Randomized Phase II Clinical Trials using Fisher’s Exact Test
Article Information
Shiwei Cao, Lu Liu, and Sin-Ho Jung *
Department of Biostatistics and Bioinformatics, Duke University
*Corresponding author: Sin-Ho Jung, Department of Biostatistics and Bioinformatics, Duke University, 2424 Erwin Road, 11070 Hock Plaza, Suite 1102, DUMC Box 2721, Durham, NC 27710.
Received: 09 March 2021; Accepted: 16 March 2021; Published: 07 April 2021
Citation: Shiwei Cao, Lu Liu, and Sin-Ho Jung. Randomized Phase II Clinical Trials using Fisher’s Exact Test. Archives of Clinical and Biomedical Research 5 (2021): 214-229.
Share at FacebookAbstract
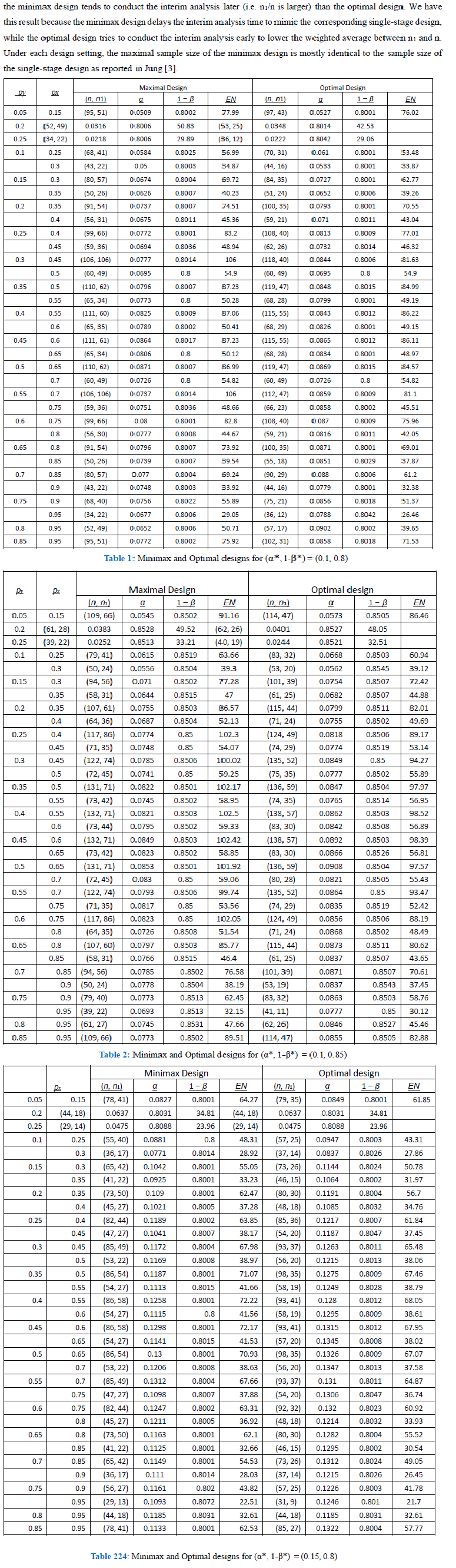
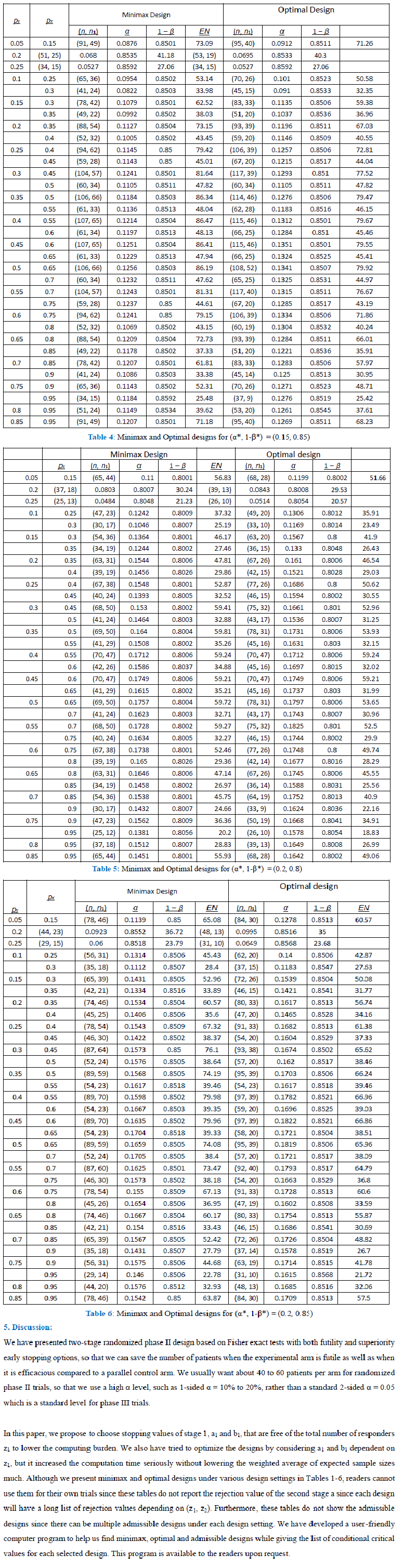
A phase II trial is to evaluate an experimental therapy using an early efficacy outcome, such as tumor shrinkage, before proceeding to a large-scale phase III trial. Traditionally, a typical phase II trial has been conducted using a single-arm design recruiting patients only to the experimental therapy to be compared with a historical control. Due to a small sample size and heterogeneity of patient population, the characteristics of the patients in a new phase II trial is often different from that of the selected historical control, so that the single-arm phase II trial results in false positive or false negative conclusions. A randomized phase II trial can resolve such problems by randomizing patients between an experimental arm and a control arm. In this paper, we propose randomized phase II trial designs based on 2-stage Fisher’s exact tests allowing for both superiority and futility early stopping options, so that we can save number of patients when experimental therapy is definitely efficacious as well as when it is futile. We propose a weighted expected sample size as a new criterion to define optimal twostage designs.
Keywords
Admissible design; Futility stopping; Minimax design; Optimal design; Superiority stopping; Weighted average sample size
Article Details