorption spectrophotometer Scopus articles flame atomic absorption spectrophotometer impact factor journals flame atomic absorption spectrophotometer Scopus journals flame atomic absorption spectrophotometer PubMed journals flame atomic absorption spectrophotometer medical journals flame atomic absorption spectrophotometer free journals flame atomic absorption spectrophotometer best journals flame atomic absorption spectrophotometer top journals flame atomic absorption spectrophotometer free medical journals flame atomic absorption spectrophotometer famous journals flame atomic absorption spectrophotometer Google Scholar indexed journals Azotized Polyethylene Terephthalat articles Azotized Polyethylene Terephthalat Research articles Azotized Polyethylene Terephthalat review articles Azotized Polyethylene Terephthalat PubMed articles Azotized Polyethylene Terephthalat PubMed Central articles Azotized Polyethylene Terephthalat 2023 articles Azotized Polyethylene Terephthalat 2024 articles Azotized Polyethylene Terephthalat Scopus articles Azotized Polyethylene Terephthalat impact factor journals Azotized Polyethylene Terephthalat Scopus journals Azotized Polyethylene Terephthalat PubMed journals Azotized Polyethylene Terephthalat medical journals Azotized Polyethylene Terephthalat free journals Azotized Polyethylene Terephthalat best journals Azotized Polyethylene Terephthalat top journals Azotized Polyethylene Terephthalat free medical journals Azotized Polyethylene Terephthalat famous journals Azotized Polyethylene Terephthalat Google Scholar indexed journals polyethylene terephthalate articles polyethylene terephthalate Research articles polyethylene terephthalate review articles polyethylene terephthalate PubMed articles polyethylene terephthalate PubMed Central articles polyethylene terephthalate 2023 articles polyethylene terephthalate 2024 articles polyethylene terephthalate Scopus articles polyethylene terephthalate impact factor journals polyethylene terephthalate Scopus journals polyethylene terephthalate PubMed journals polyethylene terephthalate medical journals polyethylene terephthalate free journals polyethylene terephthalate best journals polyethylene terephthalate top journals polyethylene terephthalate free medical journals polyethylene terephthalate famous journals polyethylene terephthalate Google Scholar indexed journals
Article Details
1. Introduction
Despite the fact that water covers 71% of the surface of the earth, there exist a serious challenge in the availability of drinking water due to harmful dissolved species in the water [1]. Such stably dispersed species make it a serious concern on the health and wellbeing of man as a consumer of that vital commodity [2, 3]. Even when the levels of the dissolved species are at trace levels and regarded to be acceptable, there is danger in the consumption of such water due to bioaccumulation of metals that can eventually be incorporated in both hard and soft body tissues [2-4]. Such dissolved metal ions typically exist as positively-charged species and can bind to negatively charged organic moieties. In such a process, if the organic moiety are in the body fluid system, they act as Lewis base by donating electrons to the metal atom that becomes a Lewis acid or electron pair acceptor. Such a reaction ends up in the denaturation of the proteins and hence disruption of their activities ending to the eventual death of cells [5]. Such binding mechanism is usually by the formation of complexes which are difficult to retrieve safely from the bound metal.
To mitigate the metal denaturation poisoning process, the scarce insufficient clean and safe water has to be provided for human consumption. The best remedial method is to remove the metals from the water before consumption, to ensure its safety. This can be achieved by using the very same method of binding the metals from the water, using a similar mechanism that causes poisoning effect before the water is consumed, so that it can be presented safe for consumption.
Adsorption is a cost-effective methods capable of removing heavy metal ions in trace levels ranging from 1 mgL-1 to 20 mgL-1 in waste have been reported [6]. This has been achieved by the use of sorbents from biological origin in packed columns [7]. The bio sorption metal removal process has been found to be very effective as the exhausted biomaterial could be regenerated for re-use. The metals complexed with the sorbent functional groups of the biomaterial can easily be stripped using a dilute acid [8]. This excellent remedial method has its challenges in that the binding happens in certain specific stoichiometric ratios that cannot be quantified when using environmental samples [8]. Thus, it is difficult to determine when the biosorbent has been exhausted unless with the use of suitable instruments such as a conductivity meter or calorimeters. Such instruments can monitor the varying concentration of species being eluted. This renders the removal method out of reach to a vast majority of the needy cases due to the limiting facilities and resources. To address this challenge, the development of safe and affordable metal concentration colour sensitive material capable of removing heavy metals from water and render it safe for domestic consumption was considered. This study explored the use of aromatic compounds with azo (-N=N-) chromophores to treat the polluted water by removal of contaminants. This was achieved by using the property of a metal concentration colour sensitive self-indicating adsorbent material. The azo compound was anchored on a solid material (PET) which is readily available found littering the environment. It was sourced at a low cost and thus a good way of cleaning the environment. The modified material is water insoluble, but capable of interacting with metals ions in water and holding them on its surface, thus removing them from water thus rendering the water safe for human consumption [9]. The mechanism is similar to stationery phase separation process as in chromatography. This enables a complete removal of dissolved metal ions from water by stripping them off from the liquid dispersing media. The application of this adsorbent has an advantage in that an efficient and easy to operate for pollution removal from water and can indicate when the synthesized azo chromophore adsorbent have their adsorption sites saturated thus alerting the operator when to regenerate it. Such a low cost investment alleviates the drawbacks of the need for high skilled personnel to monitor the metal removal process and therefore, several treatment points can be setup increasing the availability of clean water. This motivates the population to value the waste PET bottles as a material that can be used as raw material for the synthesis of a colour sensitive adsorbent for the removal of metals from water.
We report the performance of azotized polyethylene terephthalate (PET) on adsorption of copper, lead, cadmium and chromium in aqueous solution. This is as reported by Ungureanu and co-workers (2020) as the functioned PET with phenolic compounds and subsequently applied the resultant for the removal of copper ions from water [10]. This is because azo compounds are electron acceptors or radical ligands with metal–ligand orbital mixing in the singly occupied molecular orbitals thus able to bind with metal ions [10]. In this study, we thus exploited azo compounds as they represent a class of coloured compounds used in textile-processing and other industries because of their colour. These compounds possess sites capable of forming metal-complexes by coordination to develop dye-metal complexes also called premetallized dyes despite being waster [10]. Such dyes could interact with metals in ratios of either 1:1 metal complexes or 1:2 metal complexes, to form metalized azo pigments that exhibit certain colours specific to the ratio [10]. The azo pigments in this study are similar in chemical structure to azo dyes, but they lack solubilizing groups. Below shows a presentation of a PET metalized azo pigment.
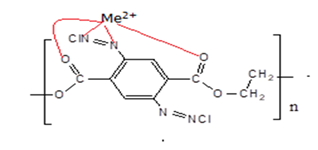
Scheme 1: Metalized azo pigment
The chromophores used in pigments are usually the same as those used in dyes, but the pigments are large molecules and do not have water solubilizing groups [11]. They contain groups that form intermolecular bonds that help to reduce water solubility. Such azo compounds are insoluble even in acidic conditions and therefore suitable for use in water treatment process [12]. Despite such azo being insoluble in water they have an affinity for hydrophobic metal ions (metallization) to produce a coloration specific to the metal. Metallization imparts a hypso or bathochromic shift to the colour of the material indicating the bonding of ligand to the metal ions through both azo nitrogen atoms [13]. Metal-complex dyes form strong coordination complexes and are an integral part of the chromophore [14]. Variation of the metal-azo ratio contribute to a variation of the colour. This is as reported by Chakraborty (2013) when he observed that the spectra of 1:1 metal-complex azo dyes displayed narrower curves, while that of 1:2 complex dyes show a broader curve exhibiting blueness and dullness [14]. That colour variation was exploited in this study to determine the exhaustion of the metallized azo material.
Although metal complexes do not have the same ligands, they are all anchored on the coordinating sites in a similar way irrespective of the electron donating group and its coordinating capacity [15]. Therefore, interaction of acids with metal complexes that include metallized azo material will result in protonation of the ligand and subsequent release of the metal similar to the corrosion process. This study exploited that property to regenerate the metal bound azo based adsorbent material for re-use by protonation of the metal pigment complex. This work presents the preparation and subsequent application of modified PET material for use in the remediation of metal laden water.
The polyethylene telephthalate material is a water insoluble chemically stable polymeric transparent solid material of high density (1.38 g cm-3) and a melting point of 260°C (500°F). This justifies its extensive use for shipping bulk food and packing of retail food products as well as a bottling material for water and soft drinks [16]. Structurally, PET contains no functional groups (sites) for metal ion sorption. For it to be applied as a metal adsorbent, a modification is required to create active sites for metal interaction. The modification process involves the attachment of an azo group that is able to chelate with metals and its colour will be influenced by the metal ion complexion. This is similar to metal complexometric indicators (pM) that undergoes a definite colour change upon interaction with some specific metal ions due to formation of weak complex with the ions present in the solution. This contributes to a significantly different colour change from the form existing outside the complex. The PET material used in this study was from disposed bottles collected from dump sites in Nairobi. Environmental water samples were obtained from five different points in triplicates from Kipsonoi River in Sotik Sub-county of Bomet County.
The study aimed in exploiting the waste non-biodegradable synthetic material to offer a solution of remediation of water for domestic consumption. The waste PTE was used as a raw material to offer a solution to metal laden waters as well as put to good use the discarded waste that litters and affects negatively the aesthetic beauty of the environment. This study exploited the variation of colour of the modified material upon interaction metal ions. That colour variation confirms the presence of adsorbed metals or the absence of the metals upon regeneration when the material reverts to its original colour upon stripping the metal ions from the adsorbent.
2. Materials and Methods
2.1 Chemicals and reagents
All the solutions were prepared in double distilled de-ionized water and all the reagents were of analytical grade. Copper (II) sulphate, lead (II) nitrate, cadmium nitrate, potassium dichromate, sulphuric acid, sodium nitrite, acetic acid (98%), hydrochloric acid (36.6 %), granulated tin, nitric acid, sodium hydroxide and sodium acetate. They were obtained from Sigma-Aldrich through its outlet Kobian (K) in Nairobi. Metal standard stock solutions (1000 mg/L) were prepared in 0.1M sodium acetate solution.
2.2 Instrumentation
The metal ions content in solution was determined by flame atomic absorption spectrophotometer (FAAS), Shimadzu AA-62000 model, while the characterization of the raw and modified sorbent material was done using Fourier transform infrared spectrometer (Bruker Tensor 27- HTS-XT MIR model). The pH of the solutions in this study was adjusted using a 3505-JENWAY (UK) pH meter. The mechanical reciprocating shaker used for the agitation of the experimental sample mixtures was a DKZ-1 NO. 1007827 model.
Procedures
2.3a Azotization of polyethylene terephthalate
The waste non-biodegradable synthetic PET bottles were cleaned, dried, uncapped and their labels removed before being crushed to small flakes. The resultant PET flakes was then modified using the method proposed by Sliman and co-workers as the functionalized PET knitted fabrics [17]. This was achieved by transferring each time a mass of 5.00 g (PET flakes) into a 250 mL round bottomed flask containing 40 mL of concentrated nitric acid and 60 mL of concentrated sulphuric acid. The mixture was maintained at a temperature below 60 oC, for 1 hour [18]. The resultant mixture containing the nitrated product was transferred into a 1.0 liter beaker half filled with ice water. The mixture was then filtered and the residue washed with double distilled water until the solution obtained as the filtrate attained a pH of 4-5. The nitrated product was reduced by mixing it with concentrated hydrochloric acid-ethanol mixture (45:50, v/v) and 50.00g of tin granules and refluxed at 90 ºC for 24 hours. The product formed was then cooled to ambient temperature, filtered and washed with acid-ethanol mixture (45:50, v/v) followed by 2.0 M sodium hydroxide and then distilled water [19]. The amino product formed was diazotized by cooling it in an ice bath (0-3 ºC) and 75 mL of 1.0 M sodium nitrite added, followed by 100 mL of hydrochloric acid. The mixture was stirred and tested with iodide paper until the paper changed colour from white to violet due to formation of diazotized product. The product was then washed with ice-cold water, dried, then analyzed using FTIR and then applied for metal sorption [19]. The plausible mechanism for the modification process is given in Scheme 1.
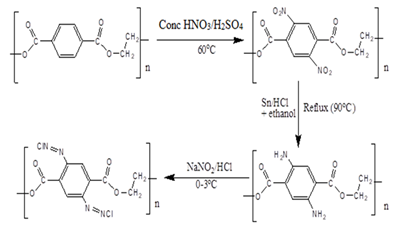
Scheme 2: Azotization of the polyethylene terephthalate material
The reaction scheme 1 shows the products obtained in each respective step of the modification process.
2.3b Optimization of sorption parameters
Batches of 20.00 ml model solutions (20 ppm) were dispensed into 100 ml polyethylene bottles containing approximately 0.03g of the azotized PET material. Thereafter, model solutions different samples (10 ml) of the metal model solutions containing 50 mg l-1 prepared in an appropriate buffer solution (0.1 M sodium acetate/acetic acid) were taken and their pH values adjusted to the desired pH values of between pH 2.0 and pH 7.0. This is because pH environments affect the stability the chelation sites of ligands and thus making the sorption selectivity the various metals being pH dependent. This is because each of the respective metal ion has its critical pH value of complexation and also again different pH values affects the chemical properties of the adsorbent differently and also at certain values induce precipitation of the metal ions. The pH for the best removal for each of the respective metal ions under study was established by FAAS. It was found that the optimum pH for the adsorption of copper, lead and chromium was 6.0 except that of cadmium was that was adsorbed at a pH value of 5.5. The pH of the model solutions was adjusted to the appropriate pH value for each metal ion under study for analysis.
The removal of metals by the adsorbent is dependent on contact time. Sufficient time needs to establish if the desired product to be formed for the available reactants. In this study, the appropriate time for the removal of the metal analyte was determined experimentally. The mixtures were equilibrated at first for 100 minutes (sufficient time) after which the metal in solution was analyzed using FAAS. The optimal sorption time was established by carrying out the experiment at room temperature while maintaining the optimum pH value for each respective metal ion and equilibrating the analytes at different time intervals followed by the determination of the metal ions under study was established by FAAS.
The effects of the other parameters such as initial metal ion concentration and sorbent dose on metal ion removal from water were also investigated at room temperature by varying the parameter under investigation while maintaining the others constant and maintaining the optimal pH value using either sodium acetate solution or 0.1 M nitric acid dropwise.
The mixture was allowed to equilibrate and then removed from the shaker at different time intervals between 5-100 minutes. The resultant mixture was then filtered off by suction and the concentration of metal ions in the filtrate determined. All the experiments were carried out in triplicates. The content of the metal ions adsorbed were calculated using equations 1 and 2 for the adsorption capacity and removal efficiency respectively [20].

Where
m=mass of adsorbent (g)
qe = Equilibrium adsorption capacity (mg/g)
Co =Initial concentration (mg/L)
Ce = Concentration at unit time (mg/L)
% R = Percentage removal
V = Volume of solution (L)
2.3c Effect of pH on complexation modified and unmodified PET
The optimum pH value for the uptake of each heavy metal ion was determined by placing 0.03 g of azotized PET into 100 mL polythene bottles and adding 20 mL of solutions containing 20 mg/L of each analyte ion prepared in 0.1 M sodium acetate/acetic acid. The pH was adjusted to different values (2-7) using 0.1 M of nitric acid or 0.1M sodium hydroxide. The mixture was then equilibrated for 2 hours in a mechanical shaker at 180 rpm, filtered and the concentrations of the metal ions in the filtrate determined using flame atomic absorption spectroscopy (FAAS)
[21, 22].
2.3d Effect of contact time on complexation
A sample containing 0.03 g of azotized PET was placed into 100 mL polyethylene bottles. Aliquots of 20 mL solutions of 20 mgL-1 concentrations initially adjusted to the optimum pH value for each metal ion under study was added into each of the bottles. The contents of the bottles were agitated on a mechanical reciprocating shaker at 180 rpm. Samples were removed at different time intervals and filtered by suction. The residual metal ion concentration in the filtrate was then determined by FAAS [22].
2.3e Effect of the initial metal ion concentration on complexation
The effect of concentration of the metal ion on the uptake by azotized PET was determined by placing 0.03 g of the adsorbent into a series of polyethylene bottles containing 20 mL of metal ions under study at different concentrations (10-100mg/L) buffered at optimum pH for each metal. The contents of the bottles were equilibrated on the mechanical shaker at 180 rpm for 50 minutes. After filtration, the residual concentration of the metal ions in the filtrate was determined by FAAS [21, 22].
2.3f Effect of adsorbent dose on removal of heavy metal ions
To determine the effect of the amount of azotized PET on the metal ions uptake, 20 mL of the model solutions containing 20 mgL-1 of metal ions buffered to optimum pH value for each metal ion were added to varying amounts (0.01-0.50 g) of the adsorbent in polyethylene bottles. They were then agitated in a mechanical shaker at 180 rpm for 50 minutes and then filtered. The concentration of metal ions in the filtrate were then determined using FAAS [21].
2.4 Adsorption kinetics
The uptake rate of metal ions and the mechanisms of binding by the adsorbents were evaluated by investigating their sorption kinetics. The kinetic models used in this study were the Lagergren’s Pseudo-first-order (Equation 3) and the Ho’s pseudo-second-order (Equation 4) [23, 24]. These models revealed the rate controlling steps and sorption mechanism.

Where qe and qt (mg g-1) represents the amount of metal ions adsorbed on the adsorbent at equilibrium and time, , respectively; (min-1) and (g mg-1min-1) are the adsorption rate constants of the pseudo-first-order and pseudo-second-order kinetics respectively. The approximate values of and can be obtained from the plots of versus and versus respectively. The experimental results were fitted into adsorption models in order to determine the kinetics and mechanisms of adsorption.
2.5 Adsorption isotherms
Adsorption isotherms describe how the adsorbate interacts with adsorbents. The model isotherms applied in this study were Langmuir and Freundlich adsorption isotherms [25, 26]. They were applied to evaluate factors such as adsorption capacity and mechanism of adsorption. Adsorption equilibrium is attained when the capacity of the complexing agent is achieved and the rate of uptake of metal ions corresponds with the rate of desorption. The maximum adsorption capacity of an adsorbent was then determined using the Langmuir and Freundlich isotherms.
The Langmuir isotherm is based on the fact that all the binding sites of an adsorbent are identical and of equal energies. This model assumes that the adsorption process takes place at specific homogeneous sites on the adsorbent. This applies to the monolayer adsorption process [27]. The linear form Langmuir model is given by equation 5.

Where (mg L-1) is the metal ion concentration at equilibrium, (mg) is the adsorption capacity, (mg g-1) is the maximum adsorbate amount that forms a complete monolayer on the surface, and (L mg-1) is the Langmuir constant related to the rate of adsorption. The higher the value of the much stronger the affinity of metal ion uptake. The values of and can be approximated from a plot of versus and determining its slope and intercept.
Freundlich isotherm is based on the equilibrium relationship between heterogeneous surfaces. This model assumes that the adsorption sites are distributed exponentially with respect to the heat of adsorption. Equation 6 below represents the linear form of Freundlich isotherm.

Where, have the same meaning as in equation 5; is the adsorption capacity while denotes the energetic heterogeneity of adsorption sites. When a graph of versus is plotted, the values of and can be estimated. The values of n should be within the range 1-10 under normal adsorption conditions.
The data obtained in the effect of initial metal ion concentration was treated with the Freundlich and Langmuir adsorption models to determine the sorption mechanism and the best adsorption model for the uptake of each of the respective heavy metal ion dispersed in the solution.
2.5 Desorption studies
Desorption studies were carried out using separate transparent polyethylene columns of capacity 10 ml which had had a frit was inserted before packing the adsorbent material. In each of the columns, 0. 3 g of modified PET was packed and a frit was inserted after parking the adsorbent. The packed (tubes) columns were washed successively with water, preconditioned and buffered to the desired pH before use. After the conditioning, 10 mL of the different sample solutions containing 10 mg L-1 of copper, lead, cadmium and chromium ions were separately loaded in each respective column and each respective eluent collected. The retained metal ions were stripped using 5 mL of 1.0 M nitric acid. The concentrations of each of the desorbed metal ions were then determined using FAAS. The demetalization of the metalized azo material can be regarded as acid treatment of the azo-metal complex as presented below.
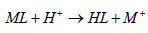
The reusability of the azotized PET was determined by repeating the adsorption-desorption cycles five times under the same experimental conditions [22, 26].
2.6 Analysis of environmental water samples
Samples of water from River Kipsonoi (Bomet County, Kenya) were collected using 1 L polyethylene bottles. They were filtered and the concentration of copper, lead, cadmium and chromium ions determined. Portions (50 mL) of water were separately spiked with known concentrations of copper, lead, cadmium and chromium ions. Each of the resulting solution was then added to a 0.03 g of azotized PET adsorbent in a 100 mL polyethylene bottle and agitated for 50 minutes on a mechanical reciprocating shaker at 180 rpm. The retained metal ions were then stripped with 10.0 mL of 1.0 M nitric acid and their concentrations determined by FAAS [21].
3. Results and Discussions
3.1 Characterization of the adsorbent material
Characterization of the parent PET and its chemically modified forms were done using FTIR. The results are discussed in the following sub-sections.
3.1a Parent PET material
The FTIR spectrum of the parent PET is given by Fig 1.
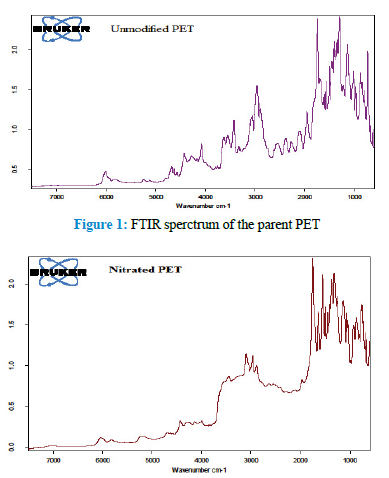
Figure 1: FTIR sperctrum of the parent PET
The spectrum (Figure 1) shows a peak at 3642.9 cm-1 which may be attributed to the terminal O-H stretching [22,29]. A peak which appeared at 3085.6 cm-1 may be due to aromatic C-H stretching [4, 27, 31,32]. The peak observed at 2973.7 cm-1 could be as a result of the aliphatic methylene asymmetric C-H stretching while the one appearing at 2910.1 cm-1 could be assigned to methylene symmetric C-H stretching [22,27,31-33].. The sharp peak at 1745.3 cm-1 corresponds to C=O stretching while the peak at 1616.1 cm-1 may be assigned to aromatic C=C stretching [22,29,31-33]. The methylene C-H bending bands were observed at the frequencies of 1459.9 cm-1 (scissoring), 1346.1 cm-1 (wagging), 1299.8 cm-1 (twisting) and 732.8 cm-1 (rocking) [31,33]. The aromatic in-plane C-H and out-of-plane bending peaks occurred at 1216.9 cm-1 and 680.8 cm-1 respectively [331]. The peak at 1137.8 cm-1 could be as a result of C-O stretching [33]. The parent material was then chemically modified by anchoring the required functional groups within its structure.
3.1b Nitrated PET
Below shows the spectrum of the nitrated PET.
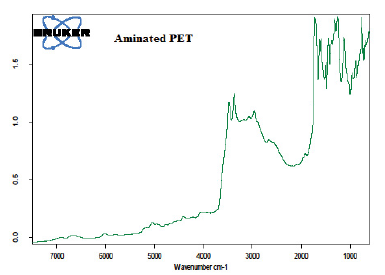
Figure 2: FTIR sperctrum of the nitrated PET
The FTIR results as in Figure 2, show a band at 3102.9 cm-1 which could be due to aromatic C-H stretching [4,27, 29]. Aliphatic C-H stretching band appeared at 2971.8 cm-1 [22, 33-35]. The band at 1756 cm-1 could be due to aromatic C=O stretching, while the one observed at 1556.3 cm-1 could be due to aromatic C=C stretching [22,27-29]. The peaks appearing at 1162.9 cm-1 and 1114.7 cm-1 may be attributed to aromatic in-plane C-H bending bands, while those observed at 885.2 cm-1, 769.5 cm-1, 725.1 cm-1 and 702.0 cm-1 may be assigned to aromatic out-of- plane C-H bending bands [31,33].
The additional peaks observed at the frequencies 1554.4 cm-1 and 1359.6 cm-1 may be due to aromatic nitro, NO2 , asymmetric and symmetric stretching frequencies respectively [21,31,38]. This indicates a successful nitration of the aromatic ring of PET.
The nitro functional group in the nitrated material was then chemically reduced to produce an amino functional group within the structure.
3.1c Aminated PET
Below shows the spectrum of the aminated PET
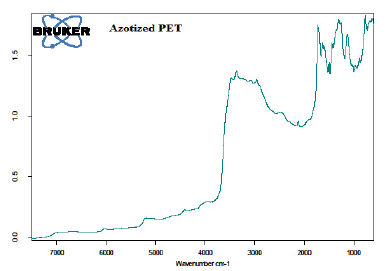
Figure 3: FTIR spectrum of the aminated PET
The results (Figure 3) shows the appearance of two sharp peaks at the frequencies 3484.8cm-1 and 3363.3 cm-1. These correspond to signals of N-H stretching [31, 38]. A peak observed at 2989.2 cm-1 may be assigned to aromatic C-H stretching [4, 28, 32]. Aromatic C=O stretching occurred at 1729.9 cm-1 while C=C stretching appeared at 1450.2 cm-1 [22, 29-38]. The bands at 1137.8 cm-1 may be due to aromatic in-plane C-H bending while those at 900.6 cm-1 and 767 cm-1 could have been as a result of aromatic out-of-plane C-H bending [26,31,39].
The signal observed at 1554.5 cm-1 for the nitrated PET (Figure 2) disappeared and a new one appeared at 1598.0 cm-1. This may be attributed to signals of N-H vibrations [29, 34]. These observations can be ascribed to the successful reduction of the nitro group to amino group in the aromatic ring of PET. The amino group was then azotized to produce an adsorbent whose colour was sensitive to metal ion concentrations.
3.1d Azotized PET
Below shows the spectrum of the azotized PET
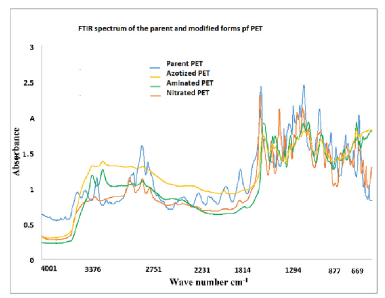
Figure 4: FTIR sperctrum of the azotized PET
The results presented in Figure 4 indicate that the signal which was at a 3300 cm-1 and a shoulder at 3400 cm-1 corresponding to 1o amine (-NH2) stretching observed in aminated PET (Figure 3) disappeared. This may be attributed to the diazotization process of aminated PET. The bands corresponding to aromatic C-H stretching was observed at 3035.4 cm-1 [4, 29- 32]. Aromatic C=O stretching band occurred at 1733.7 cm-1 [22, 29-33]. Aromatic in-plane C-H bending bands appeared at 1143.6 cm-1 and 1031.7 cm-1 , while aromatic out-of-plane bending band was noted at 771.4 cm-1 [22]. The C=C stretching band occurred at 1569.8 cm-1 [22, 29-33]. The peak corresponding to an azo (-N=N-) stretching was observed at 1532.9 cm-1 [31, 35]. Signals with frequencies between 1500 -1600 cm-1 are evidence of the azo group [36]. This signifies a successful azotization of the aminated PET. Below shows the combined results of FTIR analysis
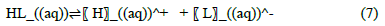
Figure 5: FTIR sperctrum of the parent and modified forms of PET
Figure 5 is presents results obtained from the FTIR analysis. The results analysis show that the findings was in agreement with the expected products in each step of the modification process as presented in the reaction scheme [31,35]. The final product was then applied for optimization of metal ions sorption parameters from water.
3.2 Optimization experiments
Optimization experiments were done in order to determine the most suitable conditions which favour the maximum removal of the heavy metal ions from aqueous solution. The results are highlighted in the following sub-sections.
3.2a Effect of pH
The metal binding efficiency of nitrogen-containing ligand is greatly influenced by the pH. This is due to the fact that pH affects the charge on the surface of the electron donating group (nitrogen), the degree of ionization, the speciation of metal ions and the level of precipitation [36,37].
The binding of metals by a chelating polymer is based on the Lewis acid-base theory. Nitrogen atom on the coordinating group acts as a Lewis base by donating electrons to metal cations which act as Lewis acids [38]. For the adsorption process to occur, the binding sites in the adsorbent dissociates according to equation 7.
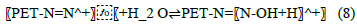
Where HL is the adsorbent which on dissociation yields a proton, H+ and conjugate base of the acid, L-. This implies that the pH of a solution has a significant effect on the dissociation of the adsorbent functional groups and hence the uptake of metal ions. The dissociation of azotized PET may thus be given by the equation.
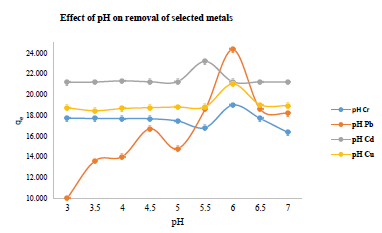
Figure 6 shows effect of pH on sorption of metal ions by azotized PET.
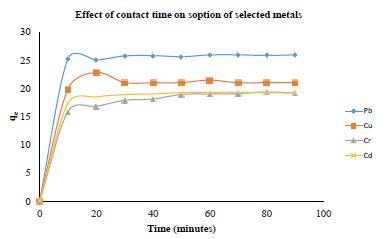
Figure 6: Effect of pH on sorption of metal ions by azotized PET
The results show that at low pH values, the uptake of metal ions was low. This may be attributed to the high concentration and mobility of H+ ions which favours the adsorption of H+ ions over the metal ions. [38]. This leads to the competition between the metal ions and the H+ ions resulting in the protonation of the chelating polymer which enhances the electrostatic repulsion of metal ions in solution [38]. The adsorption of heavy metal ions increased with the increase in pH and reached optimal values of 6.0 for copper lead and chromium while 5.5 for cadmium. Increase in the pH causes the deprotonation of the binding sites and make the surface functional groups of the complexing agents to be negatively charged. This consequently leads to in an increase in the uptake of metal ions due to electrostatic forces of attraction [21, 5-37]. It also reduces the competition between H+ ions and adsorbate cations for the binding sites on the adsorbent [21]. Beyond that optimal pH region, precipitation of the metal ions results [21, 3 6-38].
3.2b Effect of contact time on metal uptake
The rate of metal ion uptake by the azotized adsorbent was investigated when the mixture was buffered at their respective optimum pH values obtained experimentally. This was intended to monitor the efficiency of the sorbent binding sites to hold the metal, as well as the activity of the metal, thus controlling the residence time of sorbate at the solid-solution interface [39, 40]. The contact time required for adsorption to be completed is critical in designing the adsorption process since it gives the minimum time needed for the uptake of metal ions. [36].The results obtained were presented in Figure 7.
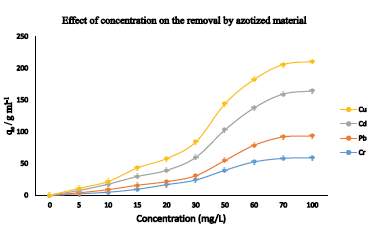
Figure 7: Effect of contact time on the adsorption of metal ions
The results show that the general rate of metal uptake up take was very high as 90% within the first 10 minutes of interaction. That rapid uptake may be attributed to the availability of numerous binding sites in the surface of the azotized PET adsorbent [38, 39]. This implies that a contact time of 10 minutes is sufficient to achieve maximum adsorption. However, in this study, sorption experiments were carried out at an equilibration time of 40 minutes. The decline in the rate of the uptake of metal ions after the equilibrium time for each metal cation could be as a result of the saturation of the coordination sites of the adsorbents [41].
3.2c Effect of the initial metal ion concentration on the uptake of metal ions
The adsorption capacity of azotized PET was determined by adding 10 mL model solutions of concentrations ranging between 2 mg L-1 and 100 mg L-1 and buffered to the optimum pH for each metal ion to 0.03 g of the adsorbent in polystyrene screw-cap bottles. The mixtures were then agitated on a mechanical shaker for 40 minutes, then filtered and the resulting filtrate analyzed for the metal ion concentration, using FAAS. The amount of metal ion adsorbed (qe) was plotted against the initial metal ion concentration (Co) and the results obtained are presented in Figure 8.
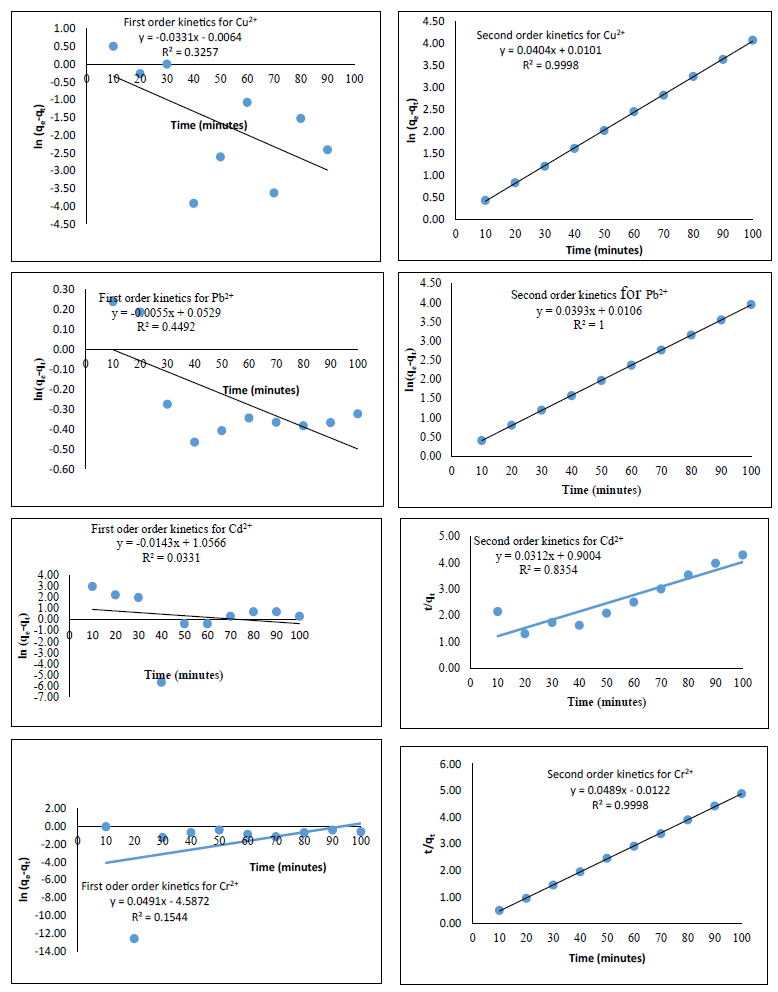
Figure 8: Effect of initial metal ion concentration on the sorption of metal ions
The results show that the metal ions uptake increased almost linearly with the increase in the concentration of the metal ions up to 80, 60, 70 and 80 mg/L for copper, lead cadmium and chromium ions respectively, then levels off. This observation could be due to the exhaustion of available binding sites [36, 37]. Further increase in the concentration beyond the equilibrium concentration, leads to slower uptake of metal ions since the available active sites in the adsorbent have become saturated [42-47]. The uptake capacities were found to be: copper (48.49 mg/g), lead (33.65 mg/g), cadmium (70.93 mg/g) and chromium (59.06 mg/g).
It was also observed that as the metal ion concentration was increased, the colour of the azotized PET changed from yellow to orange. This can be attributed to the bathochromic shift to a long-wavelength absorption band as a result of the metal azo dye complexation [39]. These colour changes show that nitrogen in the azo (-N=N) group is responsible for the adsorption of metal ions by complexation. This is in agreement with studies reported earlier [35, 47-49].
3.3 Sorption Kinetics
The kinetic data obtained was analyzed using Lagergren [23, 24] first-order and Ho’s second-order kinetics. This was to investigate the molecularity of the sorption mechanism and the rate controlling steps [23,24]. The results obtained are as presented in the Figure 9 below.
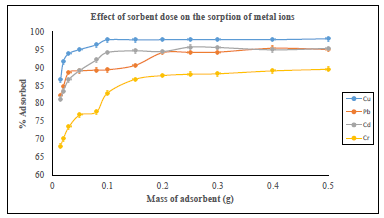
Figure 9: Kinetic data on the sorption of metal ions
The pseudo-first- and pseudo-second-order kinetic models for the binding of the considered heavy metal ions onto azotized PET are presented in Figures 9-11, while the values of the parameters are given in Table 1. The results indicate that pseudo-second-order best describe the kinetics of binding of all the metal ions studied onto the complexing agent since the R2 are closer to one. This is similar to the results obtained in several studies [37]. The pseudo-second-order rate constant ( ) followed a decreasing sequence of Cu (0.1616) > Pb (0.1457) > Cr (-0.1960) > Cd (0.00108) in mg min-1. This suggests that the chemical interaction is dependent on the affinity of metal ions to interact with the azo group (- N=N) of azotized PET.
A summary of the calculated factors from kinetic data for each metal uptake at their respective optimum parameters is as presented in a tabular form as shown in Table 1. From that information, the sorption kinetics prescribed by each respective metal is obtained based on their respective linear correlation coefficient, R2, values.
|
Metal ion |
Pseudo-First-Order |
Pseudo-Second-Order |
|||||
|
qe(mg/g) |
k1(min-1) |
R2 |
qe(mg/g) |
k2(g/mg min-1) |
R2 |
Best model |
|
|
Cu2+ |
0.9936 |
0.0331 |
0.3257 |
24.75 |
0.1616 |
0.9998 |
2nd order |
|
Pb2+ |
1.0543 |
0.0055 |
0.4492 |
25.45 |
0.1457 |
1 |
2nd order |
|
Cd2+ |
2.8766 |
0.0143 |
0.0331 |
32.05 |
1.081 x 10-3 |
0.8354 |
2nd order |
|
Cr6+ |
0.0102 |
-0.0491 |
0.1544 |
20.45 |
-0.196 |
0.9998 |
2nd order |
Table 1: Sorption kinetics for the uptake of selected heavy metals by azotized PET
The results show that the sorption for all the metal was of pseudo second order of multisite. This could be as a result of the metals being multivalent and the adsorbent having more than one active site [40].
3.3 Effect of adsorbent on the uptake of heavy metal ions
The type of the complexing agent and the functional groups it contains has intense effect on the binding of metal ions. The effect of adsorbent dose on the adsorption of metal ions was investigated at room temperature by varying the quantity of the adsorbents from 0.015 g to 0.5 g. Model solutions containing 40 mg L-1 of metal ions buffered at optimum pH value were added to the adsorbents and agitated in the mechanical shaker for 40 minutes. The mixture was then filtered and the concentration of the metal ions in the filtrate determined by FAAS [36]. The effect of the amount of the adsorbent on the % adsorption of metal ions is given by Figure 10 below.

Figure 10: Effect of sorbent dose on the sorption of metal ions
The results show that an increase in the amount of azotized PET resulted in an increase metal removal observed to be copper from 86.58 % to 98.10 %, lead from 82.17 % to 95.44 %, cadmium from 81.22 % to 95.77 % and chromium ions from 68.71 % to 89.57 %. The amount of the adsorbent required for the maximum uptake of copper, lead, cadmium and chromium were found to be 0.15 g, 0.4 g, 0.25 g and 0.4 g respectively. This could be as a result of an increase in the number of binding sites for metal sorption of the porous material [36, 37, 45-46]. An excessive increase in the amount of the adsorbent did not result in any significant changes in the % uptake of metal ions. This is probably because of the establishment of an equilibrium between the metal ions bounded to the azotized PET and those remaining in the aqueous solution [37]. The experimental data obtained for the adsorbed metal against equilibrium concentration, was analyzed using Langmuir and Freundlich equations.
3.4 Adsorption isotherms
The results from the optimization experiments were subjected to Langmuir and Freundlich isotherms. The results obtained are as presented in Figure 11.
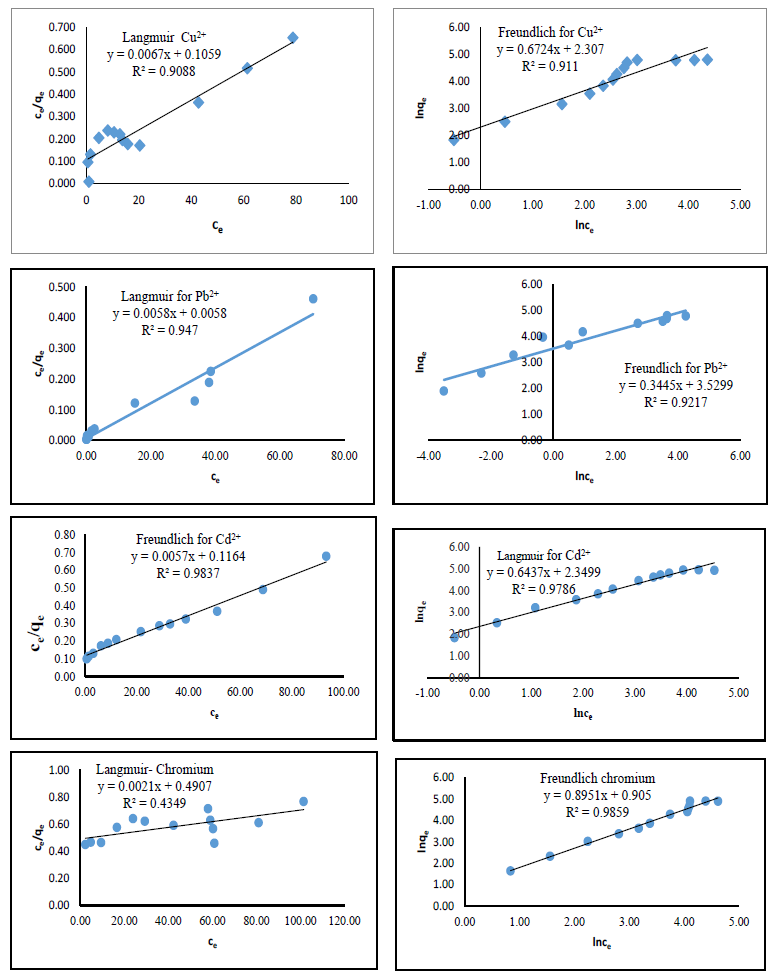
Figure 11: Adsorption isotherms for some selected metal by azotized PET
A summary of the calculated factors from linearized Langmuir and Freundlich adsorption models for each metal uptake at their respective optimum parameters is as presented in tabular form as shown in Table 2. From that information, the model prescribed by each respective metal is obtained.
The best model for the complexation of each metal ion considered is the one whose linear correlation coefficient, R2, value is closest to one. Table 2 represents the parameters of the adsorption isotherms.
|
Langmuir |
Freundlich |
||||||
|
Metal ion |
Qmax (mg/g) |
kl (L/mg) |
R2 |
1/n |
kF (mg/g) |
R2 |
Best model |
|
Cu2+ |
149.25 |
0.0633 |
0.9088 |
0.6724 |
10.04 |
0.911 |
Freundlich |
|
Pb2+ |
172.41 |
1 |
0.947 |
0.3445 |
34.12 |
0.9217 |
Langmuir |
|
Cd2+ |
175.44 |
0.049 |
0.9837 |
0.6437 |
10.48 |
0.9786 |
Langmuir |
|
Cr6+ |
476.19 |
0.0004 |
0.4349 |
0.8951 |
2.472 |
0.9859 |
Freundlich |
Table 2: Langmuir and Freundlich isotherm parameters for the binding of metal ions
The complexation of lead and cadmium ions are best described by the Langmuir isotherm model with R2 values>0.94. This indicates that azotized PET provided the homogenous surface for monolayer adsorption of lead and cadmium ions. The values of which determines the affinity of binding indicates that the binding sites of azotized PET had greater affinity for lead ions as compared to cadmium ions. This can be attributed to electronegativity of metal ions, since the binding of the metal ions on the adsorbent is also due to ion exchange at the surface [37]. The more electronegative the metal ions the higher the uptake by the adsorbent. According to Pauling’s scale, the electro negativities of lead and cadmium are 1.87 and 1.7 respectively [37].
Freundlich isotherm suited best in describing the binding of copper and chromium ions with R2 values of 0.9110 and 0.9859 respectively. The Freundlich isotherm can be applied in heterogeneous systems, especially for adsorbents with interactive species such as copper and chromium. These metal ions are small and also have multiple oxidation states, thus capable of causing polarizing effects leading to heterogeneity [37]. The behavior of and character metal ions species in solution depends on many parameters, such as the composition of dispersed complexing agents particulate matter, mineral content and pH of the dispersing media [37]. An example is the reduction of copper (II) ions in solution is distorted by nitrogen containing ligands in the presence of other functional groups. This is due to the electrostatic repulsions between copper ions and positively charged molecules at certain pH values [37]. The reason for that behaviour is existence of dissolved Cu (II) and Cu (I) oxidation states due to stabilization by the electron donation complexing agent. In that case, both species of copper are attracted by the nitrogen containing azo adsorbent. The same phenomenon is experienced in the analysis of chromium. The distortion due to electrostatic repulsions between the chromium ions and positively charged molecules of the azo adsorbent result to the metal having more one oxidation state attracted on the azo adsorbent. That contribute to the two metals prescribing to the multilayer adsorption as prescribed by the Freundlich model.
This was supported by the values for copper and chromium ions are 10.04 and 2.472 mg/g respectively. The values of being in the Freundlich model were observed to be less than 1 indicating that the adsorption of copper and chromium ions by azotized PET was heterogeneous thus multisite. The sorption of lead ions with modified azo adsorbent gave an R2 value of 0.947 for Langmuir and 0.9217 for the Freundlich model. These values have reasonably high correlation however, the sorption mechanism of lead can best prescribe to the Langmuir model. Langmuir model describes a monolayer adsorption and it indicates a chemisorption mechanism. Lead is a relatively bulky ion with low polarizing ability.
3.5 Regeneration of used adsorbent
Desorption studies were undertaken in order to ascertain the reusability of azotized PET adsorbent and thus the cost effectiveness of the treatment method. It is also useful in the recovery of valuable metals from water. This was done by loading 0.03 g of the adsorbent with 10 mL of 40 ppm metal ions [33]. Based on the fact pH affects the stability of the complexes, this study exploited that fact and used a mineral aid for the demetalization of the adsorbent by stripping of the metal ions from the PET modified material. This was the basis of regeneration of the metal loaded adsorbent. The metal ions retained by azotized PET were stripped from the adsorbent using 50 mL of 1.0 M nitric acid and the loaded metal determined using FAAS [22, 30]. The adsorption-desorption studies were repeated five times under the same experimental conditions, and the fraction recovered for each cycle was determined using equation 9.
Where Ce is the concentration of the metal ions retained while Co is the initial metal ion concentration. Figure 12 shows the adsorption-desorption cycles of the adsorbents for the metal ions.
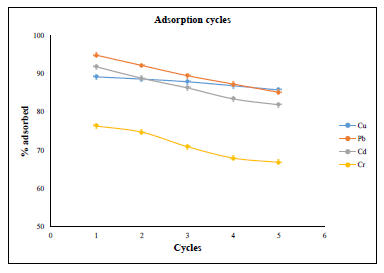
Figure 12: Adsorption cycles of the azotized PET for the metal ions.
From the results in Figure 11, it can be observed that azotized PET adsorbent could be used up to 5 cycles for the removal of copper, lead and cadmium with still over 80 % sorption efficiency. However, the adsorption efficiency of chromium ions by azotized PET declined to 66.81% by the 5th cycle. This indicate that the attached azo group is resistant to leaching and hence the regeneration ability and reusability of the azotized adsorbent. These results are in agreement with those obtained by Tharanitharan and Srinivasan as they removed Ni (II) ions from water and waste water using modified Duolite XAD-761 resin [31].
3.6 Analysis of environmental water samples
The azotized PET adsorbent was applied in real water samples obtained from Kipsonoi River. This was done by adding a 50 mL water sample previously spiked with known concentration of individual heavy metal ion to 0.03 g of the adsorbent and equilibrating for 50 minutes in a mechanical shaker. The retained metal ions were then stripped with 10.0 mL of 1.0 M nitric acid and their concentrations determined by FAAS [21, 35]. The results are given in Table 3.
|
Metal ion |
Added (mg L-1) |
Azotized PET |
|
|
Found (mg L-1) |
% recovered |
||
|
Cu2+ |
0 |
- |
|
|
2 |
1.985±0.010 |
92.00±0.459 |
|
|
4 |
3.851±0.020 |
92.62±0.476 |
|
|
Pb2+ |
0 |
- |
|
|
2 |
1.936±0.018 |
91.16±0.830 |
|
|
4 |
3.923±0.018 |
95.13±0.446 |
|
|
Cd2+ |
0 |
- |
|
|
2 |
1.978±0.018 |
98.63±0.887 |
|
|
4 |
3.613±0.027 |
90.21±0.664 |
|
|
Cr6+ |
0 |
- |
|
|
2 |
1.513±0.016 |
75.42±0.799 |
|
|
4 |
2.576±0.018 |
64.29±0.450 |
|
Table 3: Langmuir and Freundlich isotherm parameters for the binding of metal ions
The results (Table 3) obtained when the adsorbent was applied to real water sample are comparable to those obtained using the model solutions. The recovery values obtained were over 90 % for copper, lead and cadmium. However, the adsorption of chromium was found to be relatively lower for the environmental water samples. The recovery was not 100% due to the effect of interferences by species in the samples with an environmental matrix [47]. Despite that, the results indicate that there is reliability of the present method in the recovery of heavy metal ions from environmental water samples without significant interference by various matrices [35].
4. Conclusion
This study successfully functionalized PET to produce an azotized material which was confirmed by FTIR analysis. Azo compounds are electron acceptors or radical ligands with metal–ligand orbital mixing in the singly occupied molecular orbitals thus able to bind with metal ions. The modified material exhibited colour variations upon interaction with metal ions and it was observed that colour intensity depended on concentration of the adsorbed metal ions. That colour indicating metallization process was exploited as an adsorbent for the removal of the metals from water. The colour changing process of the synthesized adsorbent as it interacted with metal ions was capable of indicating when all its sites were metalized hence exhausted. Sorption parameters such as the effect of contact time, sorbent dose, pH of the sample and the adsorption capacity were investigated and optimized at fixed temperature (25 °C). The optimum pH for the adsorption of copper, lead and chromium was found to be 6.0 and that of cadmium was 5.5, thus within the physiological pH of water. The uptake of metal ions was more than 90% within the first 10 min of contact time implying that sorption was fast enough and the material was suitable for application in the removal of metals from water. The adsorption of the metals under study prescribed to the Freundlich isotherm. The adsorption capacities of copper, lead, cadmium and chromium were found to be 46.47, 33.65, 70.93 and 59.06 mg g-1 respectively. The metal ions attached on the adsorbent were easily stripped of by the use of a dilute acid, hence easily regeneratable. The regeneration was based on the fact that the stability metal ligand complexes is pH dependent. The use of a mineral acid enabled the demetalization of the adsorbent. This was the basis of regeneration of the metal loaded adsorbent and the results obtained confirmed that azotized PET has potential to be applied as an efficient and effective colour sensitive sorbent for the removal of heavy metal pollutants from water. This study achieved its dual objective of the removal of toxic metals from water using an adsorbent sourced from a non-biodegradable waste material that would otherwise pollute the environment.
Acknowledgments:
The authors appreciate Ministry of Education Science and Technology (MOEST) through African Development Bank (ADB) grant for the tuition fees.
References:
- Matt Williams. What percent of Earth is water? December 7(2021)
- Moe CL and Rheingans RD. A Novel Method to Improve Quality of Drinking Water, Based on the Eye’s Biology Water and Health 4(2006): 41-57.
- Sankhla M S, Kumari M, Nandan M, et al. Heavy Metals Contamination in Water and their Hazardous. Effect on Human Health-A Review 5 (2016): 759-766.
- Islam A, Laskar M A and Ahmad A. Efficacy of dihydroxymercaptopyrimidine functionalized polymeric resin for the trace determination of Cd by SPE coupled flame atomic absorption spectrometry. Chem. Eng 55(2010): 5553-5561.
- Samson A L, Ho B, Au A E, et al. Physicochemical properties that control protein aggregation also determine whether a protein is retained or released from necrotic cells. Open Biol 6 (2016): 160098.
- Lodeiro P, Barriada JL, Herrero R et al. The marine macroalga Cystoseira baccata as biosorbent for cadmium (II) and lead(II) removal: kinetic and equilibrium studies. Environ. Pollut 142(2) (2016): 264-73.
- Doshi B, Sillanpaa M and Kalliola S. A review of bio-based materials for oil spill treatment. Water Res 135(2018): 262-277.
- Taha MH, Masoud AM, Khawassek YM, et al. Cadmium and iron removal from phosphoric acid using commercial resins for purification purpose . Environ Sci Pollut Res 27(2020): 31278.
- Isaac Mwangi, Nicholas Cheruiyot, Ruth Wanjau, et al. Removal of Metals by Sorption with Metal Concentration Colour Sensitive Azotized Polyethylene Terephthalate Adsorbent.10 (2022): 33.
- Ungureanu O I, Bulgari, D, Mocanu A M, et al. Functionalized PET Waste Based Low-Cost Adsorbents for Adsorptive Removal of Cu (II) Ions from Aqueous Media. Water 12(9) (2020): 2624.
- Abrahart E N. Dyes and their Intermediates. Chemical Publishing, New York 132 (1977): 12.
- Hunger K, Mischke P, Rieper W, Raue R, Kunde K and Engel A. Removal of Metals by Sorption with Metal Concentration Colour Sensitive Azotized Polyethylene Terephthalate Adsorbent. Ullmann's encycl. ind. Chem 10 (2000).
- Rafet K, Erdem E and Kocaokutgen H. Synthesis and spectral characterization of some new azo dyes and their metal complexes 2007 Transit. Met. Chem 32(2007): 102–106.
- Chakraborty J N. Dye, Metal Complex. Encycl. Color Scie and Technol 18 (2013).
- Devika B G, Doreswamy B H and Tandon H C. Corrosion Behaviour of Metal Complexes of Antipyrine Based Azo Dye Ligand for Soft-Cast Steel in 1M Hydrochloric Acid J. Sci. 32(1) (2019).
- Polyethylene phthalate polymers. Substances for Use as Basic Components of Single and Repeated Use Food Contact Surfaces. Food and Drugs 10(2017).
- Sliman H, Dong X, Zhao T. Functionalization of polyethylene terephthalate knitted fabric with cowpea protein and biopolymer complex J. of Col. and Inter. Sci 565(2019): 360.
- Lemos V A and Baliza P X. Amberlite XAD-2 functionalized with 2-aminothiophenol as a new sorbent for on-line preconcentration of cadmium and copper. Talanta. Volume 67 (3)(2005): 564-570.
- Tashiro and Shimura YJ. Removal of mercuric ions by systems based on cellulose derivatives Appl. Polym. Sci 27(1982): 747.
- Hiremath PG and Theodore T. Modelling of fluoride sorption from aqueous solution using green algae impregnated with zirconium by response surface methodology. Adsorpt. Sci. Technol 35 (1-2) (2017): 194–217.
- Mwangi IW and Ngila JC. A comparative study of modified and unmodified maize tassels for removal of selected trace metals in contaminated water. Phys Chem. Earth 111 (2012): 50-52.
- Monier M, Abdel-Latif, D A. Modification and characterization of PET fibers for fast removal of Hg (II), Cu(II) and Co(II) metal ions from aqueous solutions. J. Hazard. Mater 122 (2013): 250-251.
- Lagergreg S. Ion Exchange Adsorption Kinetics of Miglitol by D001 Resins Handl 24 (918) (1898): 1-39,
- Ho YS, McKay G, Wase DJ et al. Removal of Acid Yellow 17 Dye from Aqueous Solution using Turmeric Industrial Waste Activated Carbon. Adsorpt. Sci. Technol 18(2007):
- Liu W, Yang L, Xu S, et al. Efficient removal of hexavalent chromium from water by an adsorption–reduction mechanism with sandwiched nanocomposites. RSC Adv 8 (2018):
- Langmuir I. Adsorption of Cerium (IV) from Aqueous Solutions Using Activated Carbon Developed from Rice Straw 1918 J.Ame.chem. soc 6 (2016): 1362.
- Freundlich, H. M. F. Adsorption Behavior of Acid-Leached Clays in Bleaching of Oil. J Phys Chem. 57(1906): 385-471
- Seyhan S, Colak M, Merdivan M, et al. Polymorphism of pure p-tert-butylcalix[4]arene: conclusive identification of the phase obtained by desolvation. Chim. Acta 584(2007): 462.
- Stuart B. Infrared Spectroscopy: Fundamentals and Applications. John Wiley & Sons Ltd, New York 10 (2004): 2-20.
- Huang Y. Applications of Polyvinylamine in Removal of Heavy Metals from Wastewater by Polymer-Enhanced Ultrafiltration and Adsorption. University of Waterloo, Toronto 153(2016): 42-46.
- Tharanitharan V and Srinivasan K. Removal of Ni (II) from water and wastewater using modified Duolite XAD-761 resin. Technol 16 (3) (2009): 245-253.
- Kumar M, Rathore D P S and Singh A K. Solid Phase Extraction, Preconcentration and Sequential Separation of U(VI), Th(IV), La(III) and Ce(III) by Octa-O-methoxy resorcin[4]arene based Amberlite XAD-4 Chelating Resin, Talanta 2 (2000): 31-41.
- Narin I, Soylak M, Kayakirilmaz K, et al. Speciation of Cr and Cr in tannery wastewater and sediment samples on Ambersorb 563 resin. Anal Lett. 36(2003): 641.
- Rafatullah M, Sulaiman O, Hashim R, et al. Adsorption of methylene blue on low-cost adsorbents: a review. 2010 A review. J. Hazard. Mater 177(1-3) (2010): 70-80.
- Igberase E, Osifo P, Ofomaja A. The Adsorption of Pb, Zn, Cu, Ni, and Cd by Modified Ligand in a Single Component Aqueous Solution: Equilibrium, Kinetic, Thermodynamic, and Desorption Studies. Int. J. Anal. Chem 2017(2017): 6150209
- Saad D. Development and application of polymeric materials for heavy metal ions recovery from industrial and mining wastewaters. University of Witwatersrand, Johannesburg 52(2011): 65-87
- Nguyen K M, Nguyen B Q, Nguyen HT, et al. Adsorption of Arsenic and Heavy Metals from Solutions by Unmodified Iron-Ore Sludge Appl 9(4) (2019): 619.
- Jaafar K H, Nour H, Samrani E, et al. Metal binding in soil cores and sediments in the vicinity of a dammed agricultural and industrial watershed. Monit186 (2014): 8793–8806.
- Martín D M, Ahmed M M, Rodríguez M, et al. Aminated Polyethylene Terephthalate (PET) Nanofibers for the Selective Removal of Pb(II) from Polluted Water. J. Mater 10(12) (2017): 1352.
- Ilhan S, Noubakhsh M N, Kilicarslan S and Ozdag H. Genetic characterization, nickel tolerance, biosorption, kinetics, and uptake mechanism of a bacterium isolated from electroplating industrial effluent. Turk. J. Biotech. 60(4)(2004): 50.
- Chen, H, Xiaolin Q, Ni L, et al. Study of the adsorption process of heavy metals cations on Kraft lignin. Volume 139 (2018): 248-258.
- Yusuff AS, Arab. Adsorption of hexavalent chromium from aqueous solution by Leucaena leucocephala seed pod activated carbon: equilibrium, kinetic and thermodynamic studies. J Basic Appl. Sci 26 (2019): 89
- Bartošová A, Blinová L, Sirotiak M, et al. Usage of FTIR-ATR as Non-Destructive Analysis of Selected Toxic Dyes. Published Online 25 (2017): 103-111.
- Islam A, Laskar M A and Ahmad A. Preconcentration of metal ions through chelation on a synthesized resin containing O, O donor atoms for quantitative analysis of environmental and biological samples. Environ Monit. Assess 185 (2013):
- Keche P M, Hiwase V V and Batra R D. Synthesis and thermal analysis of amberlite XAD-2 functionalized with 5-Sulfosalicylic acid. J chem. pharm 8(7) (2016):158-163.
- Mendivil-Escalante J M, Gómez-Soberón J M, Almaral-Sánchez J L, et al. Metamorphosis in the Porosity of Recycled Concretes Through the Use of a Recycled Polyethylene Terephthalate (PET) Additive. Correlations between the Porous Network and Concrete Properties. Materials 10(2) (2017): 176.
- Brown RJC, Yardley RE, Brown AS et al. Metamorphosis in the Porosity of Recycled Concretes Through the Use of a Recycled Polyethylene Terephthalate (PET) Additive. Correlations between the Porous Network and Concrete Properties. 10(2) (2017).
- Anal. At. Sample matrix and critical interference effects on the recovery and accuracy of concentration measurements of arsenic in ambient particulate samples using ICP-MS. Spectrum 19(5) (2004).
