Real-Life Efficacy of Liraglutide Therapy on Weight-Loss in Patients with Overweight and Obesity
Article Information
Michela Del Prete1*, Federico Vignati1, Gianleone Di Sacco1, Lidia Gavazzi1, Daniela Dellepiane1 and Fabrizio Muratori1
1Division of Endocrinology and Diabetology, Sant’Anna Hospital - ASST Lariana, Como, Italy
*Corresponding Author: Michela Del Prete, MD, PhD, Division of Endocrinology and Diabetology, Sant’Anna Hospital - ASST Lariana, Como, Italy/p>
Received: 13 June 2023; Accepted: 22 June 2023; Published: 28 June 2023
Citation: Michela Del Prete, Federico Vignati, Gianleone Di Sacco, Lidia Gavazzi, Daniela Dellepiane and Fabrizio Muratori. Real-Life Efficacy of Liraglutide Therapy on Weight-Loss in Patients with Overweight and Obesity. Journal of Surgery and Research. 6 (2023): 245-250.
Share at FacebookAbstract
Introduction: Liraglutide is approved for long-term weight-loss in patients with overweight and obesity as an adjunct to lifestyle modification. Here, we reported our real-life experience on long-term efficacy of liraglutide therapy in association with dietary and behavioral advice, in patients with obesity followed in our outpatient clinic.
Methods: We retrospectively assessed 109 patients with obesity (92 females and 17 males) consecutively admitted to our observation from September 2018 to October 2019 to lose weight with liraglutide. At the first visit, mean weight was 93.2±17.9 kg and mean body mass index (BMI) was 34.0±5.5 kg/m2. All patients were required to follow dietary and behavioral therapy with concomitant drug treatment. Liraglutide was administered once-daily subcutaneously at starting dose of 0.6 mg and with the achievement of 3.0 mg in two months from the starting therapy. The aim of this study was to evaluate the efficacy and safety of liraglutide in our real-life patients and how the early weight loss after 4 months of therapy with liraglutide can predict long-term weight loss.
Results: After 4-month follow up, patients had a mean weight of 83.3±16.3 kg and mean BMI of 30.4±5.0 kg/m2, with a mean percentage weight and mean BMI reduction respectively of -10.5±3.8% and -3.6±1.4 kg/m2. After 12-month follow up, 34 patients were still on treatment with liraglutide. These patients had a mean weight of 78.9±13.5 kg and mean BMI of 28.5±4.6 kg/ m2, with a mean percentage weight and mean BMI reduction respectively of -22.0±5.0% and -8.1±2.4 kg/m2. The early weight loss and BMI changes after 4-month liraglutide therapy significantly predict the weight loss and BMI changes at 6- and 12-month follow-up (p<0.0001).
Conclusions: Our results confirm the efficacy of real-life therapy with liraglutide in patients with obesity and are consistent with data obtained from the clinical trials. Our data show how early weight loss and reduction in BMI after 4 months of liraglutide therapy can significantly predict long-term weight loss.
Keywords
Real-life therapy, Liraglutide, Overweight, Obesity, Early weight loss, Long-term weight loss prediction
Real-life therapy articles; Liraglutide articles; Overweight articles; Obesity articles; Early weight loss articles; Long-term weight loss prediction articles
Real-life therapy articles Real-life therapy Research articles Real-life therapy review articles Real-life therapy PubMed articles Real-life therapy PubMed Central articles Real-life therapy 2023 articles Real-life therapy 2024 articles Real-life therapy Scopus articles Real-life therapy impact factor journals Real-life therapy Scopus journals Real-life therapy PubMed journals Real-life therapy medical journals Real-life therapy free journals Real-life therapy best journals Real-life therapy top journals Real-life therapy free medical journals Real-life therapy famous journals Real-life therapy Google Scholar indexed journals Liraglutide articles Liraglutide Research articles Liraglutide review articles Liraglutide PubMed articles Liraglutide PubMed Central articles Liraglutide 2023 articles Liraglutide 2024 articles Liraglutide Scopus articles Liraglutide impact factor journals Liraglutide Scopus journals Liraglutide PubMed journals Liraglutide medical journals Liraglutide free journals Liraglutide best journals Liraglutide top journals Liraglutide free medical journals Liraglutide famous journals Liraglutide Google Scholar indexed journals Overweight articles Overweight Research articles Overweight review articles Overweight PubMed articles Overweight PubMed Central articles Overweight 2023 articles Overweight 2024 articles Overweight Scopus articles Overweight impact factor journals Overweight Scopus journals Overweight PubMed journals Overweight medical journals Overweight free journals Overweight best journals Overweight top journals Overweight free medical journals Overweight famous journals Overweight Google Scholar indexed journals Obesity articles Obesity Research articles Obesity review articles Obesity PubMed articles Obesity PubMed Central articles Obesity 2023 articles Obesity 2024 articles Obesity Scopus articles Obesity impact factor journals Obesity Scopus journals Obesity PubMed journals Obesity medical journals Obesity free journals Obesity best journals Obesity top journals Obesity free medical journals Obesity famous journals Obesity Google Scholar indexed journals Early weight loss articles Early weight loss Research articles Early weight loss review articles Early weight loss PubMed articles Early weight loss PubMed Central articles Early weight loss 2023 articles Early weight loss 2024 articles Early weight loss Scopus articles Early weight loss impact factor journals Early weight loss Scopus journals Early weight loss PubMed journals Early weight loss medical journals Early weight loss free journals Early weight loss best journals Early weight loss top journals Early weight loss free medical journals Early weight loss famous journals Early weight loss Google Scholar indexed journals Long-term weight loss prediction articles Long-term weight loss prediction Research articles Long-term weight loss prediction review articles Long-term weight loss prediction PubMed articles Long-term weight loss prediction PubMed Central articles Long-term weight loss prediction 2023 articles Long-term weight loss prediction 2024 articles Long-term weight loss prediction Scopus articles Long-term weight loss prediction impact factor journals Long-term weight loss prediction Scopus journals Long-term weight loss prediction PubMed journals Long-term weight loss prediction medical journals Long-term weight loss prediction free journals Long-term weight loss prediction best journals Long-term weight loss prediction top journals Long-term weight loss prediction free medical journals Long-term weight loss prediction famous journals Long-term weight loss prediction Google Scholar indexed journals prediabetes articles prediabetes Research articles prediabetes review articles prediabetes PubMed articles prediabetes PubMed Central articles prediabetes 2023 articles prediabetes 2024 articles prediabetes Scopus articles prediabetes impact factor journals prediabetes Scopus journals prediabetes PubMed journals prediabetes medical journals prediabetes free journals prediabetes best journals prediabetes top journals prediabetes free medical journals prediabetes famous journals prediabetes Google Scholar indexed journals Cardiovascular Outcome articles Cardiovascular Outcome Research articles Cardiovascular Outcome review articles Cardiovascular Outcome PubMed articles Cardiovascular Outcome PubMed Central articles Cardiovascular Outcome 2023 articles Cardiovascular Outcome 2024 articles Cardiovascular Outcome Scopus articles Cardiovascular Outcome impact factor journals Cardiovascular Outcome Scopus journals Cardiovascular Outcome PubMed journals Cardiovascular Outcome medical journals Cardiovascular Outcome free journals Cardiovascular Outcome best journals Cardiovascular Outcome top journals Cardiovascular Outcome free medical journals Cardiovascular Outcome famous journals Cardiovascular Outcome Google Scholar indexed journals breastfeeding articles breastfeeding Research articles breastfeeding review articles breastfeeding PubMed articles breastfeeding PubMed Central articles breastfeeding 2023 articles breastfeeding 2024 articles breastfeeding Scopus articles breastfeeding impact factor journals breastfeeding Scopus journals breastfeeding PubMed journals breastfeeding medical journals breastfeeding free journals breastfeeding best journals breastfeeding top journals breastfeeding free medical journals breastfeeding famous journals breastfeeding Google Scholar indexed journals
Article Details
Introduction
The treatment of subjects with obesity remains problematic. To date, drugs approved for weight-loss are effective in inducing an average loss of 5 to 10% of initial weight when used as an adjunct to a reduced-calorie diet and increased physical activity [1-3]. Weight-loss with pharmacotherapy in association with lifestyle modification, stabilizes about after 6 to 9 months of treatment [1,4,5]. Liraglutide 3.0 mg is the first GLP-1 analogue to be approved for long-term weight-loss in patients with overweight and obesity as an adjunct to a reduced-calorie diet and increased physical activity, in adults with BMI ≥30 or ≥27 kg/m2 and ≥1 weight-related comorbidity. Liraglutide is 97% identical to human GLP-1 but has a longer action time [6]. Liraglutide has been used for many years at a dose of 1.8 mg for the treatment of type 2 diabetes mellitus.
The dose of liraglutide required for the treatment of obesity was first evaluated in a phase II study conducted by Astrup et al. [7] and subsequently the drug was extensively studied in the phase III SCALE studies proving effective at a dose of 3.0 mg per day compared to placebo in reducing body weight, in improving metabolic and blood pressure profile in subjects with obesity, in overweight subjects with pre-diabetes or diabetes and in subjects with obesity and sleep-apnea [8-10].
In addition, one of the SCALE studies demonstrated the efficacy of liraglutide in maintaining weight after weight loss. Liraglutide was shown to be effective in maintaining weight loss compared to placebo even after three years in patients with obesity and prediabetes [10]. The safety of liraglutide was also demonstrated by the results of the LEADER study (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) [11].
The LEADER study is a cardiovascular outcome trial on a diabetes drug, ordered by the US Food and Drug Administration. In this study, liraglutide (at a dose of 1.8 mg in diabetic patients) significantly reduced the rate of major adverse cardiovascular events (primary endpoint events, MACE) compared to placebo (3.41 versus 3.90 in 100 patients / observation year in the liraglutide and placebo groups respectively) with a 13% risk reduction, HR 0.87 [0.78, 0.97] [95% CI]) (p = 0.005) [11]. In 2018, a new cardiovascular safety study of liraglutide 3.0 mg was also published which was based on the post hoc analysis of data from the entire SCALE program, also including phase 2 studies: 5908 participants were evaluated [12]. This study also confirmed the safety of the drug [12]. In our center we decided to evaluate the use of liraglutide in subjects with overweight and obesity in real-life. This study was carried out after having used the drug for about two years after its release on the market. After two years of using liraglutide in our center and with the accumulated experience we wanted to evaluate whether a possible flexibility in the titration of liraglutide could reduce the number of initial side effects and drop-outs, the effectiveness on weight loss, if the response to therapy could be dependent on the initial body mass index (BMI) and if the response to the drug in terms of weight loss after the first 4 months could be predictive of a medium- to long-term response to the drug.
Material and Methods
From September 2018 to October 2019, 109 subjects with obesity (92 females and 17 males) were retrospectively evaluated for weight-loss with liraglutide at the Sant ’Anna Hospital of Como to assess the efficacy and safety of liraglutide treatment in this setting of patients. Patients spontaneously presented in our outpatient clinic and were examined approximately every 45 days from the beginning of the treatment. Patient characteristics are presented in table 1.
|
Patients (n=109) |
|
|
Age (years; mean ± SD) |
47.0±10.5 |
|
Sex (n) |
|
|
F |
92 |
|
M |
17 |
|
Weight (Kg; mean ± SD) |
|
|
Baseline |
93.3±17.9 |
|
4-month (102/109) |
83.3±16.3 |
|
6-month (95/109) |
80.7±16.1 |
|
12-month (34/109) |
78.9±13.5 |
|
BMI (kg/m2; mean ± SD) |
|
|
Baseline |
34.0±5.5 |
|
4-month (102/109) |
30.4±5.0 |
|
6-month (95/109) |
29.3±5.0 |
|
12-month (34/109) |
28.5±4.6 |
BMI = body mass index. SD = standard deviation
Table 1: Overall patient characteristics
Patients were aged >18 years old and at baseline mean age was 47.0±10.5 years (range: 22-75 years). At the first visit, mean weight was 93.3±17.9 kg and mean body mass index (BMI) was 34.0±5.5 kg/m2. All patients were required to follow dietary and behavioral therapy with concomitant drug treatment. Before starting liraglutide, patients were assessed to exclude any condition that contraindicate the therapy. Pregnancy and breastfeeding were also excluded. Liraglutide was administered once-daily subcutaneously at starting dose of 0.6 mg and with the achievement of 3.0 mg in two months from the starting therapy. Data on liraglutide efficacy and safety was recorded. The aim of this study was to evaluate the efficacy and safety after 4, 6, and 12 months of treatment with liraglutide. Other aims were to evaluate the pharmacological response to liraglutide compared to the initial BMI and the number of initial side effects and drop-outs related to a flexibility in the titration of liraglutide. Furthermore, in this study was evaluate if the response to the therapy in terms of weight loss after 4 months of treatment with liraglutide could be predictive of a medium- to long-term response to the drug. The titration with liraglutide was performed with the starting dose of 0.6 mg dose daily for 1 week and then titrate to 1.2 mg. After 12 days taking 1.2 mg, the dose was increased to 1.8 mg if the 1.2 mg dose was well tolerated. The 1.8 mg daily dose was increased after 15 days if well tolerated to 2.4 mg daily dose. This last dose was increased to 3.0 mg after 21 days if no side effects occurred. The efficacy was evaluated in all patients and in the following subgroups: in patients with BMI <30kg/m2, patients with BMI <35 vs ≥35kg/m2. The efficacy was assessed by calculating the percentage of weight loss after liraglutide treatment and the mean BMI change in all patients and subgroups according to the degree of obesity. Patient consents were obtained before starting the study. Ethical approval is not required for this study in accordance with local or national guidelines. Data were presented as mean ± standard deviation (SD). A difference was considered statistically significant if the corresponding p value was <0.05. The normal distribution of data was checked using the Kolmogorov–Smirnov test. According to data distribution, two-tailed t or Wilcoxon test, Pearson correlation or Spearman tests were used for comparing data concerning weight and BMI changes and to evaluate a correlation between early and final loss of body weight. Statistical analysis was performed with GraphPad Prism software (version 9, GraphPad Software Inc., La Jolla, CA).
Results
Overall patients’ efficacy
At baseline, patients had a mean weight of 93.3±17.9 kg and mean BMI was 34.0±5.5 kg/m2. Of these, 22 were overweight (BMI 28.0±1.0 kg/m2), 50 had grade I (BMI 32.2±1.5 kg/m2), 24 grade II (BMI 36.8±1.4 kg/m2) and 13 grade III (BMI 46.1±4.2 kg/m2) obesity. After 4-month follow up, 102 patients evaluable had a mean weight of 83.3±16.3 kg and mean BMI of 30.4±5.0 kg/m2, with a mean percentage weight and mean BMI reduction respectively of -10.5±3.8 % and -3.6±1.4 kg/m2. Of these, 22 patients were overweight (BMI 25.1±1.4 kg/m2), 44 had grade I (BMI 28.7±1.8 kg/m2), 24 grade II (BMI 33.2±2.1 kg/m2) and 12 grade III (BMI 40.6±4.4 kg/m2) obesity. After 6-month follow up, 95 patients were still on treatment and had a mean weight of 80.7±16.1 kg and mean BMI of 29.3±5.0 kg/m2, with a mean percentage weight and mean BMI reduction respectively of -14.1±4.7 % and -4.8±1.8 kg/m2. After 6 months of therapy, 19 patients were overweight (BMI 23.9±1.3 kg/m2), 40 had grade I (BMI 27.6±2.1 kg/m2), 22 grade II (BMI 30.8±2.1 kg/m2) and 12 grade III (BMI 39.1±4.3 kg/m2) obesity. After 12-month follow up, 34 patients were still on treatment with liraglutide. These patients had a mean weight of 78.9±13.5 kg and mean BMI of 28.5±4.6 kg/m2, with a mean percentage weight and mean BMI reduction respectively of -22.0±5.0 % and 8.1±2.4 kg/m2. Of these, 3 overweight patients at baseline achieved a normal BMI (22.8±1.4 kg/m2), 12 grade I and 11 grade II patients with obesity became overweight with a mean BMI respectively of 25.5±1.9 kg/m2 and 28.8±2.4 kg/m2. Eight grade III patients with obesity achieved a grade I obesity with a mean BMI of 34.6±4.0 kg/m2.
Overweight patients
Considering a BMI between 25 and 29.9 kg/m2, 21 over 109 patients were evaluable. At baseline, mean weight was 77.6±8.6 kg and mean BMI was 28.0±1.0 kg/m2. After 6-month follow up, 18 patients had a mean weight of 66.5±7.6 kg and mean BMI of 24.0±1.4 kg/m2, with a mean percentage weight and mean BMI reduction respectively of -14.4±3.4% and 4.0±1.0kg/m2. Two patients lost more than 5% and 16 patients more than 10% of weight. Of 18 patients, 15 (83.3%) achieved normal BMI from baseline and 3 patients were still overweight. Of these, 1 patient was lost to follow up and 2 patients achieved normal BMI at 8-month follow-up. After 12-month, 2 patients were still on treatment with liraglutide and achieved a mean BMI of 23.0±1.9 kg/m2(shown in table 2 and figure 1).
|
BMI |
|||
|
25-29.9 |
<35 |
≥35 |
|
|
Weight (Kg; mean ± SD) |
|||
|
Baseline |
77.6±8.6 |
86.5±8.1 |
111.7±16.4 |
|
6-month |
66.5±7.6 |
74.0±8.6 |
96.3±13.8 |
|
12-month |
66.0±7.1 |
69.4±8.0 |
86.6±12.1 |
|
BMI (kg/m2; mean ± SD) |
|||
|
Baseline |
28.0±1.0 |
32.1±1.5 |
39.9±5.0 |
|
6-month |
24.0±1.4 |
27.5±2.2 |
34.4±4.3 |
|
12-month |
23.0±1.9 |
25.3±2.0 |
31.2±4.2 |
BMI = body mass index. SD = standard deviation
Table 2: Mean weight and mean BMI at baseline and after 6- and 12-month follow-up according to the obesity grade
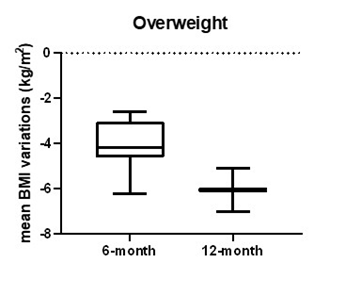
Figure 1: Mean BMI variations in patients with overweight from baseline
Patients with BMI <35 vs ≥35kg/m2
At baseline, patients with BMI <35 and ≥35 respectively had mean weight of 86.5±8.1 kg and 111.7±16.4 kg. Mean BMI was respectively 32.1±1.5 kg/m2 and 39.9±5.0 kg/m2. After 6-month follow up, 41 patients with BMI <35 and 34 patients with BMI ≥35 achieved respectively a mean weight of 74.0±8.6 kg and 96.3±13.8 kg and mean BMI of 27.5±2.2 kg/m2 and 34.4±4.3 kg/m2, with a mean percentage weight and mean BMI reduction respectively of -14.4±5.5%, -13.7±4.3%, 4.6±1.8 kg/m2 and 5.5±2.0 kg/m2. In BMI <35 group 8 patients lost more than 5% and 33 patients more than 10% of weight, while in BMI ≥35 group, 1 patient lost less than 5%, 8 more than 5% and 25 more than 10% of weight from baseline. After 12-month follow-up, 13 patients with BMI <35 and 19 patients with BMI ≥35 were still on treatment and had respectively a mean weight of 69.4±8.0 kg, 86.6±12.1 kg and mean BMI of 25.3±2.0 kg/m2 and 31.2±4.2 kg/m2, with a mean percentage weight and mean BMI reduction respectively of -28.2±9.6%, -29.4±8.4%, 7.0±1.9kg/m2 and 9.1 2.4kg/m2 from baseline (shown in table 2 and figures 2,3).
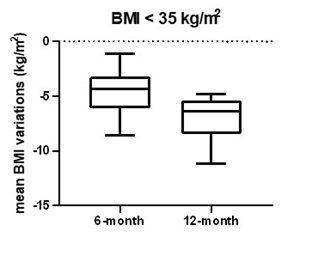
Figure 2: Mean BMI variations in patients with BMI <35 kg/m2 from baseline
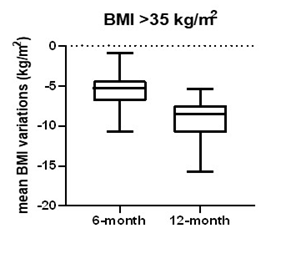
Figure 3: Mean BMI variations in patients with BMI ≥35 kg/m2 from baseline
Long-term weight loss
There was a significant correlation between percentage weight loss changes at 4, and 6- and 12-month follow-up after liraglutide therapy (6 months: R2= -0.88; p<0.0001; 12 months: R2= -0.67; p<0.0001) in the whole examined subjects. BMI changes were also statistically significant after 4, 6 and 12 months of therapy compared to baseline (4 months: degree of freedom (df)= 101, p<0.0001; 6 months: df= 92, p<0.0001; 12 months: df= 33, p<0.0001).
Overall safety
Six patients discontinued liraglutide due to response failure and 4 patients for cost reasons. Liraglutide was well tolerated, and no serious adverse events were recorded. Only 4 patients experienced mild adverse events as nausea and vomiting and discontinued the treatment.
Discussion
This study confirms that in real-life, therapy with liraglutide, associated with diet and exercise, is significantly effective and safe in patients with obesity ensuring a mean overall weight loss of 9.8 kg, 13.2 kg and 22.4 kg after 4, 6 and 12 months of treatment from baseline. In literature and to date, there are not many real-life studies on liraglutide treatment in patients living with obesity [13-15]. Our results on efficacy and safety of liraglutide confirm those reported in the literature and proved that liraglutide was effective in all subgroups of treated patients regardless of initial BMI. The adherence to the therapy and high percentage of weight loss are related in our study, presumably, not only to the effectiveness of liraglutide and the motivation of patients to lose weight, but also to the fact that patients were always examined by the same physician. In real life, the percentage of weight loss is greater than that obtained in clinical trials because in everyday reality, patients who do not respond to therapy do not continue it for a period established at the outset. A recent Canadian study on 310 subjects with a baseline BMI of 40.7 kg/m2 reported a change in body weight of -6.3 and -7.1% at 4- and 6-month follow up respectively [13]. A significant weight loss was also observed in a Swiss patient court with a mean percentage weight change of -4.7% and -5.3% from baseline after 4 and 7 months of treatment with liraglutide [15]. In our study, we observed a greater mean weight loss respectively of -10.5% and -14.1% from baseline in all patients. Considering the subgroups of patients according to the BMI, mean weight loss changes was still more than 10% at 4- and 6-month follow-up. In a recent real-life study, the authors reported the efficacy of liraglutide on weight loss in more than 60 % of patients with obesity [14]; 69.8% of patients lost at least 5% of the initial weight [14]. In our study, we also observed an overall weight loss in all patients with an early weight loss > 5% in 4.0% and > 10% in 56.8% of patients after 4 months of treatment and respectively in 2.1% and 79.5% of patients after 6 months of treatment. After 12 months of treatment all patients still on treatment achieved more than 10% of weight loss from baseline. We also found a significant early weight loss predictive of long-term weight loss and in line with results reported in literature [14]. An observation on this topic was made on the post hoc results of the SCALE maintenance study. In the Scale maintenance obese/overweight subjects who lost more than 5% of initial weight during a low-calorie diet run-in were randomly assigned to liraglutide 3.0 mg per day or placebo for 56 weeks. Participants lost a mean 6.0% of weight during run-in and from randomization to week 56, weight decreased an additional mean 6.2% with liraglutide and 0.2% with placebo [10]. In a post hoc study carried out on SCALE maintenance data, patients who were treated with liraglutide were divided into two groups: those who at 16 weeks had lost more than 4% and those who had lost less than 4%. By dividing the responders to liraglutide in this way, it was seen that the weight loss in the responders was not 6.1%, but 9% at the end of 56 weeks [16]. The efficacy of liraglutide have also been confirmed in patients with type 2 diabetes mellitus in comparison with other glucagon-like peptide 1 receptor agonists [17,18]. However, in our study, we focused on patients with obesity and without diabetes or other metabolic disorders. In our setting of patients liraglutide treatment was confirmed to be safe, well tolerated with no severe adverse events. These observations confirmed that our strategy on flexibility in the titration of liraglutide can effectively reduce the number of initial side effects and drop-outs.
Conclusions
In conclusion, our data confirm the efficacy and safety of treatment with liraglutide in association with lifestyle modification after 4, 6 and 12 months of therapy in patients with overweight and obesity regardless the initial BMI. Furthermore, the early weight loss after 4 months of liraglutide therapy can also be predictive of long-term weight loss.
Statement of ethics
Ethical approval is not required for this study in accordance with local or national guidelines. This retrospective review of patient data did not require ethical approval in accordance with local/ national guidelines.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Funding sources
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author contributions
MDP collected the data, performed statistical analysis, and drafted the study. FM proposed and supervised the study, collected the data, and edited the manuscript. All authors contributed to the manuscript editing, read and approved the final manuscript.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 100 (2015): 342-362.
- Khera R, Murad MH, Chandar AK, et al. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA 315 (2016): 2424-2434.
- Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 311 (2014): 74-86.
- Jensen MD, Ryan DH, Apovian CM, et al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 63 (2014): 2985-3023.
- Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med 376 (2017): 1492.
- Barrera JG, Sandoval DA, D'Alessio DA, et al. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol 7 (2011): 507-516.
- Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 36 (2012): 843-854.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med 373 (2015): 11-22.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA 314 (2015): 687-699.
- Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 37 (2013): 1443-1451.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 375 (2016): 311-322.
- Davies MJ, Aronne LJ, Caterson ID, et al. Liraglutide and cardiovascular outcomes in adults with overweight or obesity: A post hoc analysis from SCALE randomized controlled trials. Diabetes Obes Metab 20 (2018): 734-739.
- Wharton S, Liu A, Pakseresht A, et al. Real-World Clinical Effectiveness of Liraglutide 3.0 mg for Weight Management in Canada. Obesity (Silver Spring) 27 (2019): 917-924.
- Ferrari F, Fierabracci P, Salvetti G, et al. Weight loss effect of liraglutide in real-life: the experience of a single Italian obesity center. J Endocrinol Invest 43 (2020): 1779-1785.
- Haase CL, Serratore AMG, Lucrezi G, et al. Use of Liraglutide 3.0 mg for Weight Management in a Real-World Setting in Switzerland. Obes Facts 14 (2021): 568-576.
- Muratori F, Vignati F, Wharton S, et al. Early responders to liraglutide 3.0 mg as adjunct to diet and exercise from the scale maintenance trial. Eat Weight Disord 34 (2018): 72.
- Tanaka K, Okada Y, Tokutsu A, et al. Real-world effectiveness of liraglutide versus dulaglutide in Japanese patients with type 2 diabetes: a retrospective study. Sci Rep 12 (2022): 154.
- Garcia-Perez LE, Boye KS, Rosilio M, et al. The Real-World Observational Prospective Study of Health Outcomes with Dulaglutide and Liraglutide in Type 2 Diabetes Patients (TROPHIES): Design and Baseline Characteristics. Diabetes Ther 12 (2021): 1929-1946.
