Reducing the Unintentional Impact of Triazole Fungicides on Aspergillus fumigatus: Are Cyclodextrins a Solution?
Article Information
Chloé Godeau1, Nadia Morin-Crini1, Éva Fenyvesi2, Fatemeh Haghighi1, Marc Fourmentin3, Laurence Millon4, Christophe Ramseyer1, David Landy5, Grégorio Crini1*, Steffi Rocchi1,4
1UMR 6249 Chrono-environnement, Université Bourgogne Franche-Comté, 16 route de Gray, 25030 Besançon Cedex, France
2CycloLab Cyclodextrin Research and Development Ltd., Illatos 7, Budapest, H-1097, Hungary
3Laboratoire de Physico-chimie de l’Atmosphère, EA 4493, Université du Littoral Côte-d’Opale, 59140, Dunkerque, France
4Parasitologie Mycologie, Centre Hospitalier Régional Universitaire, 25030 Besançon Cedex, France
5Unité de Chimie Environnementale et Interactions sur le Vivant (UCEIV, UR 4492), SFR Condorcet FR CNRS 3417, ULCO, F-59140, Dunkerque, France
*Corresponding Author: Grégorio Crini, UMR 6249 Chrono-environnement, Université Bourgogne Franche-Comté, 16 route de Gray, 25030 Besançon Cedex, France
Received: 16 December 2020; Accepted: 23 December 2020; Published: 03 March 2021
Citation:
Chloé Godeau, Nadia Morin-Crini, Éva Fenyvesi, Fatemeh Haghighi, Marc Fourmentin, Laurence Millon, Christophe Ramseyer, David Landy, Grégorio Crini, Steffi Rocchi. Reducing the Unintentional Impact of Triazole Fungicides on Aspergillus fumigatus: Are Cyclodextrins a Solution? Journal of Environmental Science and Public Health 5 (2021): 114-136.
Share at FacebookAbstract
Graphical Abstract
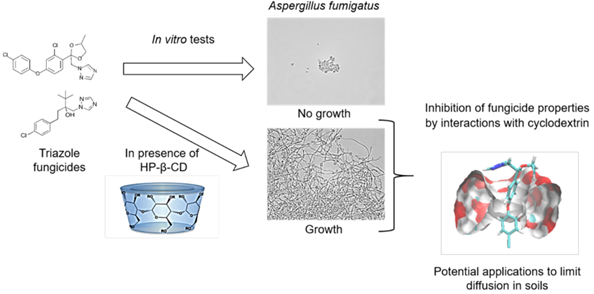
Triazoles are among the most widely used fungicides in agriculture, for the protection of materials and crops. Their broad spectrum of action makes them the substances of choice for preventing and curing fungal diseases such as Septoria or Fusarium wilt. However, their spread in the environment can lead to the selection of triazole resistance not only in crop pathogens, but also in non-target organisms, such as a non-phytopathogenic fungus Aspergillus fumigatus. This fungus is an opportunistic human pathogen associated with high mortality in cases of resistance to pharmacological treatments based on triazoles. There is therefore a need to find ways of limiting the unintended impact of fungicides on this pathogen. We describe here the impact of linear (maltodextrin) or cyclic (cyclodextrins, CDs) dextrins and their interaction with two fungicides (difenoconazole and tebuconazole), through measurements of growth in 20 A. fumigatus strains. Three native (α-CD, β-CD and γ-CD) and two modified (hydroxypropyl-β-CD (HP- β-CD) and heptakis-(2,3,6-tri-O-methyl)-β-CD (TRIMEB)) cyclodextrins were used. In each experiment, the minimum inhibitory concentration (MIC) was determined according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) reference method. Microscopical observations were also performed to highlight the effect of the presence of dextrins. HP-β-CD was the most effective cyclic dextrin. It reduced the antifungal activity of triazoles, whereas maltodextrin and α-CD had no effect. Those observations were more investigated thanks to molecular modeling in order to clarify cyclodextrin/fungicide interactions. The use of cyclodextrins is a promising approach for limiting the emergence of resistance to triazole antifungal drugs.
Keywords
Triazoles; Cyclodextrins; Interaction; Aspergillus fumigatus; Molecular Dynamics Simulation
Triazoles articles; Cyclodextrins articles; Interaction articles; Aspergillus fumigatus articles; Molecular Dynamics Simulation articles
Triazoles articles Triazoles Research articles Triazoles review articles Triazoles PubMed articles Triazoles PubMed Central articles Triazoles 2023 articles Triazoles 2024 articles Triazoles Scopus articles Triazoles impact factor journals Triazoles Scopus journals Triazoles PubMed journals Triazoles medical journals Triazoles free journals Triazoles best journals Triazoles top journals Triazoles free medical journals Triazoles famous journals Triazoles Google Scholar indexed journals Cyclodextrins articles Cyclodextrins Research articles Cyclodextrins review articles Cyclodextrins PubMed articles Cyclodextrins PubMed Central articles Cyclodextrins 2023 articles Cyclodextrins 2024 articles Cyclodextrins Scopus articles Cyclodextrins impact factor journals Cyclodextrins Scopus journals Cyclodextrins PubMed journals Cyclodextrins medical journals Cyclodextrins free journals Cyclodextrins best journals Cyclodextrins top journals Cyclodextrins free medical journals Cyclodextrins famous journals Cyclodextrins Google Scholar indexed journals Interaction articles Interaction Research articles Interaction review articles Interaction PubMed articles Interaction PubMed Central articles Interaction 2023 articles Interaction 2024 articles Interaction Scopus articles Interaction impact factor journals Interaction Scopus journals Interaction PubMed journals Interaction medical journals Interaction free journals Interaction best journals Interaction top journals Interaction free medical journals Interaction famous journals Interaction Google Scholar indexed journals Aspergillus fumigatus articles Aspergillus fumigatus Research articles Aspergillus fumigatus review articles Aspergillus fumigatus PubMed articles Aspergillus fumigatus PubMed Central articles Aspergillus fumigatus 2023 articles Aspergillus fumigatus 2024 articles Aspergillus fumigatus Scopus articles Aspergillus fumigatus impact factor journals Aspergillus fumigatus Scopus journals Aspergillus fumigatus PubMed journals Aspergillus fumigatus medical journals Aspergillus fumigatus free journals Aspergillus fumigatus best journals Aspergillus fumigatus top journals Aspergillus fumigatus free medical journals Aspergillus fumigatus famous journals Aspergillus fumigatus Google Scholar indexed journals Molecular Dynamics Simulation articles Molecular Dynamics Simulation Research articles Molecular Dynamics Simulation review articles Molecular Dynamics Simulation PubMed articles Molecular Dynamics Simulation PubMed Central articles Molecular Dynamics Simulation 2023 articles Molecular Dynamics Simulation 2024 articles Molecular Dynamics Simulation Scopus articles Molecular Dynamics Simulation impact factor journals Molecular Dynamics Simulation Scopus journals Molecular Dynamics Simulation PubMed journals Molecular Dynamics Simulation medical journals Molecular Dynamics Simulation free journals Molecular Dynamics Simulation best journals Molecular Dynamics Simulation top journals Molecular Dynamics Simulation free medical journals Molecular Dynamics Simulation famous journals Molecular Dynamics Simulation Google Scholar indexed journals Pesticides articles Pesticides Research articles Pesticides review articles Pesticides PubMed articles Pesticides PubMed Central articles Pesticides 2023 articles Pesticides 2024 articles Pesticides Scopus articles Pesticides impact factor journals Pesticides Scopus journals Pesticides PubMed journals Pesticides medical journals Pesticides free journals Pesticides best journals Pesticides top journals Pesticides free medical journals Pesticides famous journals Pesticides Google Scholar indexed journals Fungicides articles Fungicides Research articles Fungicides review articles Fungicides PubMed articles Fungicides PubMed Central articles Fungicides 2023 articles Fungicides 2024 articles Fungicides Scopus articles Fungicides impact factor journals Fungicides Scopus journals Fungicides PubMed journals Fungicides medical journals Fungicides free journals Fungicides best journals Fungicides top journals Fungicides free medical journals Fungicides famous journals Fungicides Google Scholar indexed journals antifungal drugs. articles antifungal drugs. Research articles antifungal drugs. review articles antifungal drugs. PubMed articles antifungal drugs. PubMed Central articles antifungal drugs. 2023 articles antifungal drugs. 2024 articles antifungal drugs. Scopus articles antifungal drugs. impact factor journals antifungal drugs. Scopus journals antifungal drugs. PubMed journals antifungal drugs. medical journals antifungal drugs. free journals antifungal drugs. best journals antifungal drugs. top journals antifungal drugs. free medical journals antifungal drugs. famous journals antifungal drugs. Google Scholar indexed journals Food articles Food Research articles Food review articles Food PubMed articles Food PubMed Central articles Food 2023 articles Food 2024 articles Food Scopus articles Food impact factor journals Food Scopus journals Food PubMed journals Food medical journals Food free journals Food best journals Food top journals Food free medical journals Food famous journals Food Google Scholar indexed journals microorganisms articles microorganisms Research articles microorganisms review articles microorganisms PubMed articles microorganisms PubMed Central articles microorganisms 2023 articles microorganisms 2024 articles microorganisms Scopus articles microorganisms impact factor journals microorganisms Scopus journals microorganisms PubMed journals microorganisms medical journals microorganisms free journals microorganisms best journals microorganisms top journals microorganisms free medical journals microorganisms famous journals microorganisms Google Scholar indexed journals
Article Details
1. Introduction
Pesticides use is increasing worldwide, to intensify agricultural production to meet the needs of growing populations requiring more and more food [1]. Fungicides are widely used in agricultural development, to prevent or cure fungal infections of plants that can decrease production yields [2]. Triazole fungicides are often the treatment of choice, because of their broad spectrum of action and their specific targeting of fungi [3, 4]. Almost 30 different molecules have been authorized by national, European and international agencies (e.g. the US Food and Drug Administration) and are used to protect crops or materials against fungal damage [5, 6]. New formulations are continually being released onto the market [7].
Following their application on crops, triazoles act on the aerial parts of the plant and persist in soils for a few days to several months, depending on the molecule used, the characteristics of the soil and meteorological conditions [8, 9]. During this period, these fungicides may have unintended effects on Aspergillus fumigatus, a non-phytopathogenic fungus naturally present in soils, through the exertion of selection pressure [10]. This can lead to an increase in the size of the population of A. fumigatus strains with pan-resistance to triazoles [11].
This resistance phenomenon is a matter of concern, because this fungus can cause disease in humans [12]. Indeed, A. fumigatus is an opportunistic pathogen, and is the fungus most frequently implicated in invasive pulmonary aspergillosis, a fungal infection with a high mortality rate [13, 14]. The phenomenon of resistance to medical treatments based on triazoles has been known for more than 20 years and is frequently described [15]. This resistance has been reported worldwide and is linked to the use of triazole fungicides in the environment, given the close chemical similarities of medical and agricultural triazoles [16-19]. The number of people at risk of developing opportunistic diseases is steadily increasing around the world. Over the last decade, medical advances in immunosuppression techniques, chemotherapy, organ transplantation and the use of biotherapies in chronic inflammatory diseases, in particular, have resulted in a major expansion of the population of immunocompromised individuals [20]. This observation highlights the importance of limiting the emergence of resistance in opportunistic pathogens. New solutions to limit the spread of triazole resistance are therefore required, to limit public health implications [21].
One possible approach to preventing triazole selection pressure would be to limit the overdispersion of triazole fungicides in the soil, which isn’t needed, and to prevent contact between active fungicides and A. fumigatus. Cyclodextrins (CDs), synthetic cyclic molecules derived from starch by enzymatic degradation [22, 23], can be used to catch fungicides before their uncontrolled dissipation in the environment. These oligosaccharides have a remarkable ability to form host-guest interactions in solution and in the solid state (possible formation of inclusion complex). Their specific properties and their lack of toxicity and biocompatibility with humans have led to their use in numerous applications in the food industry, pharmaceuticals, medicine, biotechnology, cosmetics, hygiene and toiletries, the textile industry, catalysis, chromatography, and remediation, for example [24-34]. Crini et al. [35] have shown that cyclodextrin polymers can interact with five triazole molecules. In a preliminary study, we showed that some dextrins interacted with difenoconazole, limiting it antifungal activity [36]. Complex formation and stability of inclusion compounds in CDs can be studied by molecular dynamics simulation [37-41], which are especially useful when guest solubility limits experimental investigations.
Here, we pursue our work on this topic, broadening the experimental conditions used and performing A. fumigatus growth tests to assess the impact of linear and cyclic dextrins and their interaction with two triazole fungicides, difenoconazole (DIFENO) and tebuconazole (TEBU), which are particularly widely used in the environment. Molecular dynamics simulations are also conducted to better investigate interactions between cyclodextrins and these fungicides.
2. Materials and Methods
2.1 Fungal strains
Twenty A. fumigatus strains were used, isolated in 2017 from a sampling campaign carried out on market gardens in the east of France. These strains were characterized by sequencing the β-tubulin and cyp51A genes, to check species identification and the absence of genetic mutations conferring triazole resistance [42-43].
2.2 Fungicides
Difenoconazole (DIFENO, 1-[[2-[2-chloro-4-(4-chlorophenoxy) phenyl]-4-methyl-1,3-dioxolan-2-yl]methyl]-1,2,4-triazole) and tebuconazole (TEBU, 1-(4-chlorophenyl)-4,4-dimethyl-3-(1,2,4-triazol-1-ylmethyl)pentan-3-ol) were purchased from Sigma Aldrich (Saint Quentin Fallavier, France). Their structure and main characteristics are presented in Table 1.
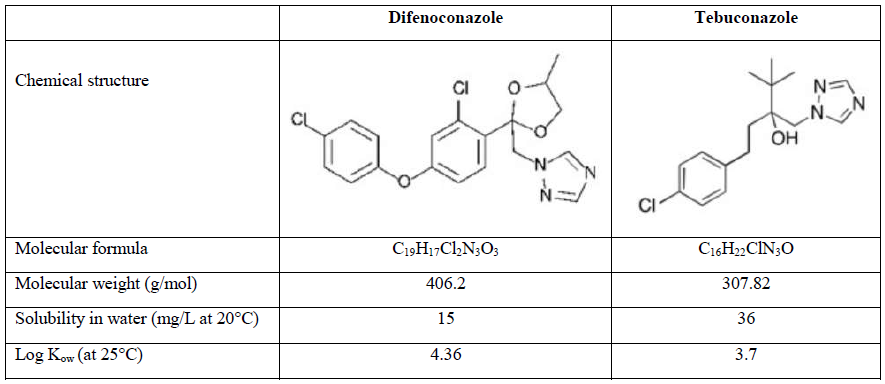
Table 1: Chemical characteristics of difenoconazole and tebuconazole.
2.3 Commercial dextrins
A linear dextrin (maltodextrin, MALTO, Glucidex® D19 from Roquette, Lestrem, France), three native (α-CD, β-CD and γ-CD, purity ≥ 98%) and two modified (HP-β-CD and TRIMEB, purity ≥ 95%) cyclodextrins (from CycloLab, Hungary) were used in this study. MALTO (purity ≥ 99%) has a dextrose equivalent value of 19 (n » 6 in Figure 1). α-CD, β-CD and γ-CD contain six, seven and eight glucose units, respectively. HP-β-CD (hydroxypropyl-β-cyclodextrin) and TRIMEB (heptakis-(2,3,6-tri-O-methyl)-β-cyclodextrin) are commercial products derived from β-CD. Their structures are shown in Figure 1 and they are described in Table 2.
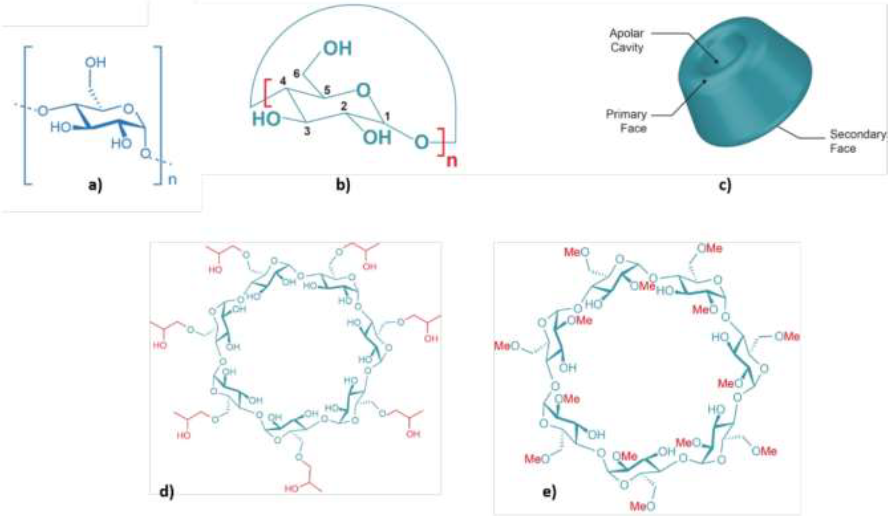
Figure 1: Chemical structures of linear and cyclic dextrins; a) maltodextrin (MALTO; n » 6); b) α-cyclodextrin (α-CD, n = 6), β-cyclodextrin (β-CD, n = 7) and γ-cyclodextrin (γ-CD, n = 8); c) general structure of native α-, β-, and γ-cyclodextrins; and of two modified β-cyclodextrins: d) hydroxypropyl-β-cyclodextrin (HP-β-CD) and e) heptakis-(2,3,6-tri-O-methyl)-β-cyclodextrin (TRIMEB).
|
Cyclodextrin |
Degree of substitution1 |
Molecular weight2 |
Solubility3 |
|
α-cyclodextrin (α-CD) |
972 |
145 |
|
|
β-cyclodextrin (β-CD) |
1135 |
18.5 |
|
|
g- cyclodextrin (γ-CD) |
1297 |
232 |
|
|
Hydroxypropyl-β-cyclodextrin (HP-β-CD) |
0.65 |
1400 |
>600 |
|
Randomly methylated β-cyclodextrin (TRIMEB) |
3.0 |
1430 |
>500 |
1Mean number of substituents per glucopyranose repeat unit
2Molecular weight in Daltons
3Solubility in pure water at 25°C, in g/L
Table 2: General description of cyclodextrins.
2.4 Determination of minimum inhibitory concentrations
All 20 strains were tested with the EUCAST method to determine minimum inhibitory concentrations (MICs) for medical triazoles [44]. For itraconazole and voriconazole, clinical breakpoints were used to define resistance (a strain was defined as resistant if the MIC for these two molecules exceeded 2 mg/L [45]).
In each EUCAST plate, two reference strains were used (a sensitive strain: CBS 101355, and an environmental strain with the TR34/L98H mutation, pan-resistant to antifungal triazoles). Briefly, the different strains were plated in 96-well microplates and exposed to a range of antifungal concentrations, from 0.0312 to 16 mg/L generated by a 10-well two-fold dilution series in RPMI 1640 (Roswell Park Memorial Institute; Sigma Aldrich, Saint Louis, USA) medium. MICs were determined, by eye, after 48 hours of incubation at 37°C, as the lowest triazole concentration inhibiting fungal growth.
We then performed the EUCAST method with DIFENO and TEBU in same conditions, to measure the MICs of triazole fungicides for the same strains. In addition to the reference RPMI medium used for the EUCAST method, we used RPMI medium supplemented with maltodextrin and each of the five CDs to estimate the dextrin/triazole interaction potential and its limits. These media were prepared such that there were 10 molecules of dextrin per molecule of fungicide, in accordance with our preliminary results [36].
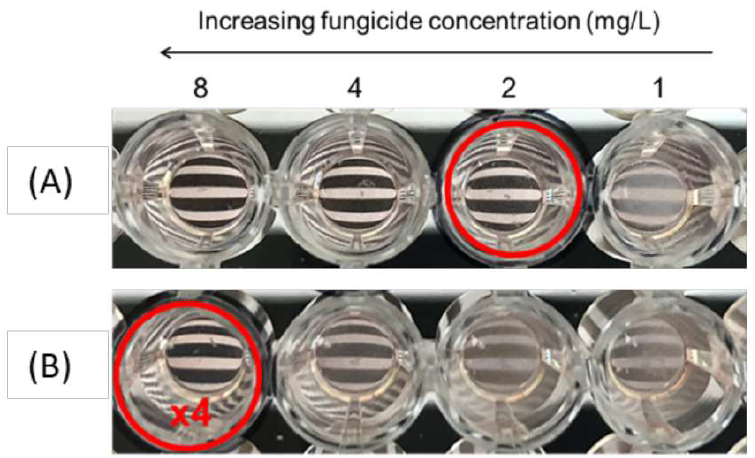
Figure 2: Picture of part of the EUCAST plate for the 4E007 strain with difenoconazole in (A) RPMI medium, (B) medium supplemented by HP-β-CD at a molar ratio of 10 dextrin molecules per molecule of fungicide. Minimum inhibitory concentrations are indicated by a red circle for each set of conditions: “x4” (increase by a factor of 4), corresponding to the increase in MIC relative to the triazole in RPMI alone.
The MICs obtained with culture medium consisting solely of RPMI with DIFENO or TEBU were compared with those obtained with each of the dextrin-supplemented media. For each fungicide, we assessed the impact of MALTO or CDs in culture media by observing the increase in MIC (Figure 2).
2.5 Microscopy analysis of fungal growth
The effect of each culture medium on fungal strain growth was also investigated in more detail, by performing a microscopy analysis. After the 48 hours of incubation required for the susceptibility test, 40 µL of culture medium present in the well were transferred to a microscope slide and the slide was sealed. The slides were observed under a light microscope (Leica microsystems, France), and image analysis was performed with Leica LAS V4.9 software. Microscopy observations were performed in every media at the concentration of fungicides determined as MIC for the strain concerned with the medium containing only RPMI: 1 mg/L for DIFENO and 2 mg/L for TEBU.
2.6 Statistical analysis
Log2-transformed MIC values were used for statistical analysis. The Akaike information criterion (AIC) was used to select the most parsimonious models (including random effects and/or interactions between variables). A linear mixed-effects model (LMM) was applied incorporating a random effect of strain, to assess differences in fungal growth according to the dextrin used (relative to RMPI medium without dextrins) and fungicides. Statistical analyses were performed, and table and graphical display were generated with R-3.4.4 statistical software for Microsoft® Windows and lme4 [46], nlme [47], MuMIn [48], gtsummary and ggeffects [49] libraries.
2.7 Simulation methodology
Simulations were conducted between α-CD, β-CD, γ-CD or HPβ-CD on the one hand and TEBU or DIFENO on the other hand. The high-resolution X-ray structures of α-CD, β-CD and γ-CD were used as initial 3D models for molecular dynamics simulations. The following protein data bank (PDB) codes were taken from research collaboratory for structural bioinformatics (RCSB) as 2XFY for α-CD [50], 5MK9 for β-CD and 5MKA for γ-CD [51]. The structures of TEBU and DIFENO molecules were also downloaded from RCSB [52]. HP-β-CD structure used had one side substitution of seven hydroxypropyl, it was converted from PubChem to PDB format using Online SMILES Translator of NIH site (https://cactus.nci.nih.gov/). All structures were then subjected to quantum chemistry calculations using the Gaussian09 suite of programs [53]. Optimization of all molecules was done at DFT/B3LYP /6-31g* level of theory. Partial charges were calculated within the same level of theory using the restrained electrostatic potential scheme [54]. The software package Antechamber [55] was used for the automatic parameterization of force field parameters with general Amber force field atom type [56,57]. Several systems were built for molecular dynamics (MD) which are presented in supplementary data (Table S-2) together with their equilibrium protocol.
Finally, production runs for the complexes of each systems were performed for 200 nanoseconds (ns) under the condition of constant pressure and temperature. An integration step of 2 femtoseconds was used in all simulations with the bonds to hydrogen atoms converted to rigid constraints. The trajectories for structural coordinates were saved every 1 picosecond for data analysis. MD trajectories were analysed using visual molecular dynamics [58] and Chimera [59]. The molecular mechanics Poisson–Boltzmann surface area (MM-PBSA) method [60, 61] were applied to estimate the binding free energy of the complexes.
3. Results
3.1 Characteristics of the fungal strains
The susceptible reference strain, CBS 101355, had a MIC of 4 mg/L for both fungicides, whereas the resistant reference strain had a MIC of 16 mg/L for both fungicides. The 20 strains tested had MICs no higher than that of the susceptible reference strain, for both DIFENO and TEBU. MICs were between 1 (12/20 strains) and 2 (8/20 strains) mg/L for DIFENO, and between 2 (12/20 strains) and 4 (8/20 strains) mg/L for TEBU, depending on the strain tested. Thus, for statistical analysis, strains were considered to be the random factor accounting for MIC variations and were added to the random part of the model.
3.2 Effect of the presence of cyclodextrins in the culture medium
The MICs of DIFENO and TEBU in dextrin-free RPMI culture medium were not significantly different from those obtained in RPMI medium supplemented with MALTO (p = 0.8) or α-CD (p= 0.3).
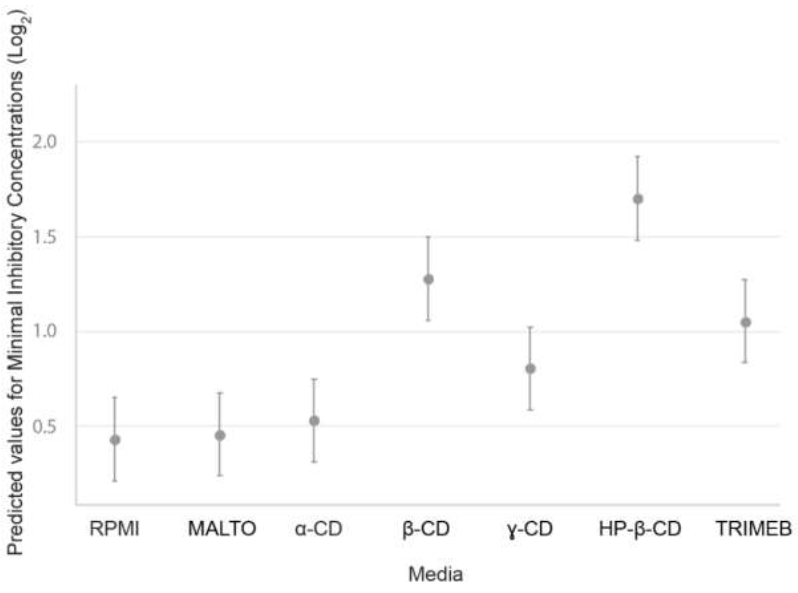
Figure 3:Predicted values of minimum inhibitory concentrations as a function of medium (RPMI reference medium or RPMI supplemented with dextrins), with strain integrated into the mixed-effects model as a random effect. The dextrins used were MALTO: maltodextrin; α-CD: α-cyclodextrin; γ-CD: γ-cyclodextrin; TRIMEB: heptakis-(2,3,6-tri-O-methyl)-β-cyclodextrin; β-CD: β-cyclodextrin; and HP-β-CD: hydroxypropyl-β-cyclodextrin.
By contrast, the MICs obtained in media supplemented with the other dextrins tested (β-CD, γ-CD, HP-β-CD and TRIMEB) were significantly higher than those obtained in dextrin-free RPMI medium (p < 0.001). The outputs of the LMM (estimates, confidence intervals and p-values) are presented in the supplemental data (Table S-1). Strains (random effects) accounted for 0.40 of the variance, and the fixed model accounted for 0.396. The predicted MICs for the fixed part of the model (medium) are shown in Figure 3.
In several strains, the MICs of both DIFENO and TEBU were higher in media supplemented with TRIMEB, β-CD, or HP-β-CD than in dextrin-free RPMI medium (Table 3). For DIFENO, an increase in MIC was observed for 14/20, 17/20 and 20/20 for TRIMEB, β-CD and HP-β-CD, respectively. The effect seemed particularly strong with HP-β-CD, increasing MICs relative to TRIMEB and β-CD.
For TEBU, the effects on growth of TRIMEB, β-CD and HP-β-CD were less pronounced than for DIFENO: an increase was observed for 9/20, 16/20 and 19/20 strains, respectively. Only one strain displayed an increase in MIC by a factor of 4, on medium supplemented with HP-β-CD against 9 for DIFENO. MICs for DIFENO were higher in γ-CD-supplemented medium than in the reference medium (higher MICs for 12/20 strains), whereas no effect on the MIC of TEBU was observed. Overall, increase in MICs were larger for DIFENO than for TEBU. Regardless of the fungicide considered, an order of effectiveness, in terms of the increase in MIC, was observed for the dextrins added to the culture medium: MALTO » α-CD < γ-CD < TRIMEB < β-CD << HP-β-CD (Figure 3).
|
Media supplemented with cyclodextrins* |
||||||||
|
γ-CD |
TRIMEB |
β-CD |
HP-β-CD |
|||||
|
Strains |
TEBU |
DIFENO |
TEBU |
DIFENO |
TEBU |
DIFENO |
TEBU |
DIFENO |
|
4E007 |
- |
x2 |
- |
x2 |
x2 |
x2 |
x2 |
x4 |
|
4E008 |
- |
- |
- |
- |
x2 |
x2 |
x2 |
x2 |
|
4E009 |
- |
x2 |
x2 |
x2 |
x2 |
x2 |
x2 |
x4 |
|
4E010 |
- |
- |
- |
- |
x2 |
x2 |
x2 |
x4 |
|
4E011 |
- |
x2 |
x2 |
- |
x2 |
x2 |
x2 |
x4 |
|
4E012 |
- |
- |
x2 |
x2 |
x2 |
- |
x2 |
x2 |
|
4E013 |
- |
- |
- |
- |
x2 |
x2 |
x4 |
x4 |
|
4E014 |
- |
x2 |
- |
x2 |
x2 |
x2 |
x2 |
x2 |
|
4E015 |
- |
x2 |
- |
x2 |
- |
x2 |
x2 |
x2 |
|
4E016 |
- |
x2 |
x2 |
x2 |
- |
x2 |
x2 |
x4 |
|
4E017 |
- |
x2 |
- |
x2 |
- |
x2 |
x2 |
x2 |
|
4E018 |
- |
- |
x2 |
- |
x2 |
x2 |
x2 |
x4 |
|
4E019 |
- |
x2 |
- |
x2 |
x2 |
x2 |
x2 |
x2 |
|
4E020 |
- |
- |
- |
- |
x2 |
- |
x2 |
x2 |
|
4E022 |
- |
- |
x2 |
x2 |
- |
x2 |
x2 |
x2 |
|
4E023 |
- |
x2 |
- |
x2 |
x2 |
- |
x2 |
x2 |
|
4E024 |
- |
- |
x2 |
x2 |
x2 |
x2 |
x2 |
x2 |
|
4E025 |
- |
x2 |
- |
x2 |
x2 |
x2 |
x2 |
x2 |
|
4E026 |
- |
x2 |
x2 |
x2 |
x2 |
x2 |
x2 |
x4 |
|
4E027 |
- |
x2 |
x2 |
x2 |
x2 |
x2 |
x2 |
x4 |
*γ-CD: γ-cyclodextrin; TRIMEB: heptakis-(2,3,6-tri-O-methyl)-β-cyclodextrin; β-CD: β-cyclodextrin; HP-β-CD: hydroxypropyl-β-cyclodextrin
“x2” or “x4” indicates the factor increase in MIC in each set of conditions
Table 3:Increases in minimum inhibitory concentrations (MICs) observed on EUCAST plates for 20 A. fumigatus strains in different conditions, in the presence of the fungicide difenoconazole (DIFENO) or tebuconazole (TEBU). MICs were measured in the presence of four cyclodextrins added to the culture media at a molar ratio of 10:1.
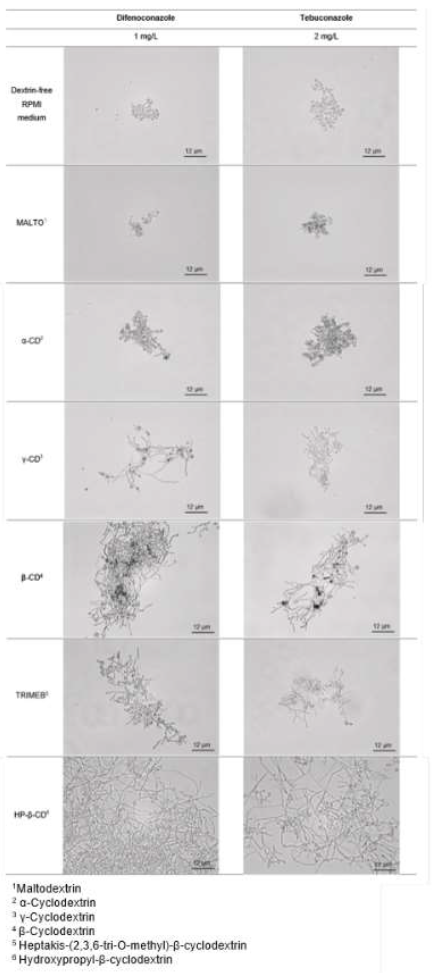
Figure 4: Micrographs of A. fumigatus (strain 4E026), comparing its growth between dextrin-free RPMI medium and RPMI medium supplemented with linear or cyclic dextrins at a molar ratio of 10:1. The observations presented were obtained with a fungicide concentration of 2 mg/L for tebuconazole and 1 mg/L for difenoconazole, corresponding to the MICs of these fungicides in dextrin-free RMPI medium. The slides were observed under a light microscope, at a magnification of x 400.
Microscopy analysis of strain 4E026 were performed to assess the germination of fungal spores in each set of conditions, images are presented in Figure 4. Those observations were performed at fungicide concentrations found to inhibit fungal growth in dextrin-free RPMI: 1 mg/L DIFENO and 2 mg/L TEBU. The absence of an effect of α-CD and MALTO on the recovery of fungal growth was confirmed by microscopy (Figure 4), for both fungicides. For γ-CD-supplemented medium, hyphal development was visible in the presence of DIFENO. For β-CD and its derivatives, in the presence of DIFENO or TEBU, fungal growth was greater in medium supplemented with HP-β-CD than in media supplemented with β-CD or TRIMEB, in accordance with Table 3. Similar increases in MIC relative to the reference medium were observed with β-CD, TRIMEB and HP-β-CD with TEBU for the 4E026 strain (Table 3), but microscopy revealed more extensive hyphal development with HP-β-CD.
3.3 Incorporation of antifungal agents in cyclodextrins
In order to test the stability of cyclodextrins in our simulations, the root mean square deviation was calculated (RMSD) for the optimized CDs as a function of MD simulations time. Whatever the system, cyclodextrins were found very stable since RMSD displayed values less than 2Å. γ-CD and HP-β-CD being a bit more mobile due to the larger diameter of γ-CD and the high flexibility of hydroxypropyl groups of HP-β-CD. The interaction of antifungal agents with cyclodextrins and their random incorporation were investigated on the course of the MD simulation runs. Distances between the centers of mass of the CDs and the ligands were analyzed as a function of time (Figure 5). During the 200 ns of MD simulation, DIFENO was found to enter in β-CD, γ-CD and HP-β -CD but not in α-CD. The time scale necessary for the molecules to incorporate into CDs is also a marker of the strength of the interaction between antifungals and CDs. Lesser this time is and stronger the antifungal/CD interaction is. DIFENO incorporated β-CD after about 70 ns, γ-CD after 20 ns and bound in less than 10 ns to HP-β-CD.
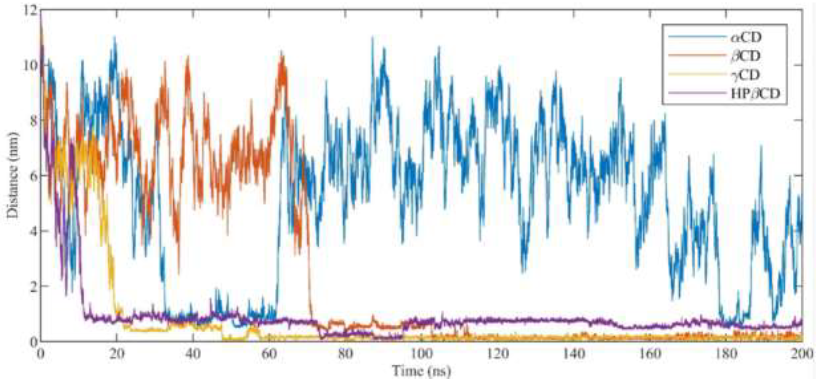
Figure 5:Distance between the center of mass of cyclodextrins (α-CD, β-CD, γ-CD and HP-β-CD) and difenoconazole.
For TEBU, no incorporation in α-CD occurred during the MD simulation run. The interaction of TEBU with CDs is found weaker than the one observed with DIFENO. Indeed, the time scales which characterize the binding and insertion of the ligands are found greater for TEBU with respect to DIFENO, for example the highest time scale was 140 ns for γ-CD/TEBU interaction instead of 20 ns for γ-CD/DIFENO interaction.
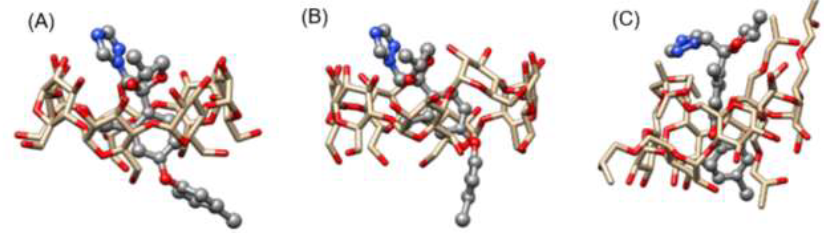
Figure 6:Most favorable configurations of DIFENO obtained in (A) β-CD, (B) γ-CD and (C) HP-β-CD.
A visual inspection of the fungicide/CD complexes have been carried out together with the analysis of all changes of the distances between CDs and antifungal drugs. Figure 6 illustrates the most favourable configurations of DIFENO obtained in β-CD, γ-CD and HP-β-CD: whatever the CD, the chlorobenzene group of DIFENO, which due to its hydrophobicity seems predisposed to interact with the hydrophobic cavity of the CD, appears to have a strong interaction with it. The binding free energy of all systems that formed complexes were also calculated according to the MM-PBSA method.
The binding energies of DIFENO with β-CD and γ-CD (-26.74 ± 0.04 and -27.10 ± 0.04 Kcal/mol, respectively) looked very similar. They were found greater than those obtained for TEBU which were between -23,76 ± 0,03 Kcal/mol for β-CD and -19.69 ± 0.04 Kcal/mol for γ-CD. Due to the large flexibility of the hydroxypropyl chains which move constantly, two main conformations of HP-β-CD were observed during the simulations: a “closed” one where one or two hydroxypropyl group(s) block the access to the cavity and an “open” conformation where hydroxypropyl groups point toward the solvent and do not prevent the entry of the ligands into the CD cavity. For DIFENO, in the open situation of the cavity we found the highest binding energy value (-30.46 ± 0.1 Kcal/mol) while the other configuration gave only -22.30 ± 0.05 Kcal/mol.
4. Discussion
An effect of the interaction between dextrins and triazole molecules on the growth of A. fumigatus was demonstrated in this study. A decrease in the antifungal activity of DIFENO and TEBU fungicides in the presence of cyclodextrins was observed in vitro. The inhibitory effect was greatest for HP-β-CD, whereas maltodextrin had no effect. A lower biocidal activity in the presence of CDs than in the presence of the biocide alone has already been reported in several studies reviewed by Nardello-Rataj and Leclercq [62]. The authors reported an antagonistic effect of the formation of a complex between HP-β-CD and a series of p-hydroxybenzoic acid esters against Candida albicans: antimicrobial activity was lower in the presence of the CD, whereas solubility in water was higher and the concentration of free p-hydroxybenzoic acid esters was lower, with an increase in the binding constant, suggesting that the degree of inactivation depended entirely on the proportion of the biocide encapsulated. The authors also reported that biocides with a higher solubility in water (e.g. thimerosal and bronopol) displayed only slight inactivation, whereas lipophilic substances (e.g. phenolic residues) were strongly inactivated in the presence of HP- β-CD, in assays with various strains.
In details, with native cyclodextrins, fungal growth remained inhibited with α-CD sign that there is probably no interaction with fungicides, surely because its cavity is too small for the formation of complexes with these two triazole molecules. For γ-CD, a resumption of fungal growth was visible only with DIFENO, suggesting that the cavity of this molecule is too large and too flexible for the formation of a stable inclusion compound with TEBU but more appropriate for the inclusion of DIFENO. Indeed, the binding energies obtained in the present work for DIFENO are larger than those obtained for TEBU. The effect on fungal growth was greatest in the presence of β-CD, resulting in an increase in MIC for 17/20 strains for DIFENO and 16/20 strains for TEBU. The β-CD cavity size was, therefore, considered optimal relative to the other native cyclodextrins. We infer from these results that a cyclodextrin/triazole interaction occurs, potentially through the formation of an inclusion complex in the hydrophobic cavities of the cyclodextrin molecules. This mechanism would also explain the lack of effect of linear maltodextrin, which has no cavity.
Several studies have already reported interactions between CDs and fungicides [63-68]. However, to our knowledge, publications on the interaction between triazole fungicides and CDs are rare. Our results are consistent with those of Stepniak et al. [69], who studied the host-guest interaction of β-CD with TEBU and demonstrated, by calculating the constant for complex formation, a high affinity of β-CD molecules for the non-polar functional groups of TEBU (para-chlorobenzyl and tert-butyl groups) present in the hydrophobic cavity. Balmas et al. [70] also demonstrated the formation of a stable complex between TEBU and β-CD, arguing that this complex could be used to control plant diseases in agriculture through the prolonged release of fungicide. However, our results temper the statements of Stepniak et al. [69]. These authors examined the effect of TEBU within β-CD macromolecules in cultures of only one strain of A. fumigatus; they observed a toxic action not only of the free fungicide at a given dose, but also of the fungicide included in β-CD at the same dose. We found that DIFENO and TEBU had clear fungicidal activity against 20 strains of A. fumigatus, but that this activity was weaker in the presence of β-CD, as shown by increases in both MICs (Table 3) and hyphal development, as observed under the microscope (Figure 4, comparison between reference medium and medium implemented with β-CD). However, the presence of β-CD molecules in the culture medium had a statistically significant effect on fungal growth.
These observations led us to test two β-CD derivatives, TRIMEB and HP-β-CD, both of which allowed a recovery of fungal growth. TRIMEB was less efficient than β-CD, but the hydroxypropyl groups grafted onto the glucose units in HP-β-CD seemed to promote interactions with TEBU and DIFENO (Table 3). Indeed, HP-β-CD had an effect on all strains, with both fungicides, resulting in a loss of antifungal efficacy against A. fumigatus. This effect was particularly marked with DIFENO, and this interaction was probably the most favorable theoretical “host-guest” interaction in this study. Indeed, DIFENO has a higher electron density than TEBU and is more lipophilic (see log KOW values in Table 2), favoring its interaction with the hydrophobic cavities of CDs. The HP-β-CD-DIFENO system showed the highest binding energy value (-30.46 ±0.1 Kcal/mol) in molecular simulation in the open state, supporting the hypothesis of the formation of an inclusion complex between these two molecules. It results in the significant loss of antifungal efficiency observed, reflected in the higher MICs obtained (Table 3), together with the higher rates of spore germination and hyphal development (Figure 4). The interaction rendered the fungicides less effective, probably by blocking their active sites.
5. Conclusion
Triazoles are used for the treatment of foliar fungal diseases. Their presence in the soil is not required and has an undesirable side effect on A. fumigatus, potentially favoring the emergence of resistance in this microorganism. In this study, we highlight the potential role of cyclodextrins in limiting this phenomenon. Indeed, cyclodextrins can encapsulate triazoles before they spread in the soil, without affecting the efficacy of foliar treatment. This would eliminate the potential selection pressure exerted on A. fumigatus by triazoles in the soil. This study sheds light on the cyclodextrin/triazole interaction and the effect on A. fumigatus, providing a strong first argument in favor of their use in the environment. Molecular modeling confirmed the interaction between cyclodextrins and fungicides. They reveal that the binding energy of fungicides to HP-β-CD is significantly larger than to β-CD and γ-CD, a-CD being not favorable at all. DIFENO has a better affinity than TEBU to these CDs. The chlorobenzene group of this fungicide appears to be a key chemical group for their binding to CDs. Other experiments are underway to investigate the interaction between cyclodextrins and other triazoles.
Acknowledgment
This work received financial support from the Observatoire des Sciences de l’Univers Terre Homme Environnement Temps Astronomie of Franche-Comté-Bourgogne, France. Numerical resources provided by the mesocentre de calcul de Franche-Comté are greatly acknowledged.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Silva V, Mol HGJ, Zomer P, et al. Pesticide residues in European agricultural soils-A hidden reality unfolded. Science of The Total Environment 653 (2019): 1532-1545.
- Pang J, Mei M, Yuan D, et al. Development of on-line monolith-based in-tube solid phase microextraction for the sensitive determination of triazoles in environmental waters. Talanta 184 (2018): 411-417.
- Chowdhary A, Sharma C, Kathuria S, et al. Prevalence and mechanism of triazole resistance in Aspergillus fumigatus in a referral chest hospital in Delhi, India and an update of the situation in Asia. Frontiers in Microbiology 6 (2015): 428.
- Chen Y, Lu Z, Zhao J, et al. Epidemiology and molecular characterizations of azole resistance in clinical and environmental Aspergillus fumigatus isolates from China. Antimicrobial Agents and Chemotherapy 60 (2016): 5878-5884.
- Maertens JA. History of the development of azole derivatives. Clinical Microbiology and Infection 10 (2004): 1-10.
- Fisher MC, Hawkins NJ, Sanglard D, et al. Worldwide emergence of resistance to antifungal drugs challenges human health and food security Science 360 (2018): 739-742.
- Tesh SA, Tesh JM, Fegert I, et al. Innovative selection approach for a new antifungal agent mefentrifluconazole (Revysol®) and the impact upon its toxicity profile. Regulatory Toxicology and Pharmacology 106 (2019): 152-168.
- El Azhari N, Dermou E, Barnard RL, et al. The dissipation and microbial ecotoxicity of tebuconazole and its transformation products in soil under standard laboratory and simulated winter conditions. The Science of the Total Environment 637 (2018): 892-906.
- Šudoma M, Neuwirthová N, Hvezdová M, et al. Fate and bioavailability of four conazole fungicides in twelve different arable soils - Effects of soil and pesticide properties. Chemosphere 230 (2019): 347-359.
- Snelders E, Camps SMT, Karawajczyk A, et al. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS ONE 7 (2012): e31801.
- Lestrade PPA, Meis JF, Melchers WJG, et al. Triazole resistance in Aspergillus fumigatus: recent insights and challenges for patient management. Clinical Microbiology and Infection 25 (2019): 799-806.
- Behnsen J, Hartmann A, Schmaler J, et al. The opportunistic human pathogenic fungus Aspergillus fumigatus evades the host complement system. Infection and immunity 76 (2008): 820-827.
- Dagenais TRT, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive Aspergillosis. Clinical Microbiology Reviews 22 (2009): 447-465.
- Latgé JP, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clinical Microbiology Reviews 33 (2019): e00140-18.
- Denning DW, Venkateswarlu K, Oakley KL, et al. Itraconazole resistance in Aspergillus fumigatus. Antimicrobial Agents and Chemotherapy 41 (1997): 1364-1368.
- Snelders E, Huis In 't Veld RAG, Rijs AJMM, et al. Possible environmental origin of Resistance of Aspergillus fumigatus to medical triazoles. Applied and Environmental Microbiology 75 (2009): 4053-4057.
- Chowdhary A, Kathuria S, Xu J, et al. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathogens 9 (2013): e1003633.
- Loeffert ST, Melloul E, Dananché C, et al. Monitoring of clinical strains and environmental fungal aerocontamination to prevent invasive aspergillosis infections in hospital during large deconstruction work: a protocol study. BMJ Open 7 (2017): e018109.
- Jeanvoine A, Rocchi S, Bellanger AP, et al. Azole-resistant Aspergillus fumigatus: A global phenomenon originating in the environment?. Medecine Et Maladies Infectieuses 50 (2019): 389-395.
- Gonçalves S, Oliveira C, Cunha C, et al. The Lung Microbiome, Metabolome, and Breath Volatolome in the Diagnosis of Pulmonary Disease. Microbiome and Metabolome in Diagnosis, Therapy, and other Strategic Applications, ch. 31 (2019): 297-305.
- Vermeulen E, Lagrou K, Verweij PE. Azole resistance in Aspergillus fumigatus: a growing public health concern. Current Opinion in Infectious Diseases 26 (2013): 493-500.
- Szejtli J. Cyclodextrin Technology. Springer Netherlands (1988).
- Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chemical Reviews 98 (1998): 1743-1754.
- Del Valle EMM. Cyclodextrins and their uses: a review. Process Biochemistry 39 (2004): 1033-1046.
- Application of cyclodextrins in agrochemistry. In Cyclodextrins and Their Complexes, H. Dodziuk, Ed. Wiley-VCH Verlag GmbH and Co. KGaA (2006): 459-467.
- Bilensoy E. Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications. Wiley (2011).
- Crini G. Review: A history of cyclodextrins. Chemical Reviews 114 (2014): 10940-10975.
- Euvrard É, Morin-Crini N, Druart C, et al. Cross-linked cyclodextrin-based material for treatment of metals and organic substances present in industrial discharge waters. Beilstein Journal of Organic Chemistry 12 (2016): 1826-1838.
- Adeoye O, Figueiredo A, Marques HC, et al. Cyclodextrins and Skin Disorders: Therapeutic and Cosmetic Applications. Carrier-Mediated Dermal Delivery New York: Jenny Stanford Publishing, ch.13, Ed. Ascenso A, Ribeiro H and Simões S (2017).
- Cova TFGG, Murtinho D, Pais AACC, et al. Cyclodextrin-based materials for removing micropollutants from wastewater. Current Organic Chemistry 22 (2018): 2150-2181.
- Crini G, Fourmentin S, Fenyvesi É, et al. Cyclodextrins, from molecules to applications. Environmental Chemistry Letters 16 (2018): 1361-1375.
- Sikder MdT, Rahman MdM, Jakariya Md, et al. Remediation of water pollution with native cyclodextrins and modified cyclodextrins: A comparative overview and perspectives. Chemical Engineering Journal 355 (2018): 920-941.
- Yao X, Huang P, Nie Z. Cyclodextrin-based polymer materials: From controlled synthesis to applications. Progress in Polymer Science 93 (2019): 1-35.
- Fenyvesi É, Barkács K, Gruiz K, et al. Removal of hazardous micropollutants from treated wastewater using cyclodextrin bead polymer – A pilot demonstration case. Journal of Hazardous Materials 383 (2020): 121181.
- Crini G, Exposito Saintemarie A, Rocchi S, et al. Simultaneous removal of five triazole fungicides from synthetic solutions on activated carbons and cyclodextrin-based adsorbents. Heliyon 3 (2017): e00380.
- Rocchi S, Morin-Crini N, Léchenaut-Bergerot C, et al. Effet de l’interaction cyclodextrine-difénoconazole sur la croissance d’une moisissure responsable d’infections fongiques graves. Environnement, Risques and Santé 18 (2019): 411-417.
- Raffaini G, Ganazzoli F. A molecular modeling study of complex formation and self-aggregation behavior of a porphyrin-β-cyclodextrin conjugate. Journal of Inclusion Phenomena Macrocyclic Chemistry 76 (2013): 213-221.
- Brocos P, Díaz-Vergara N, Banquy X, et al. Similarities and differences between cyclodextrin-sodium dodecyl sulfate host-guest complexes of different stoichiometries: Molecular dynamics simulations at several temperatures. The Journal of Physical Chemistry B 114 (2010): 12455-12467.
- Zhang H, Ge C, Van Der Spoel D, et al. Insight into the structural deformations of beta-cyclodextrin caused by alcohol cosolvents and guest molecules. The Journal of Physical Chemistry B 116 (2012): 3880-3889.
- Raffaini G, Ganazzoli F. A molecular dynamics study of the inclusion complexes of C60 with some cyclodextrins. The Journal of Physical Chemistry B 114 (2010): 7133-7139.
- Gaburjakova J, Gaburjakova M. Coupled gating modifies the regulation of cardiac ryanodine receptors by luminal Ca2+. Biochimica et Biophysica Acta - Biomembranes 1838 (2014): 867-873.
- Mellado E, Diaz-Guerra TM, Cuenca-Estrella M, et al. Identification of two different 14-α sterol demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. Journal of Clinical Microbiology 39 (2001): 2431-2438.
- Alanio A, Sitterlé E, Liance M, et al. ‘Low prevalence of resistance to azoles in Aspergillus fumigatus in a French cohort of patients treated for haematological malignancies. The Journal of Antimicrobial Chemotherapy 66 (2011): 371-374.
- Arendrup, MC, Meletiadis J, Mouton JW, et al. EUCAST definitive document E.Def 9.3.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. (2020).
- Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, et al. Breakpoints for antifungal agents: An update from EUCAST focussing on echinocandins against Candida spp. and triazoles against Aspergillus spp. Drug Resistance Updates 16 (2013): 81-95.
- Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67 (2015): 1-48.
- Pinheiro J, Bates D, DebRoy S, et al. nlme: Linear and nonlinear mixed effects models (2019).
- Barton K. MuMIn: Multi-Model Inference (2020).
- Lüdecke D. ggeffects: Tidy data frames of marginal effects from regression models. Journal of Open Source Software 3 (2018): 772.
- Rejzek M, Stevenson CE, Southard AM, et al. Chemical genetics and cereal starch metabolism: structural basis of the non-covalent and covalent inhibition of barley β-amylase. Molecular BioSystems 7 (2011): 718-730.
- Homburg C, Bommer M, Wuttge S, et al. Inducer exclusion in Firmicutes: insights into the regulation of a carbohydrate ATP binding cassette transporter from Lactobacillus casei BL23 by the signal transducing protein P-Ser46-HPr. Molecular Microbiology 105 (2017): 25-45.
- Tyndall JDA, Sabherwal M, Sagatova AA, et al. Structural and functional elucidation of yeast lanosterol 14α-demethylase in complex with agrochemical antifungals. Plose One 11 (2016): 1-18.
- Frisch MJ, Nielsen AB. M. J. Gaussian 09 Programmer ’ s Reference.
- Bayly CI, Cieplak P, Cornell WD, et al. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. The Journal of Physical Chemistry 97 (1993): 10269-10280.
- Wang J, Wang W, Kollman PA, et al. Automatic atom type and bond type perception in molecular mechanical calculations. Journal of Molecular Graphics and Modelling 25 (2006): 247-260.
- Wang J, Wolf RM, Caldwell JW, et al. Development and testing of a general Amber force field. Journal of Computational Chemistry 25 (2004): 1157-1174.
- Kutzner C, Páll S, Fechner M, et al. Best bang for your buck: GPU nodes for GROMACS biomolecular simulations. Journal of Computational Chemistry 36 (2015): 1990-2008.
- Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. Journal of Molecular Graphics 14 (1996) 33-38.
- Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera - A visualization system for exploratory research and analysis. Journal of Computational Chemistry 25 (2004): 1605-1612.
- Kumari R, Kumar R, Lynn A. G-mmpbsa -A GROMACS tool for high-throughput MM-PBSA calculations. Journal of Chemical Information and Modeling 54 (2014): 1951-1962.
- Baker NA, Sept D, Joseph S, et al. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proceedings of the National Academy of Sciences 98 (2001): 10037-10041.
- Nardello-Rataj V, Leclercq L. Encapsulation of biocides by cyclodextrins: toward synergistic effects against pathogens. Beilstein Journal of Organic Chemistry 10 (2014): 2603-2622.
- Pospišil L, Sokolová R, Hromadová M, et al. Inclusion complex of fungicide vinclozoline and β-cyclodextrin: The influence of host–guest interaction on the reduction mechanism. Journal of Electroanalytical Chemistry 517 (2001): 28-36.
- Lezcano M, Al-Soufi W, Novo M, et al. Complexation of several benzimidazole-type fungicides with α- and β-cyclodextrins, Journal of Agricultural and Food Chemistry 50 (2002): 108-112.
- Zhu XL, Wang HB, Q Chen, et al. Preparation and characterization of inclusion complex of iprodione and β-cyclodextrin to improve fungicidal activity. Journal of Agricultural and Food Chemistry 55 (2007): 3535-3539.
- Ge X, Huang Z, Tian S, et al. Complexation of carbendazim with hydroxypropyl-β-cyclodextrin to improve solubility and fungicidal activity. Carbohydrate Polymers 89 (2012): 208-212.
- Fernandes C, Encarnação I, Gaspar A, et al. Influence of hydroxypropyl-β-cyclodextrin on the photostability of fungicide pyrimethanil. International Journal of Photoenergy 2014 (2014): 8.
- Gao S, Liu Y, Jiang J, et al. Physicochemical properties and fungicidal activity of inclusion complexes of fungicide chlorothalonil with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. Journal of Molecular Liquids 293 (2019): 111513.
- Stepniak A, Belica-Pacha S, Rozalska S, et al. Study on a host–guest interaction of β-cyclodextrin with tebuconazole in water. Journal of Molecular Liquids 211 (2015): 288-293.
- Balmas V, Delogu G, Sposito S, et al. Use of a complexation of tebuconazole with beta-cyclodextrin for controlling foot and crown rot of durum wheat incited by Fusarium culmorum. Journal of Agricultural and Food Chemistry 54 (2006): 480-484.
Supplementary Materials
|
Effects |
Characteristics |
Estimates |
95% CI1 |
p-value |
|
Fixed |
||||
|
Fungicides: |
||||
|
Difenoconazole (reference) |
- |
- |
- |
|
|
Tebuconazole |
0.84 |
0.75, 0.94 |
<0.001 |
|
|
Media2: |
||||
|
RMPI (reference) |
- |
- |
- |
|
|
MALTO |
0.03 |
-0.15, 0.20 |
0.8 |
|
|
α-CD |
0.10 |
-0.07, 0.27 |
0.3 |
|
|
β-CD |
0.85 |
0.68, 1.0 |
<0.001 |
|
|
γ-CD |
0.38 |
0.20, 0.55 |
<0.001 |
|
|
HP-β-CD |
1.3 |
1.1, 1.4 |
<0.001 |
|
|
TRIMEB |
0.63 |
0.45, 0.80 |
<0.001 |
|
|
Random |
||||
|
Strains |
0.40 |
- |
- |
|
|
Residuals |
0.396 |
- |
- |
1CI = Confidence interval
2RPMI (reference medium used), supplemented with α-cyclodextrin (α-CD), β-cyclodextrin (β-CD), γ-cyclodextrin (γ-CD), hydroxypropyl-β-cyclodextrin (HP-β-CD) or heptakis-(2,3,6-tri-O-methyl)-β-cyclodextrin (TRIMEB).
Table S-1: Outputs of the linear mixed-effects model using strains, media and fungicides to explain observed minimal inhibitory concentrations. In the model, RMPI medium and difenoconazole were used as references.
|
System* |
Cyclodextrin |
Ligand |
Side of cubic box (Å) |
Water |
|
αCD |
α |
- |
13.31 |
75776 |
|
βCD |
β |
- |
13.51 |
78159 |
|
γCD |
γ |
- |
13.68 |
80987 |
|
HPβCD |
β |
- |
13.90 |
84595 |
|
αCD-DIFENO |
α |
DIFENO |
13.31 |
75249 |
|
βCD-DIFENO |
β |
DIFENO |
13.51 |
78141 |
|
γCD-DIFENO |
γ |
DIFENO |
13.68 |
80970 |
|
αCD-TEBU |
α |
TEBU |
13.31 |
75259 |
|
βCD-TEBU |
β |
TEBU |
13.51 |
78141 |
|
γCD-TEBU |
γ |
TEBU |
13.68 |
80969 |
|
HPβCD-DIFENO |
β |
DIFENO |
13.90 |
84579 |
Table S-2: Box size and molecular content of each studied system.
* α-cyclodextrin (α-CD), β-cyclodextrin (β-CD), γ-cyclodextrin (γ-CD), hydroxypropyl-β-cyclodextrin (HP-β-CD), difenoconazole (DIFENO), tebuconazole (TEBU). For all systems, the following equilibration protocol was applied. All simulations were performed using the Gromacs 5.1.2 MD package [1] with the AMBER14sb force field [2]. All systems were immersed into a cubic periodic box of approximately 14 nanometer (nm) side filled of TIP3P water molecules [3]. The cut-off distance for non-bonded interactions was set to 1.2 nm. All systems were first relaxed by steepest descent method at 5000-step with an energy convergence criterion of 10 kJ/mol. . They were then heated from 0 K to 310 K for 100 picoseconds (ps) and equilibrated at 1 atmosphere (atm) with a relaxation time of 2 ps for 150 ps at the NPT ensemble. Pressure of 1 atm was maintained by the isotropic Parrinello-Rhaman barostat [4] using Langevin dynamics. Velocity rescale thermostat [5] was used at the temperature of 310 K. All bonds were constrained using the LINCS algorithm [6].
References
- Jorgensen WL, Chandrasekhar J, Madura JD, et al. Comparison of simple potential functions for simulating liquid water. Journal of Chemical Physics 79 (1983): 926-935.
- Maier JA, Martinez C, Kasavajhala K, et al. Journal of Chemical Theory and Computation 11 (2015): 3696-3713.
- Essmann U, Perera L, Berkowitz ML, et al. A smooth particle mesh Ewald method. The Journal of Chemical Physics 103 (1995): 8577-8593.
- Baker NA, Sept D, Joseph S, et al. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proceedings of the National Academy of Sciences 98 (2001): 10037-10041.
- Bussi G, Donadio D, Parrinello M. Canonical sampling trhough velocity rescaling. The Journal of Chemical Physics 126 (2007): 014101.
- Hess B, Bekker H, Berendsen HJC, et al. LINCS: A linear constraint solver for molecular simulations. Journal of Computational Chemistry 18 (1997): 1463-1472.
