Processing and Application of Starch in Food in Japan
Article Information
Tomoya Shintani1,2*
1United Graduate School of Agricultural Science, Ehime University, Matsuyama, Japan
2Department of Nutritional Representative-Supplement Adviser, The Japanese Clinical Nutrition Association, Tokyo, Japan
*Corresponding Author: Tomoya Shintani, United Graduate School of Agricultural Science, Ehime University, 3-5-7, Tarumi, Matsuyama, Ehime 790–8507, Japan
Received: 17 May 2020; Accepted: 01 June 2020; Published: 06 July 2020
Citation: Tomoya Shintani. Processing and Application of Starch in Food in Japan. Journal of Food Science and Nutrition Research 3 (2020): 140-152.
Share at FacebookAbstract
Starch is the most common carbohydrate in human diet, and its major sources worldwide are cereals and root vegetables. As starch is inexpensive and easy to process chemically, many starch-based products have been developed. Modified starch has been contributing to a rich diet for several years. Asia, especially Japan, has always led the way in research and development pertaining to starch, including the application of glucoamylase in glucose production, endorsement of high fructose syrup, and application of glucose isomerase in fructose production. Additionally, erythritol, D-allulose, trehalose, and resistant maltodextrin were the representative starch-derived molecules to be commercialized and are now consumed globally. More novel healthy starch-based products will continue to be discovered in the future. In this review, we describe the current status of processing and utilization of starch.
Keywords
Starch, Sugar, Isomerized sugar, Glucose, Fructose, D-Allulose, Maltodextrin, Modified starch
Starch articles, Sugar articles, Isomerized sugar articles, Glucose articles, Fructose articles, D-Allulose articles, Maltodextrin articles, Modified starch articles
Starch articles Starch Research articles Starch review articles Starch PubMed articles Starch PubMed Central articles Starch 2023 articles Starch 2024 articles Starch Scopus articles Starch impact factor journals Starch Scopus journals Starch PubMed journals Starch medical journals Starch free journals Starch best journals Starch top journals Starch free medical journals Starch famous journals Starch Google Scholar indexed journals Sugar articles Sugar Research articles Sugar review articles Sugar PubMed articles Sugar PubMed Central articles Sugar 2023 articles Sugar 2024 articles Sugar Scopus articles Sugar impact factor journals Sugar Scopus journals Sugar PubMed journals Sugar medical journals Sugar free journals Sugar best journals Sugar top journals Sugar free medical journals Sugar famous journals Sugar Google Scholar indexed journals Isomerized sugar articles Isomerized sugar Research articles Isomerized sugar review articles Isomerized sugar PubMed articles Isomerized sugar PubMed Central articles Isomerized sugar 2023 articles Isomerized sugar 2024 articles Isomerized sugar Scopus articles Isomerized sugar impact factor journals Isomerized sugar Scopus journals Isomerized sugar PubMed journals Isomerized sugar medical journals Isomerized sugar free journals Isomerized sugar best journals Isomerized sugar top journals Isomerized sugar free medical journals Isomerized sugar famous journals Isomerized sugar Google Scholar indexed journals Glucose articles Glucose Research articles Glucose review articles Glucose PubMed articles Glucose PubMed Central articles Glucose 2023 articles Glucose 2024 articles Glucose Scopus articles Glucose impact factor journals Glucose Scopus journals Glucose PubMed journals Glucose medical journals Glucose free journals Glucose best journals Glucose top journals Glucose free medical journals Glucose famous journals Glucose Google Scholar indexed journals Fructose articles Fructose Research articles Fructose review articles Fructose PubMed articles Fructose PubMed Central articles Fructose 2023 articles Fructose 2024 articles Fructose Scopus articles Fructose impact factor journals Fructose Scopus journals Fructose PubMed journals Fructose medical journals Fructose free journals Fructose best journals Fructose top journals Fructose free medical journals Fructose famous journals Fructose Google Scholar indexed journals D-Allulose articles D-Allulose Research articles D-Allulose review articles D-Allulose PubMed articles D-Allulose PubMed Central articles D-Allulose 2023 articles D-Allulose 2024 articles D-Allulose Scopus articles D-Allulose impact factor journals D-Allulose Scopus journals D-Allulose PubMed journals D-Allulose medical journals D-Allulose free journals D-Allulose best journals D-Allulose top journals D-Allulose free medical journals D-Allulose famous journals D-Allulose Google Scholar indexed journals Maltodextrin articles Maltodextrin Research articles Maltodextrin review articles Maltodextrin PubMed articles Maltodextrin PubMed Central articles Maltodextrin 2023 articles Maltodextrin 2024 articles Maltodextrin Scopus articles Maltodextrin impact factor journals Maltodextrin Scopus journals Maltodextrin PubMed journals Maltodextrin medical journals Maltodextrin free journals Maltodextrin best journals Maltodextrin top journals Maltodextrin free medical journals Maltodextrin famous journals Maltodextrin Google Scholar indexed journals Modified starch articles Modified starch Research articles Modified starch review articles Modified starch PubMed articles Modified starch PubMed Central articles Modified starch 2023 articles Modified starch 2024 articles Modified starch Scopus articles Modified starch impact factor journals Modified starch Scopus journals Modified starch PubMed journals Modified starch medical journals Modified starch free journals Modified starch best journals Modified starch top journals Modified starch free medical journals Modified starch famous journals Modified starch Google Scholar indexed journals
Article Details
1. Introduction
Starch is the most common carbohydrate in human diet and is present in many staple foods. The major sources of starch intake worldwide are cereals, such as rice, wheat, and corn, and root vegetables, such as potatoes, sweet potatoes, and cassava (Figure 1). Several starch-based products have been developed, as starch is inexpensive and easy to process chemically. In addition, modified starch-based products are used in many processed foods, for example, as starch sugars, such as glucose or isomerized sugars, which are the most widely produced starch-based products and are used mainly as sweeteners. In Japan, which has a population of 130 million, these sweeteners account for about 65% of Japan’s starch use, or 1.95 million tons of starch [1]. About 0.42 million tons of starch are used in the form of modified starch, mainly in diet. Although starch is an important source of energy for humans, it is now evident that obesity caused by excessive intake of sugars and other nutrients is a trigger for metabolic disorders worldwide [2]. This review describes the methods that are employed for production of starch sugars such as glucose, isomerized sugar, glucose syrup, maltodextrin, and modified starch as well as their current application status in Japan. Ingredients with low-calorie content and low-glycemic index can also be produced from starch and will be discussed in this review.

Figure 1: Rice, wheat, corn, potatoes, sweet potatoes and cassava.
2. Starch-related sugars
2.1 Glucose and high fructose syrup
Glucose is a component of starch (Figure 2). The sugar in starch is liquefied by heat-resistant α-amylase and decomposed by glucoamylase or a combination of glucoamylase and pullulanase. The resulting sugar solution is then decolorized with activated carbon, desalted with ion exchange resin, purified, and concentrated to produce liquid glucose (approximately 96% purity). This liquid glucose is then converted to anhydrous glucose via crystallization using the decoction method, crystallization, beeswaxing, and drying in crystal cans, and to refined glucose by spray drying [3]. Liquid glucose is used as a raw material for sorbitol and caramel production as well as a fermentation substrate.
Crystalline glucose has many pharmaceutical applications, such as a sweetener in confectionery. Its use in powdered seasonings is unique because of its powdery properties (fluidity). Glucose is predominantly used as isomerized sugar, which is produced via reaction of glucose produced through a series of processes such as starch liquefaction, saccharification, and purification with the immobilizing enzyme (glucose isomerase), which consequently leads to the formation of a glucose syrup with 42% fructose content. In addition, the purity of fructose is increased through chromatography using a strong acidic ion exchange resin to produce high fructose corn syrup with 55% fructose content and high fructose syrup with 95% fructose content [4]. Almost 1 million tons of solid isomerized sugar is produced, making it the second most consumed sweetener after sugar (sucrose). Isomerized sugar is most often used in beverages because it produces higher sweetness at a lower temperature than sugar, followed by bread, seasonings, and frozen desserts. However, the metabolism of fructose as a food component, which is produced and consumed in large quantities in the U.S., is different from that of glucose. Then, because of the difference, it is often cited as a cause of obesity, and is therefore considered a controversial topic [5, 6].
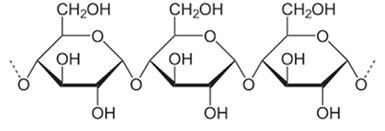
Figure 2: Molecular structure of starch.
2.2 Glucose syrup and maltodextrin
Hydrolysis with acids and enzymes leads to the decomposition of starch into glucose. However, depending upon the reaction method and conditions, intermediate products with different sugar compositions can also be obtained [7]. These products are classified based on their Dextrose Equivalent (DE) value, which is defined as the degree of hydrolysis of the products. Intermediate products with DE above 20 are classified as glucose syrup, and those with DE below 20 are classified as maltodextrins [8]. In Japan, products with DE of 10 and below are classified as dextrins. Glucose syrup has been used as a sweetener for a long time. Glucose syrup can be of two types depending on the manufacturing method (acid saccharification and enzyme saccharification). After it is hydrolyzed under pressure using oxalic acid or hydrochloric acid as a catalyst, the glucose syrup is neutralized, decolorized with activated carbon, and desalted using ion exchange resin The resulting sugar solution is concentrated into a liquid product (DE30-60). In enzymatic saccharification method, glucose syrup is first hydrolyzed using α-amylase, and then it is decomposed (DE40-55) using β-amylase to produce a sugar solution with approximately 50% maltose content. Then, using the same purification method as in the first method, the final liquid product is obtained [9].
Due to these differences in manufacturing methods, there are slight differences in sugar composition, sweetness, and viscosity [10], leading to subtle differences in taste in the resulting confectionery and chilled desserts. Enzymatic saccharified glucose syrup has recently been used in large quantities, especially as a raw material for production of foamed liquor. Corn syrup solids (DE20-35) are made by spray-drying a sugar solution with relatively low DE, which is obtained by acid or enzyme (α-amylase) treatment. Corn syrup solids are used in various powdered foods, meat products, and infant milk powder. Similar to the production of enzymatic saccharified glucose syrup, the combination of β-amylase and branching enzyme yields high maltose content and high maltose syrup. The maltose content was further enhanced by ion exchange chromatography to produce crystalline maltose [11]. Crystalline maltose could be used to replace sugar as a low-sweetener in Japanese and Western confectionaries, and could be used for infusions (pharmaceuticals) based on its properties as a disaccharide. Trehalose is a disaccharide similar to maltose (Figure 3), which is produced in large quantities industrially by the action of three enzymes, including isoamylase, maltooligosyl trehalose synthase, and maltooligosyl trehalose trehalohydrolase, on liquefied starch. Trehalose exhibits excellent hydration properties in food products and is used to inhibit the aging of starch in confectioneries and denaturation of proteins in livestock and marine products. It is classified as a food additive since it is used in the manufacturing of food products [12]. Other special glucose syrups such as maltotetraose and isomaltoligosaccharides are also produced and sold [13].
Maltodextrin (including dextrin) has lower DE than corn syrup solids and it is produced by the acid-enzyme two-step liquefaction method or the enzyme-enzyme two-step liquefaction method in order to reduce the aging of the sugar solution. Hydrochloric acid or oxalic acid is used as the acid and heat-resistant α-amylase is used as the enzyme. The purification is performed using the same way as for glucose syrup, and the final powder is made into a product by spray drying [14]. The low DE (8-15) commodities are mainly used as spray base materials for dried foods, such as powdered soy sauce and miso seasoning. They are also used as a milk substitute (fat substitute) because of their milk-like texture. Products with a relatively high DE (15-20) are used as a carbohydrate source in nutritional (medical) and fluidized diet concentrates due to their moderate osmotic pressure [14].
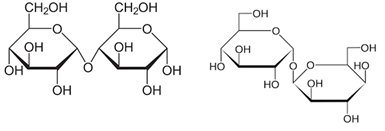
Figure 3: Molecular structure (Haworth projection) of maltose and trehalose.
3. Sugar alcohols
The hydrogenation (reduction) of various starch sugars in the presence of a catalyst yields sugar alcohols according to the raw material [15]. D-sorbitol is produced from glucose and D-mannitol is produced from fructose (Figure 4). Maltitol is also produced from maltose, which is a dimer of D-glucose. Reduced glucose syrup is also produced from glucose syrup and maltodextrin. The change in the reducing end makes the syrup less prone to browning and more moisturizing. High-purity maltitol is a non-carcinogenic, non-digestible sweetener with 90% sweetness and 2 kcal/g [16]. It is often used in combination with xylitol to make sugarless gum and candies.
4. Modified starch
Starch is an indispensable food material for processed foods because of its viscosity and hydrocolloidal properties that appear upon heating. As starch requires heating when used in food products and has unavoidable negative properties such as aging, modified starch is made by a variety of processes (enzymatic, physical, and chemical) to improve the original structure and physical properties of starch and to add functionalities (Figure 5). In Japan, 11 of these chemically modified starches were designated as food additives in October 2008 and ingredient standards were established [7]. Accordingly, chemically-treated modified starch is labeled on foods to distinguish it from enzyme-treated or physically-treated starch, which is considered a food ingredient.
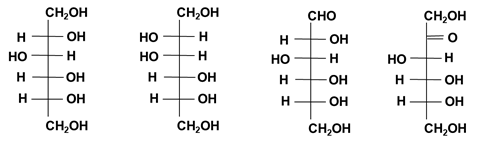
Figure 4: Molecular structure (Fischer projection) of D-sorbitol, D-mannitol, glucose and fructose.
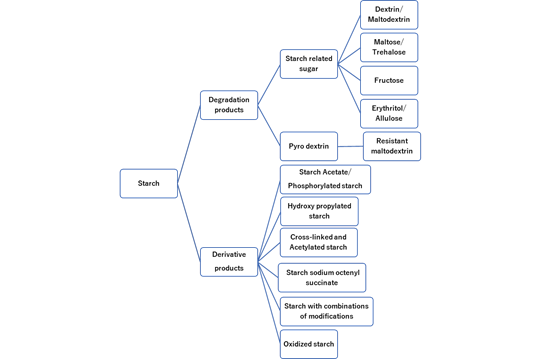
Figure 5: Varieties of starch products.
4.1 Modified starch as a food additive in Japan
For the production of the 11 modified starches, the glucose chain in starch is subjected to esterification, etherification, or oxidation, or a combination of these processes. Based on the similarity in chemical structure, the modified starches can be classified into 10 additive derivative starches and 1 soluble degradable starch. The chemical processing of starch can be divided into wet and dry methods depending on the conditions at the time of the reaction [17], but the reaction is usually carried out in a state where the starch is dispersed in water (wet method). After the addition of chemicals to the starch emulsion (suspension) consisting of water and starch, its mixture is rinsed with water to remove foreign substances and chemicals and is purified, followed by a drying process to yield the final product. Modified starch with various properties can be obtained by varying the concentration of chemicals and reaction conditions [18]. Processed starch can be combined with various raw starches and can be treated with any of the 11 different processing methods. By maintaining the degree of processing within the standard range, a wide variety of products can be produced. Therefore, in order to achieve the desired effect more efficiently, it is important to use a suitable addition technology. The formulations and basic performance of the 11 products and their applications in food industry are described below.
4.1.1 Starch acetate: Starch acetate (Figure 6) is obtained by esterification of starch with acetic anhydride (or one other type of reagent) [19]. The product has low pasting temperature, strong adhesion property, and excellent aging stability and transparency. It is widely used for improving the texture and physical properties of food products, viscosity stability of sauces, and texture of frozen noodles.
4.1.2 Phosphorylated starch: Phosphorylated starch is obtained by esterification of starch with sodium tripolyphosphate (or three other reagents). The reaction yields a highly viscous glue liquid with excellent hydrophilicity and freezing resistance [20]. Phosphorylated starch is mainly used to control the aging of frozen and refrigerated products and to prevent water release in sauces and fillings. However, there is little use of this processed starch in the market.
4.1.3 Hydroxy-propylated starch: It is obtained by the chemical reaction of starch with propylene oxide (Figure 7) [21]. It has low pasting temperature, with excellent aging resistance, and freezing and thawing resistance. It is widely used in foodstuffs, mainly to improve texture, physical properties, and aging resistance as well as to add stability to frozen and bakery foods.
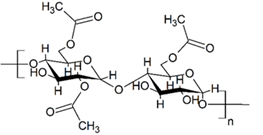
Figure 6: Molecular structure of starch acetate.
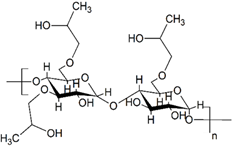
Figure 7: Molecular structure of hydroxy-propylated starch.
4.1.4 Phosphorylated cross-linked starch: Phosphorylated cross-linked starch is obtained by esterification of the starch with trimethylated sodium (or one other reagent) [22]. The pasting property of this starch is suppressed, but it has excellent shear and acid resistance. Depending on the degree of cross-linking, a variety of viscous liquids can be obtained using this starch. It is widely used in food products, mainly for improving physical properties, but also for improving the texture of snack foods, tempura flours, and bakery products.
4.1.5 Phosphate cross-linked and acetylated starch: It is obtained by esterification of the starch with sodium trimethylated (or any other agent) and acetic anhydride (or any other reagent) [23]. It has properties of both starch acetate and phosphate cross-linked starch. It has low pasting temperature, and excellent shear resistance and acid resistance, and it is resistant to swelling during heating. It is used to improve the cooking resistance of retort foods (sauce portions) and to improve the mechanical resistance of sauces and fillings, mainly to improve viscosity stability.
4.1.6 Acetylated adipic acid cross-linked starch: It is obtained by esterification of starch with acetic anhydride and adipic anhydride [24]. Its properties and food applications are similar to those of acetylated phosphate cross-linked starch described above. Acetylated adipic acid cross-linked starch is stable at low pH and hence can be used in dressings.
4.1.7 Phosphated distarch phosphate: It is obtained by esterification of the starch with sodium tripolyphosphate (or three other reagents) and sodium trimetaphosphate (or any other reagent) [25]. It can be produced as a highly viscous glue liquid, which is hydrophilic in nature, and it has has excellent freezing resistance. However, there is little use of this processed starch in the market. It is also used to improve the appearance of sauces and fillings, to prevent water release in frozen foods, and to prevent aging in chilled foods.
4.1.8 Hydroxypropyl distarch phosphate: It is obtained by esterification of the starch with sodium trimetaphosphate (or any other reagent) and etherification with propylene oxide [26]. It has properties of both hydroxypropyl starch and phosphate cross-linked starch, a low pasting temperature as well as excellent freezing resistance, heating stability, shear resistance, and acid resistance. It is mainly used to prevent water leakage in frozen foods, and aging in chilled foods, and to improve the stability of sauces and fillings.
4.1.9 Starch sodium octenyl succinate: It is obtained by esterification of starch with anhydrous octenyl succinic acid [27]. Because of the introduction of hydrophobic groups, it has an emulsification capacity (surface activity). In addition, the pasting temperature of this starch is slightly lower than the non-modified raw starch, and the storage stability of the starch is improved. It is used to improve the stability of dressings and as a base material for emulsified flavors, mainly to suppress the release of fats and oils and to add emulsification stability to dressings.
4.1.10 Oxidized starch: It is obtained by oxidizing starch with sodium hypochlorite [28]. The properties of this starch include low viscosity, excellent viscosity stability, low aging resistance, low pasting start temperature, and excellent transparency. It is widely used in food products, mainly to improve the texture and physical properties as well as to improve the taste of snacks. It is also used as a powdered base material and as a glazing agent for rice crackers.
4.1.11 Acetylated starch oxidized: It is obtained by oxidizing starch with sodium hypochlorite and then esterifying it with acetic anhydride [29]. It has the properties of both acetic acid starch and oxidized starch, including low pasting temperature and excellent stability and transparency. The viscosity of the glue liquid is suppressed, and it has excellent resistance to aging. Acetylated starch oxidized is mainly used to improve physical properties, inhibit aging, and stabilize salts, and to make body agents for gummy candies.
5. Starch-derived anti-obesity materials
5.1 Erythritol
Zero-calorie carbohydrates are also produced from starch. Erythritol (Figure 8) is a sugar alcohol produced from glucose by the fermentation process of Trichosporonoides megachiliensis, a type of basidiomycete yeast [15]. It is endothermic (tastes cold) in the mouth and is 70-80% sweeter than sugar and less likely to cause tooth decay. Although erythritol does not has a reducing end, its energy value is the most important. About 90% of ingested erythritol is excreted in the urine and about 10% reaches the colon. Fermentation of the entire amount in the colon results in the production of 0.2 kcal/g energy, which is 10% of the 2 kcal/g energy produced by fermentation with intestinal bacteria. Erythritol is treated as a zero-kilocalorie sweetener in many countries' nutrition labeling systems [30].
5.2 D-allulose
D-allulose, a monosaccharide with various physiological functions [31], has recently been developed. It is a ketose produced by the action of D-tagatose-3-epimerase on fructose [32]. D-allulose (Figure 8) has 70% sweetness for sucrose, and has non-carcinogenic and anti-carcinogenic properties in the oral cavity. The energy value of this sugar has been assessed in the upper and lower gastrointestinal tracts [33]. The upper gastrointestinal tract was evaluated by measuring the urinary excretion rate of absorbed D-allulose and carbohydrate energy expenditure (CEE) using indirect calorimetry. The lower gastrointestinal tract was evaluated by measuring the energy production during the fermentation process using human enterobacteria and by measuring the concentration of exhaled hydrogen gas in humans after ingestion of D-allulose. The results revealed that there was no increase in CEE for up to 3 h after administration of D-allulose in humans (20 g ingestion), suggesting that the administered (absorbed) D-allulose does not turn into energy like glucose. Conversely, the excretion rate of D-allulose in urine was evaluated for 48 h as excretion rate (1) 0-12 h, (2) 12-24 h, and (3) 24-48 h after ingestion. More than 50% of D-allulose was excreted by 12 hours after ingestion and more than 60% was excreted in 24 hours, and the cumulative excretion rate for 48 h of evaluation was 66 ± 13% for 20 g of D-allulose, 78 ± 11% for 10 g of D-allulose, and 79 ± 12% for 5 g of D-allulose. These results showed that about 70% of the intake was absorbed from the upper gastrointestinal tract and excreted in the urine without being metabolized. Conversely, in an intestinal bacterial assimilation test of D-allulose in the lower gastrointestinal tract, 31 out of 35 strains were not assimilated, and the remaining 4 strains were weakly assimilated. In an exhaled hydrogen gas test after ingestion of D-allulose, fructo-oligosaccharide (2 kcal/g) (FOS: 20 g, 10 g, 5 g), which is considered to be completely fermented, was used. As a result, the fermentation ratio of 1 for FOS was 0.16 for 20 g, 0.04 for 10 g, and 0.05 for 5 g of D-allulose, and almost no fermentation was observed in D-allulose. From these results, it was estimated that D-allulose adds zero kilocalories in food. This means that D-allulose, like erythritol, is a zero-calorie ingredient. In addition, D-allulose has excellent physiological functions and has been confirmed to have inhibitory and antioxidant effects on blood glucose in animals and humans [34]. In addition, the anti-obesity effects of D-allulose have been confirmed in many studies, and the mechanism of their
expression has been studied well [35].
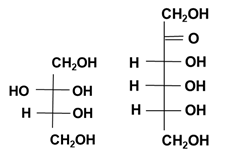
Figure 8: Molecular structure of erythritol and D-allulose.
5.3 Syrup containing D-allulose
A syrup containing D-allulose, named Rare Sugar Syrup (RSS), was produced by further isomerization of isomerized sugars using the alkaline method [36]. RSS was developed as an ideal balanced sweetener with fewer calories and better taste [37]. The development of the aforementioned method for the production of single D-allulose was primarily based on the use of an enzyme followed by chromatographic separation. In contrast, rare sugar-containing syrups are prepared by making the sugar alkaline without using enzymes; this method was originally developed to make use of mixed sugars. The anti-obesity effect of RSS in humans has been confirmed [38].
A double-blind, 12-week intake study comparing two groups using a control diet was conducted in 34 healthy adults. The test diet consisted of a jelly beverage containing 40 g of RSS, and the control diet consisted of a jelly containing isomerized sugar. The diets were consumed once a day before breakfast to achieve the same calorie content. The results showed a significant decrease in body weight and body fat percentage in the group consuming the test diet. This study showed that rare sugar-containing syrup has an anti-obesity effect in humans. Another clinical trial confirmed that the rare sugar-containing syrup is a low GI sweetener [39]. This rare sugar-containing syrup is used in Japan and other Asian countries.
5.4 Resistant maltodextrin
Resistant maltodextrin is produced by fractionating and purifying the amylase-resistant components (transitions and reverse synthesis) that are produced during roasting into the indigestible fraction from acid pyrodextrin, prepared by heating [40]. Its component is glucan with an average molecular weight of 2000 Da, which is similar to that of glucose syrup and maltodextrin, but has a more branched structure. The majority of resistant maltodextrin is classified as dietary fiber because it is not digested and absorbed in the upper gastrointestinal tract up to the small intestine, and reaches the large intestine as is, with an energy value as low as 1 kcal/g, as measured by indirect calorimetry [41]. Resistant maltodextrin slows the absorption of carbohydrates in the upper gastrointestinal tract and suppresses the absorption of lipids, thereby slowing the rise of blood glucose after meals and the rise of triglycerides in the serum. Animal and human studies also confirmed the long-term anti-obesity effects of these products [42]. It is also known to have intestinal regulating effects and accelerated absorption of minerals [43], and is used in many health-oriented foods, including Food for Specified Health Uses.
5.5 Highly cross-linked starch and highly etherized starch
Some modified starches are classified as indigestible starches (resistant starch), which are starches that cannot be degraded by human digestive enzymes and are classified as RS1 (starch covered with a strong outer skin), RS2 (enzyme-resistant starch), RS3 (aging starch), and RS4 (modified starch) and is considered a dietary fiber [44], as shown in Table 1. At present, there are two types of indigestible starch used in processed foods, RS2 and RS4. RS2 is derived from honey corn starch, which is difficult to process due to its physical properties and has a poor texture, limiting its use. Phosphate-cross-linked starch, which is a type of modified starch, gradually becomes indigestible because its swelling is inhibited by heating as the cross-linking is strengthened and human digestive enzymes become ineffective. The dietary fiber content (according to the Prosky method) of cross-linked phosphate starch with a phosphorus content of 0.4% or more is 80% or more in anhydrous form. When humans ingest this highly cross-linked phosphate starch, no increase in blood glucose level is observed, and only a very small amount of hydrogen gas is excreted during exhalation, and the energy conversion factor has been estimated to be less than 1 kcal/g. It is also known that highly etherized starch is a low GI starch that is digested more slowly in the human gastrointestinal tract and has a slower increase in blood glucose than unmodified starch. The energy value of the indigestible fraction of highly etherized starch was also estimated to be 0 kcal/g. This is because the indigestible fraction, which escapes digestion and absorption in the small intestine, is almost never fermented and decomposed and is excreted in the feces. These two modified starches also have anti-obesity effects, which are difficult to use for human energy.
|
Resistant Starch |
Types |
Food examples |
|
RS1 |
Cereals and legumes that are poorly crushed and are wrapped in the cell wall are physically incapable of being acted upon by digestive enzymes |
Whole grains, less refined grains, and foods with high starch density (e.g., pasta) |
|
RS2 |
Raw starch with average amylose content, but has a B-type crystalline structure and has not been cooked or glued. It can be digested by cooking and gluing. |
Unripe banana (green banana) and fresh potato |
|
Starch with high amylose content (high amylose starch) |
High amyl-roasted maize starch, high amylose rice starch, and food to which they have been added |
|
|
RS3 |
Starch formed when starch, once glued, is left to cool |
Starch aging |
|
RS4 |
Enzymatically, physically, and chemically modified starch. |
Processed foods (snacks, bread, frozen foods, noodles, sauce, dessert, etc.) |
Table 1: Types of resistant starch and examples of foods that contain resistant starch.
6. Conclusions
In this review, the current status of processing and utilization of starch was described. For many years, modified starch has been supporting a rich diet. Asia, especially Japan, has always led the research and development of starch processing, including the use of glucoamylase in glucose production, endorsement of isomerized sugar, and use of glucose isomerase in fructose production [45]. In addition, erythritol, D-allulose, trehalose, and resistant maltodextrin, which were the first starch-derived ingredients to be commercialized, are now consumed worldwide. New carbohydrates based on starch will continue to be researched and developed in the future. More value-added materials will certainly be developed in the future by combining the excellent production technologies described in this review, especially physiological evaluation in humans.
Acknowledgments
The author would like to express their deep thanks to the late Dr. Kazuhiro Okuma (Matsutani Chemical Industry Co., Ltd., Hyogo, Japan). Dr. Kazuhiro Okuma was an authority on starch processing and utilization. This review was based on his Japanese article [45]. Also, the author would like to thank the The Japanese Society of Applied Glycoscience.
Funding
The author declares that no funding was received for this study.
Conflicts of Interest
The author declares no conflicts of interest. Tomoya Shintani is an employee of Matsutani Chemical Industry Co., Ltd. (Hyogo, Japan). However, the company provided no financial support for this review. This work was almost completed while the author was at Ehime University.
References
- Agriculture and Livestock Industries Corporation. Alic (2019).
- Johnson JR, Segal SM, Sautin Y, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86 (2007): 899-906.
- Newkirk WB. Development and Production of Anhydrous Dextrose. Ind Eng Chem 28 (1936): 760-766.
- Johnson R, Padmaja G, Moorthy SN. Comparative production of glucose and high fructose syrup from cassava and sweet potato roots by direct conversion techniques. Innovative Food Science & Emerging Technologies 10 (2009): 616-620.
- Gross SL, Li L, Ford SE, et al. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr 79 (2004): 774-
- Bocarsly EM, Powell SE, Avena MN, et al. High-fructose Corn Syrup Causes Characteristics of Obesity in Rats: Increased Body Weight, Body Fat and Triglyceride Levels . Pharmacol Biochem Behav 97 (2010): 101-106.
- Agriculture and Livestock Industries Corporation. Alic (2017).
- Food and Drug Administration. Code of federal regulation 21.184.1444. FDA (2019).
- Norman BE. A novel debranching enzyme for application in the glucose syrup industry. Starch 34 (1982): 340-346.
- Sun J, Zhao R, Zeng J, et al. Characterization of Destrins with Different Dextrose Equivalents. Molecules 15 (2010): 5162-5173.
- Yoshino Z. Production of powdery maltose (1983) US4595418A.
- Ohtake S, Wang YJ. Trehalose: Current Use and Future Applications. Journal of Pharmaceutical Sciences 100 (2011): 2020-2053.
- Kimura T, Nakakuki T. Maltotetraose, a new saccharide of tertiary property. Starch 42 (1990): 151-157.
- Hofman LD, Buul JVJ, Brouns JPHF. Nutrition, Health, and Regulatory Aspects of Digestible Maltodextrins. Crit Rev Food Sci Nutr 56 (2016): 2091-2100.
- Shintani T. Food Industrial Production of Monosaccharides Using Microbial, Enzymatic, and Chemical Methods Fermentation 5 (2019): 47.
- Grembecka M. Sugar alcohols-their role in the modern world of sweeteners: a review. European Food Research and Technology 241 (2015): 1-14.
- Rutenberg WM, Solarek D. Starch derivatives: Production and uses Starch. Chemistry and Technology (Second Edition) Food Science and Technology (1984): 311-388.
- Ackar D, Babic J, Jozinovic A, et al. Starch Modification by Organic Acids and Their Derivatives: A Review. Molecules 20 (2015): 19554-19570.
- Khalil MI, Hashem A, Hebeish A. Preparation and characterization of starch acetate. Starch 47 (1995): 394-398.
- Sitohy ZM, Ramadan FM. Degradability of different phosphorylated starches and thermoplastic films prepared from corn starch phosphomonoesters. Starch 53 (2001): 317-322.
- Kaur L, Singh N, Singh J. Factors influencing the properties of hydroxypropylated potato starches. Carbohydrate Polymers 55 (2004): 211-223.
- Manoia K, Rizvia SHS. Physicochemical characteristics of phosphorylated cross-linked starch produced by reactive supercritical fluid extrusion. Carbohydrate Polymers 81 (2010): 687-694.
- Mirmoghtadaie L, Kadivar M, Shahedi M. Effects of cross-linking and acetylation on oat starch properties. Food Chemistry 116 (2009): 709-713.
- Raina CS, Singh S, Bawa SA, et al. Some characteristics of acetylated, cross-linked and dual modified Indian rice starches. European Food Research and Technology 223 (2006): 561-570.
- Lim S, Seib PA. Preparation and pasting properties of wheat and corn starch phosphates. Cereal chemistry 70 (1993): 137-144.
- Wootton M, Bamunuarachchi A. Water binding capacity of commercial produced native and modified starches. Starch 30 (1978): 306-309.
- Wang X, Li X, Chen L, et al. Preparation and characterisation of octenyl succinate starch as a delivery carrier for bioactive food components, Food Chemistry 126 (2011): 1218-1225.
- Wang Y, Wang L. Physicochemical properties of common and waxy corn starches oxidized by different levels of sodium hypochlorite. Carbohydrate Polymers 52 (2003): 207-217.
- Halal LMES, Colussi R, Pinto ZV, et al. Structure, morphology and functionality of acetylated and oxidised barley starches. Food Chemistry 168 (2015): 247-256.
- Goossens J, Gonze M. Nutritional properties and applications of erythritol: a unique combination? Advances in Sweeteners (1996): 150-186.
- Izumori K. Bioproduction strategies for rare hexose sugars. Naturwissenschaften 89 (2002): 120-124.
- Izumori K. Izumoring: A strategy for bioproduction of all hexoses. J. Biotechnol 124 (2006): 717-722.
- Iida T, Hayashi N, Yamada T, et al. Failure of D-Psicose Absorbed in the Small Intestine to Metabolize Into Energy and Its Low Large Intestinal Fermentability in Humans. Metabolism 59 (2010): 206-214.
- Izumori K. Production and Application of Functional Monosaccharides Including Rare Sugar. Oleoscience. 13 (2013): 421-422.
- Hossain A, Yamaguchi F, Matsuo T, et al. Rare Sugar D-allulose: Potential Role and Therapeutic Monitoring in Maintaining Obesity and Type 2 Diabetes Mellitus, Pharmacology and Therapeutics 155 (2015): 49-59.
- Takamine S, Nakamura M, Iida T, et al. Manufacturing method of rare sugar syrup through alkali isomerization and its inhibitory effect of α-glucosidase. Bull. Appl. Glycosci 5 (2015): 44-49.
- Shintani T, Yamada T, Hayashi N, et al. Rare sugar syrup containing d-allulose but not high-fructose corn syrup maintains glucose tolerance and insulin sensitivity partly via hepatic glucokinase translocation in Wistar rats. J. Agric. Food Chem 65 (2017): 2888-2894.
- Hayashi N, Yamada T, Takamine S, et al. Weight reducing effect and safety evaluation of rare sugar syrup by a randomized double-blind, parallel-group study in human. Journal of Functional Foods 11 (2014): 152-159.
- Yamada T, Shintani S, Iida T, et al. Effect of ingestion of rare sugar syrup on the blood glucose response in humans. J. Jpn. Soc. Nutr. Food Sci 70 (2017): 271-278.
- Okuma K, Matsuda I. Production of Indigestible Dextrin from Pyrodextrin. J. Appl. Glycosi 50 (2003): 389-394.
- Goda T, Kajiya Y, Suruga K, et al. Availability, fermentability, and energy value of resistant maltodextrin: modeling of short-term indirect calorimetric measurements in healthy adults. Am J Clin Nutr 83 (2006): 1321-1330.
- Okuma K, Matsuda I, Katsuta Y, et al. Development of Indigestible Dextrin. J. Appl. Glycosi 53 (2006): 65-69.
- Miyazato S, Nakagawa C, Kishimoto Y, et al. Promotive effects of resistant maltodextrin on apparent absorption of calcium, magnesium, iron and zinc in rats. Eur J Nutr 49 (2010): 165-171.
- Fred B, Bernd K, Eva A. Resistant starch and "the butyrate revolution. Trends in Food Science & Technology 13 (2002): 251-261.
- Okuma K. Denpun-no-kakou-to-shokuhin-riyou (available in Japanese). Bull. Appl. Glycosci 1 (2011): 34-38.
