Predictors of Neurodevelopmental Outcome in Hyperbilirubinemic Neonates Admitted in NICU
Article Information
A. Mannan1*, Md. Arif Hossain2, Shimul Mandal3, Sadeka Choudhury Moni4, Ismat Jahan5, Mohammad Kamrul Hassan Shabuj4, Mohammod Shahidullah1, Shaheen Akhter6
1Professor, Department of Neonatology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
2Neonatologist, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
3Resident, Department of Neonatology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
4Associate Professor, Department of Neonatology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
5Assistant Professor, Department of Neonatology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
6Professor, Department of Paediatric Neurology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
*Corresponding Author: M. A. Mannan, Professor, Department of Neonatology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
Received: 01 March 2022; Accepted: 09 March 2022; Published: 01 April 2022
Citation:
M. A. Mannan, Md. Arif Hossain, Shimul Mandal, Sadeka Choudhury Moni, Ismat Jahan, Mohammad Kamrul Hassan Shabuj, Mohammod Shahidullah, Shaheen Akhter. Predictors of Neurodevelopmental Outcome in Hyperbilirubinemic Neonates Admitted in NICU. Journal of Pediatrics, Perinatology and Child Health 6 (2022): 200-218.
Share at FacebookAbstract
Background: Neonatal hyperbilirubinemia is an important cause of preventable brain damage among infants. Neurodevelopmental assessment may help in the early identification and management of neurodevelopmental sequelae.
Objectives: The aim of this study were to identify the predictors of abnormal neurodevelopment at 3 & 12 months in babies having birth weight ≥1800 g and gestational age >34 weeks with neonatal hyperbilirubinemia.
Methods: This prospective observational study was conducted at Department of Neonatology and Institute of Pediatric Neurodisorder and Autism (IPNA), Bangabandhu Sheikh Mujib Medical University (BSMMU), Shahbag, Dhaka, Bangladesh from July 2019 to June 2021. Hyperbilirubinemic newborns were followed up and their neurodevelopmental assessment was done by using BSID III method at 3 and 12 months of age. All the collected data was tabulated and statically analyzed by using SPSS software.
Results: A total of 90 newborns were enrolled, and among them 72 completed the first follow up and 67 completed second follow up. Average gestational age was 37.28±1.4 and mean birth weight was 2870.97 ± 458 g. There was slight female predominance 42 (58.3%) and 69 (95.8%) babies were inborn and only 3 (4.2%) were outborn. Out of 72 neonates, 9 (12.5%) had abnormal neurodevelopment results at 3 months, whereas 2 (3%) had neurodevelopmental abnormalities at 12 months. Neurodevelopmental follow up is suggesting reversibility of adverse neurodevelopment outcome. Perinatal and clinical data were compared between age appropriate neurodevelopment group and delayed neurodevelopment group. This study found that hemolytic jaundice, need for exchange transfusion, jaundice within first 24 hours, peak serum bilirubin > 20 mg/dl and longer duration of phototherapy > 48 hours were not significantly associated with abnormal neurodevelopment.
Keywords
Neonatal hyperbilirubinemia, Neurode-velopmental Outcome, Neonatal hyper-bilirubinemia
Neonatal hyperbilirubinemia articles; Neurode-velopmental Outcome articles; Neonatal hyper-bilirubinemia articles
Article Details
1. Introduction
Neonatal jaundice is the most common clinical problem among newborns, especially in the south East Asia [1]. Approximately 60-70% of term and 80% of preterm infants develop jaundice in the first week of life, yet only 5-10% of all newborns require intervention for pathological jaundice [2]. The overall incidence of neonatal jaundice in Bangladesh is about 33% and reported by various Indian workers varies from 4.6% to 77% [3]. The pathophysiological mechanism that predisposes a newborn infant to hyperbilirubinemia are- 1) Increased bilirubin synth-esis from larger RBC mass and shorter lifespan of neonatal RBC, 2) Decreased hepatic uptake, 3) Impaired conjugation and excretion due to reduced UDPGT activity and 4) Increased absorption from enhanced enterohepatic circulation [4]. Neonatal jaundice is a cause of concern for the physicians and a source of anxiety for the parents due to its complications. However, if significant jaundice is untreated and timely intervention is not initiated, bilirubin may cross the blood-brain barrier and cause reversible damage (called early acute bilirubin encephalopathy) or a devastating, permanently disa-bling damage (called kernicterus) [5]. "Kernicterus" refers to the neurological consequences of the deposition of unconjugated bilirubin in brain tissue by damaging and scarring of the basal ganglia and brain stem nuclei [6]. Clinicians usually suffer from “Vigintiphobia” i.e. a bilirubin level of more than 20mg/dl where there may be a high probability of development of ‘’Kernicterus’’ [7]. By pathological criteria, kernicterus develops in 30% of infants with bilirubin levels > 25 – 30 mg/dl. Recent data suggest that much lower levels can also be associated with harm, such as neuro-developmental impairment, cerebral palsy, and hearing deficits, in the most immature neonates [8]. Worldwide phototherapy has emerged as the most widely used and relatively safe therapeutic method for the treatment of unconjugated hyperbilirubinemia. It is estimated that in the United States alone more than 350,000 newborns undergo this treatment every year [9]. More severe neonatal jaundice and jaundice due to hemolytic disease of newborns are usually managed by exchange trans-fusion. Today, most patients with high bilirubin levels do not experience neurological damage due to the modern treatment methods and because patients can benefit more from medical services. However, there are few patients who still develop mild neurological damage, and detecting these patients is critical. Essentially, the population in which we can make a difference is among patients with little damage. Therefore, in addition to the hearing assessment, which is usually what we pay the most attention to during follow-up, neurodevelopmental screening tests should be carried out diligently. Serum bilirubin > 17.64 mg/dl was associated with increased risk of complex minor neurological dysfunction [10]. Another study showed that need for exchange transfusion, Rh incom-patibility and onset of jaundice within 2 days of birth are independent predictors of abnormal neurodeve-lopment at 6 months [11]. Hyperbilirubinemia causes adverse neurodevelopmental outcome 10.3% at 3 months and 6.25% at 12 months [12]. So from previous study it is said that many of these neurological disability caused by hyperbilirubinemia can be reversible by early intervention. However there was little data in our country upon predictors of neurological outcome of hyperbilirubonemic neona-tes. In this study, we made a neurodevelopmental evaluation of newborns with different bilirubin levels, etiologies and modalities of treatment. We used the Bayley Scales of Infant and Toddler Development (VersionIII), which included cognition, fine motor, language and gross motor assessments. The aim of this study is to see the predictos of neurodevelopmental outcome in hyper-bilirubinemic neonates.
1.1 Objectives
1.1.1 General: To see the predictors of neuro-developmental outcome in hyperbilirubinemic term and late preterm infants.
1.1.2 Specific:
To assess the neurodevelopmental outcome of hyperbilirubinemic neonates having following characteristics:
Rh isoimmunization
ABO incompatibility
Those required exchange transfusion Additional:
To see other factors for abnormal neuro-developmental outcome of hyperbiliru-binemic neonates like: Duration of phototherapy:
Peak serum bilirubin (mg/dl)
Age of onset of jaundice
2. Materials and Methods
2.1 Study design
Prospective observational study.
2.2 Place of study
Department of Neonatology and Institute of Pediatric Neurodisorder and Autism (IPNA), Bangabandhu Sheikh Mujib Medical University (BSMMU), Shahbag, Dhaka, Bangladesh.
2.3 Study period
July 2019 to June 2021 (two years): Enrollment period: July 2019 to July 2020- Follow up period: July 2020 to June 2021.
2.4 Study population
Admitted neonates with significant unconjugated hyperbilirubinemia in the NICU, BSMMU during the study period.
2.5 Inclusion criteria
- Gestational age 35-42 weeks.
- Birth weight between1800-4000 grams.
- Neonates with significant hyperbilirubine-mia (According to the American Academy of Pediatrics, Subcommittee on Hyperbiliru-binemia. Management of Hyperbilirubinem-ia in the newborn infant 35 or more weeks of gestation) requiring phototherapy or exchange transfusion within 2 first weeks of life.
2.6 Exclusion criteria
- Preterm neonates with gestational age < 35 weeks.
- Neonates with major congenital anomalies (e.g. -omphalocele, gastroschisis, trachea-esophageal fistula, congenital diaphragmatic hernia, anorectal malformation, gross hydronephrosis, neural tube defect etc.).
- Sick neonates: Like- sepsis, seizure, perinatal asphyxia & hypothyroidism.
- Small for gestational age.
- Conjugated hyperbilirubinemia (Conjugated bilirubin >2 mg/dl or more than 20% TSB).
2.7 Variables
2.7.1 Main outcome variable: Adverse develop-mental outcome defined as significant delay in one or more developmental domains: cognition, language (receptive & expressive language) and motor functions (fine & gross motor function).
2.7.2 Predictor variables:
Antenatal factors:
- Gestational age
- Mode of delivery (vaginal/ Caesarian/ instrumental)
- Place of delivery
- Parity (Primi or multipara)
Neonatal factors:
- Birth weight (gram)
- Sex (male/ female)
- Presence or absence of blood group incompatibility (ABO/ Rh)
- Treatment modalities (photoyherapy/ excha-nge transfusion)
- Age of onset of jaundice
- Age on admission
- Peak serum bilirubin
- TSB level on admission
- Presence or absence of rebound hyper-bilirubinemia
2.8 Operational Definition
2.8.1 Gestational age: The duration of gestation was measured from the first day of the last menstrual period. Gestational age was expressed in completed days or completed weeks [13].
2.8.2 Term neonate: From 37 completed weeks to less than 42 completed weeks (259 to 293 days) of gestation [13].
2.8.3 Late preterm: Late preterm infants are defined as born at 34-0/7 to 36-6/7 weeks’ gestational age.
2.8.4 Preterm neonate: Less than 37 completed weeks (less than 259 days) of gestation [13].
2.8.5 Birth weight: The first weight of the fetus or newborn obtained after birth. For live births, birth weight should preferably be measured within the first hour of life before significant postnatal weight loss has occurred [13].
2.8.6 Fetal growth at birth can be assessed by using chart [14, 15]: Appropriate for gestational age (AGA) was defined as a birth weight between 10th and 90th percentile for infant’s gestational age. Small for gestational age (SGA) was defined as a birth weight below 10th percentile for infant’s gestational age. Large for gestational age was defined as a birth weight above 90th percentile for infant’s gestational age.
2.8.7 Apgar score: A numerical expression of the condition of a newborn on a scale of 010. The scores are recorded at 1 and 5 minutes after delivery and become a permanent part of the health record. A score of 7-10 is normal [16].
Perinatal asphyxia: Perinatal asphyxia was defined as failure to initiate and sustain breathing at birth (WHO). As per American Academy of Pediatrics and American College of Obstetrics and Gynecology perinatal asphyxia was defined when following criteria were present: a) profound metabolic or mixed academia, pH<7 in cord; b) persistence of Apgar score 0-3 for longer than 5 minutes; c) neonatal neurologic sequelae and d) multi-organ involvement.
Favorable outcome: Favorable outcome was defined as normal neurologic development by physical examination and developmental assessment by Bayley Scales of Infant and Toddler Development Third edition (BSID-III).
Adverse outcomes: Adverse outcomes was defined as significant delay in one or more developmental domains: cognition, language (receptive & expressive language) and motor functions (fine & gross motor function).
2.8.8 The bayley scales of infant and toddler development, third edition: Scale used to assess 5 domains in neurodevelopmental scale, such as: cognitive development, expressive and receptive language, and fine and gross motor development. Developmental delay is classified as “delayed” if a Bayley III score below 70 on any of the subscales.
2.8.9 Major congenital anomaly: Congenital abnormality that requires medical or surgical treatment, has a serious adverse effect on health and development, or has significant cosmetic impact [17].
2.8.10 Significant hyperbilirubinemia: Hyperbili-rubinemia requiring admissions in the neonatal ward for intervention either phototherapy or exchange transfusion (According to the American Academy of Pediatrics, Subcommittee on Hyperbili-rubinemia. Management of Hyperbilirubinemia in the newborn infant 35 or more weeks of gestation, 2004).
2.8.11 Conjugated Hyperbilirubinaemia: Conju-gated Hyperbilirubinaemia is defined as a measure of direct reacting bilirubin of >1mg/dl if total serum bilirubin is ≤ 5 mg/dl, or serum conjugated bilirubin level > 2 mg/dl or > 20% of total serum bilirubin. (AAP 2004).
2.8.12 Rebound hyperbilirubinemia: Rebound hyperbilirubinemia is defined as postphototherapy rise serum bilirubin level needing the reinstitution of phototherapy [18].
2.8.13 ABO incompatibility: If the following criteria are present:
- Mother’s blood group O positive.
- Baby’s blood group A or B positive.
- Presence of significant jaundice and
- Onset of jaundice within 24 hours [18].
2.8.14 Rh incompatibility: If the following criteria are present:
- Mother’s blood group Rh-negative.
- Baby’s blood group Rh-positive.
- Presence of significant jaundice and
- Onset of jaundice within 24 hours [18].
2.9 Ethical consideration
There was minimum physical, psychological, social and legal risk during taking history, physical examination and investigations. Proper safety measures were taken in every step of the study. Only researcher was allowed to access the collected data. Ethical clearance was obtained from Institutional Review Board (IRB) of BSMMU to undertake the study. According to Helsinki Declaration for Medical Research involving Human Subjects 1964, all the parents were informed about the study design, the right of the participants to withdraw themselves from the research at any time, for any reason. Informed written consent were obtained from parents who voluntarily provided consent to participate in this study. The following ethical issues were addressed accordingly:
- Strict confidentiality and security of data related to patient were maintained. The presentation of data and information related to patient was documented anonymously.
- The data analysis was completed on the subjects who completed the study according to protocol after recruitment of subjects with valid informed consent.
- There was no additional risk or safety concern due to the research process to either patient or researcher.
- There was no potential conflict of interest in this study and entirely an academic research project.
2.10 Study procedure
This prospective observational study was conducted in the Department of Neonatology and Institute of Pediatric Neurodisorder and Autism (IPNA), Bangabandhu Sheikh Mujib Medical University (BSMMU), Shahbag, Dhaka, Bangladesh from July 2019 to June 2021, over a period of 2 years after approval by the Institutional Review Board. All inborn and outborn neonates admitted due to significant unconjugated hyperbilirubinemia within first 2 weeks of life with gestational age 35-42 weeks and birth weight between 1800- 4000gm were included in this study. Babies with gestational age <35 wks, post term, small for date, babies with intra – uterine growth restriction, those with hypoglycemia, conjugated hyperbilirubinemia, perinatal asphyxia, respiratory distress, sepsis, seizure, hypothyroidism and major congenital anomalies affecting the neurodevelopmental outcome were excluded from the study. Written informed consent was taken from parents and assurance about confidentiality was given. During hospitalization, details of maternal and perinatal medical history and physical examination were recorded for each neonate. Gestational age (completed 35–42 weeks) was assessed using the last menstrual period and available ultrasonography scans. Gestational age was confirmed by the New Ballard scoring in neonates of age <7 days. Anthropometric measurements were taken of all enrolled neonates. The newborn infants weight was taken without clothing soon after birth on an electronic scale with a precision of 10 gm [Model 914, SALTER].
Blood sample collection was done by venepuncture under all aseptic precautions at the time of admission. Serum was separated by centrifugation at ×3000 RPM for 5 min and then, serum bilirubin estimation was done by colorimetric assay using a fully automated analyzer (SIEMENS Healthineers Atellica CH Analyzer, Germany). Other investigations including blood group, direct Coombs test, thyroid profile, liver function test, complete blood count and C-reactive protein were also done. Newborns with hyperbilirubinemia were managed as per the guide-lines for phototherapy and exchange transfusion published by the American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Six hours from the start of phototherapy, 1 ml of blood was sent for estimating total serum bilirubin (TSB), and after that every 24 hourly and peak serum bilirubin level was considered. Phototherapy was discontinued when the TSB level was below 3 mg/dl lower than the phototherapy threshold for that postnatal age in the phototherapy graph. All neonates were followed up clinically till discharge and 24 hours of stoppage of phototherapy. TSB concentration was checked to see rebound hyperbilirubinemia. Data about birth history, gestational age, antenatal history, maternal history, total and direct serum bilirubin, peak serum bilirubin, day of onset of jaundice, blood grouping, sepsis screening, thyroid profile, treatment history, condi-tion at discharge were collected in a predesigned questionnaire. An infant once discharged was then followed up at 3 and 12 months of age by detail neurological examination and developmental assess-ment. To evaluate development, Bayley scales of Infant and Toddler Development, Third edition was used. This assessment was done by clinical psycho-logists assigned from Institute for Pediatric Neuro-disorder and Autism (IPNA), BSMMU, who were blinded about patient’s diagnosis. In the Bayley III, cognitive development, expressive and receptive language, and fine and gross motor development all were evaluated. Developmental delay was classified as “delayed” if a Bayley III score below 70 on any of the subscales. Outcome was recorded as favorable outcome (normal neurologic development) and adverse developmental outcome defined as signifi-cant delay in one or more developmental domains: cognition, language (receptive & expressive lang-uage) and motor functions (fine & gross motor function).
2.11 Sample size
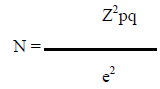
Where, n = the desired sample size z = the standard normal deviate, usually set at 1.96 at 5% level which corresponds to 95% confidence level. The prevalence of abnormal neurodevelopmental outcome of hyper-bilirubinemic neonates in previous study (Monika Sharma et al. 2016 ) is 6.25% So prevalence rate, p = .062 q = 1 – p = = (1- 0.062)= 0.938 e = Acceptable error (usually set as 5%) = 0.05
Putting the values in the above equation the sample size ‘n’ is estimated as –
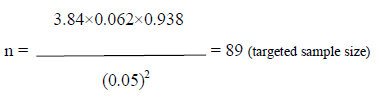
Total 89 case are required for this study
2.12 Data analysis
Data were analyzed using the statistical package for social sciences (SPSS) version 20. Quantitative data were expressed as mean ± SD and categorical data were presented as proportion/percentage. All quantitative variables (between the groups of favorable and adverse outcome) were compared by unpaired t-test; categorical variables were compared by Chi-square test or Fisher’s exact test. P value < 0.05 was considered as significant.
3. Results
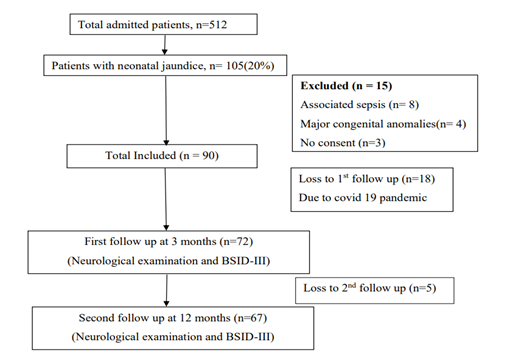
During the period of enrollment, total 512 patients were admitted. 105 patients (20%) had neonatal jaundice who were assessed for eligibility. Among them, 8 patients were excluded due to sepsis, 4 infants were excluded due to major congenital anomalies and 3 patient declined to participate in the study. Finally 90 patients were included. After getting treatment for neonatal jaundice all 90 patients were discharged to home. Among them 72 patients came for first follow-up and 67 patients completed up to 2nd follow-up (Figure 1). Baseline characteristics of the studied infants are presented in Table 1. Most of the babies were born at term (73.6%). Mean birth weight was 2870.97 ± 458 g. Gender distribution reflected slight female predominance (58.3%). Most of the infants were born by LUCS 67 (93.1%). About 69 (95.8%) babies were inborn and 3 (4.2%) were outborn. Average age of the mother were 27.76 ± 7 years.
Table 2 showing clinical characteristics of neonatal jaundice patient. Average age of patient at admission was 56.17 ± 39 hours. Etiologies of neonatal jaundice were Exacerated Physiologic 49 (68.2%), ABO incompatibility 12 (16.7%) and Rh incompatibility were 11 (15.3%). Average total serum bilirubin (TSB) at admission and average peak serum bilirubin at admission were 12.96 ± 5.5 and 15.08 ± 4 mg/dl respectively. Most of the patient 55 (76.4%) develops jaundice after 24 hours of age. Only 11 (15.3) patient were treated by exchange transfusion and rest of them by phototherapy and average duration of phototherapy were 55.57 ± 29.190 hours. Half of the patient received phototherapy < 48 hours. Only 9 (12.5%) patients had peak serum bilirubin ≥ 20mg/dl. Rebound hyperbilirubinemia were found in 9 (12.5) patients. Among total 72 infants whose outcome in 1st follow up were analyzed, 9 patients (12.5%) had adverse neurodevelopmental outcome and rest 63 patients (87.5%) had normal neurologic development (favorable outcome) (Table 3). When perinatal characteristics were compared between adverse and favorable outcome group (Table 4), no significant difference was noted between term and preterm babies (p = 0.76). Also, birth weight, sex, place & mode of delivery and parity had no impact on outcome.
Table 5 is showing the comparison of clinical parameters between the adverse and favorable outcome group. The p value was not significant for any of the etiologies of jaundice. There was no significant difference between adverse and favorable outcome groups regarding the onset of jaundice, peak serum bilirubin, treatment modality, duration of phototherapy and rebound hyperbilirubinemia. Total 67 infants completed 2nd follow up. Only 2 patients (3%) had adverse neurodevelopmental outcome and rest 65 patients (97%) had normal neurologic development (favorable outcome) (Figure 6). Table 7 is showing the comparison of perinatal factors between adverse and favorable neurodevelopmental outcome group. None of the factors, gestational age, birth weight, sex, place & mode of delivery and parity were found to be significantly related to adverse outcome. Table 8 is showing the results of univariate analysis of the clinical parameters of the neonates in adverse and favorable outcome group. None of the clinical factors, causes of jaundice, age of onset of jaundice, peak serum bilirubin, and treatment modality, duration of phototherapy and rebound hyperbilirubinemia were found to be significantly related to adverse outcome.
|
Characteristics |
Findings |
|
GA, n(%) |
|
|
Term |
53 (73.6) |
|
Pre-term |
19 (26.4) |
|
GA(weeks), mean±SD |
37.28 ± 1.4 |
|
BW, n(%) |
|
|
1800 – 2499 grams |
13 (18.1) |
|
2500 – 4000 grams |
59 (81.9) |
|
BW(grams), mean±SD |
2870.97 ± 458 |
|
Sex, n(%) |
|
|
Male |
30 (41.7) |
|
Female |
42 (58.3) |
|
Mode of delivery, n(%) |
|
|
LUCS |
67 (93.1) |
|
NVD |
5 (6.9) |
|
Place of delivery, n(%) |
|
|
Inborn |
69 (95.8) |
|
Out born |
3 (4.2) |
|
Parity, n(%) |
|
|
Primi |
31 (43.1) |
|
Multi |
41 (56.1) |
|
Age of mother (years), mean ±SD |
27.76 ± 7 |
GA: Gestational Age, BW: Birth Weight, LUCS: Lower Uterine Caesarean section, NVD: Normal Vaginal Delivery, SD: Standard Deviation. Quantitative data are presented as mean±SD and Qualitative data as the number and percentage.
Table 1: Baseline characteristics of perinatal factors with neonatal jaundice (N=72).
|
Characteristics |
Finding |
|
Age at admission (hours), mean±SD |
56.17 ± 39 |
|
Causes of jaundice, n (%) |
|
|
Exacerbated physiological |
49 (68.1) |
|
ABO incompatibility |
12 (16.7) |
|
Rh incompatibility |
11 (15.3) |
|
TSB at admission(mg/dl), mean±SD |
12.96 ± 5.5 |
|
Peak TSB (mg/dl), mean ±SD |
15.08 ± 4 |
|
Onset of jaundice, n (%) |
|
|
Within 24 hours |
17 (23.6) |
|
After 24 hours |
55 (76.4) |
|
Treatment modality, n (%) |
|
|
Phototherapy |
61 (84.7) |
|
Exchange transfusion |
11 (15.3) |
|
Duration of photo(hours), mean±SD |
55.57 ± 29.190 |
|
No of exchange, n (%) |
|
|
Single |
8 (72.72) |
|
Multiple |
3 (27.27) |
|
Rebound hyperbilirubinemia, n (%) |
|
|
Yes |
9 (12.5) |
|
No |
63 (87.5) |
|
Peak TSB, n (%) |
|
|
<20 |
63 (87.5) |
|
≥20 |
9 (12.5) |
|
Duration of photo, n (%) |
|
|
<48 hours |
36 (50.0) |
|
≥48 hours |
36 (50.0) |
TSB: Total Serum Bilirubin, SD: Standard Deviation, Quantitative data are presented as mean±SD and Qualitative data as the number and percentage
Table 2: Clinical characteristics of neonates with neonatal jaundice (N=72).
|
Variable |
Outcome |
|
Average age at 1st follow up (months), mean±SD |
3.6279 ± 0.43269 |
|
Overall outcome, n (%) |
|
|
Adverse (Score, 40 – 69) |
9 (12.5) |
|
Favorable (Score, 70 – 160) |
63 (87.5) |
|
Cognition |
|
|
Adverse (Score, 40 – 69) |
7 (10) |
|
Favorable (Score, 70 – 160) |
65 ( 90) |
|
Language |
|
|
Adverse (Score, 40 – 69) |
2 (3) |
|
Favorable (Score, 70 – 160) |
70 (97) |
|
Motor |
|
|
Adverse (Score, 40 – 69) |
3 (4) |
|
Favorable (Score, 70 – 160) |
69 (96) |
Quantitative data are presented as mean±SD and Qualitative data as the number and percentage
Table 3: Outcome in 1st follow up at 3 months of age (N=72).
|
Characteristics |
Favorable(N=63) |
Adverse(N=9) |
P value |
|
Gestational age, n(%) |
0.762ns |
||
|
Term |
46(87.8) |
7(13.2) |
|
|
Pre-term |
17(89.5) |
2(10.5) |
|
|
Birth weight, n(%) |
0.728ns |
||
|
1800 – 2499 |
11(84.6) |
2(15.4) |
|
|
2500 – 4000 |
52(88.1) |
7(11.9) |
|
|
Sex, n(%) |
0.857ns |
||
|
Male |
26(86.7) |
4(13.3) |
|
|
Female |
37(88.1) |
5(11.9) |
|
|
Place of delivery, n(%) |
0.504ns |
||
|
Inborn |
60(87) |
9(13) |
|
|
Out born |
3(100) |
0 |
|
|
Mode of delivery, n(%) |
0.381ns |
||
|
NVD |
5(100) |
0 |
|
|
LUCS |
58(86.6) |
9(13.4) |
|
|
Parity, n(%) |
0.418ns |
||
|
Primi |
26(83.9) |
5(16.1) |
|
|
Multi |
37(90.2) |
4(9.8) |
|
Qualitative data are presented as the number and percentage, Statistical test: Chi-square test and Fisher’s exact test for categorical data, p < 0.05 were considered as significant. ns- not significant.
Table 4: Comparison of perinatal characteristic between adverse and favorable outcome at 3 months of age (N=72).
|
Characteristics |
Favorable(N=63) |
Adverse(N=9) |
P value |
|
Causes of jaundice, n(%) |
0.856ns |
||
|
Exacerbated physiological |
43(87.8) |
6(12.2) |
|
|
ABO incompatibility |
10(83.3) |
2(16.7) |
|
|
Rh incompatibility |
10(90.9) |
1(9.1) |
|
|
Age of onset, n(%) |
0.345ns |
||
|
Within 24 hours |
16(94.1) |
1(5.9) |
|
|
After 24 hours |
47(85.5) |
8(14.5) |
|
|
Peak TSB(mg/dl), n(%) |
0.346ns |
||
|
1 – 20 |
56(88.9) |
7(11.1) |
|
|
>20 |
7(77.8) |
2(22.2) |
|
|
Treatment modality, n(%) |
0.710ns |
||
|
Phototherapy |
53(86.9) |
8(13.1) |
|
|
Exchange transfusion |
10(10.9) |
1(9.1) |
|
|
Duration of photo, n(%) |
0.722ns |
||
|
24 – 48 hours |
31(86.1) |
5(13.9) |
|
|
>48 hours |
32(88.9) |
4(11.1) |
|
|
Rebound hyperbilirubinemia, n(%) |
0.225ns |
||
|
Yes |
9 (100) |
0 |
|
|
No |
54 (85.7) |
9 (14.3) |
|
Qualitative data are presented as the number and percentage, Statistical test: Chi-square test and Fisher’s exact test for categorical data, p < 0.05 were considered as significant. ns- not significant.
Table 5: Comparison of clinical features between adverse and favorable outcome at 3 months of age (N=72).
|
Variables |
Outcome |
|
Average age at 2nd follow up (months), mean±SD |
13.19 ± 1.15 |
|
Overall outcome, n (%) |
|
|
Adverse (Score, 40 – 69) |
2 (3) |
|
Favorable (Score, 70 – 160) |
65 (97) |
|
Cognition |
|
|
Adverse (Score, 40 – 69) |
00 (00) |
|
Favorable (Score, 70 – 160) |
67 (100) |
|
Language |
|
|
Adverse (Score, 40 – 69) |
1 (1.5) |
|
Favorable (Score, 70 – 160) |
66 (98.5) |
|
Motor |
|
|
Adverse (Score, 40 – 69) |
1 (1.5) |
|
Favorable (Score, 70 – 160) |
66 (98.5) |
Quantitative data are presented as mean±SD and Qualitative data as the number and percentage
Table 6: Outcome in 2nd follow up at 12 months of age (N=67).
|
Characteristics |
Favorable(N=65) |
Adverse(N=2) |
P value |
|
Gestational age, n(%) |
0.491ns |
||
|
Term |
47 (97.9) |
1 (2.1) |
|
|
Pre-term |
18 (94.7) |
1 (5.3) |
|
|
Birth weight, n(%) |
0.481ns |
||
|
1800 – 2499 |
13 (100) |
0 |
|
|
2500 – 4000 |
52 (96.3) |
2 (3.7) |
|
|
Sex, n(%) |
0.224ns |
||
|
Male |
28 (100) |
0 |
|
|
Female |
37 (94.9) |
2 (5.1) |
|
|
Place of delivery, n(%) |
0.756ns |
||
|
Inborn |
62 (96.9) |
2 (3.1) |
|
|
Outborn |
3 (100) |
0 |
|
|
Mode of delivery, n(%) |
0.683ns |
||
|
NVD |
5 (100) |
0 |
|
|
LUCS |
60 (96.8) |
2 (3.2) |
|
|
Parity, n(%) |
0.100ns |
||
|
Primi |
27 (93.1) |
2 (6.9) |
|
|
Multi |
38 (100) |
0 |
|
Qualitative data are presented as the number and percentage, Statistical test: Chi-square test and Fisher’s exact test for categorical data, p < 0.05 were considered as significant. ns- not significant.
Table 7: Comparison of perinatal characteristic between adverse and favorable outcome at 12 months of age (N=67).
|
Characteristics |
Favorable(N=65) |
Adverse(N=2) |
P value |
|
Causes of jaundice, n(%) |
0.604ns |
||
|
Exacerbated physiological |
43 (95.6) |
2 (4.4) |
|
|
ABO incompatibility |
11 (100) |
0 |
|
|
Rh incompatibility |
11 (100) |
0 |
|
|
Age of onset, n(%) |
0.402ns |
||
|
Within 24 hours |
17 (100) |
0 |
|
|
After 24 hours |
48 (96.0) |
2(4) |
|
|
Peak TSB(mg/dl), n(%) |
0.063ns |
||
|
1 – 20 |
59 (98.3) |
1 (1.7) |
|
|
>20 |
6 (85.7) |
1 (14.3) |
|
|
Treatment modality, n(%) |
0.525ns |
||
|
Phototherapy |
54 (96.4) |
2 (3.6) |
|
|
Exchange transfusion |
11 (100) |
0 |
|
|
Duration of photo, n(%) |
0.145ns |
||
|
24 – 48 hours |
31 (93.9) |
2 (6.1) |
|
|
>48 hours |
34 (100) |
0 |
|
|
Rebound hyperbilirubinemia, n(%) |
0.572ns |
||
|
Yes |
9 (100) |
0 |
|
|
No |
56 (96.6) |
2 (3.4) |
|
Qualitative data are presented as the number and percentage, Statistical test: Chi-square test and Fisher’s exact test for categorical data, p < 0.05 were considered as significant. ns- not significant.
Table 8: Comparison of clinical features between adverse and favorable outcome at 12 months of age (N=67).
4. Discussion
Neonatal hyper-bilirubinemia is a common problem requiring medical attention in newborn and a leading cause of preventable brain damage, physical and mental handicap and early deaths among infants. Unconjugated bilirubin crosses blood– brain barrier and causes neuro toxicity and subsequent death or lifelong neurological sequelae in surviving infants. The phenomenon of deposited indirect bilirubin in the basal ganglia causes a neurological syndrome called kernicterus. The cause and effect relationship between neurodevelopment and risk factors of hyperbilirubinemia is more complex and we cannot simply presume that neonatal hyperbilirubinemia and presence of various risk factors will always lead to adverse neurodevelopmental outcome. Because of having treatable risk factors of neonatal hyper-bilirubinemia, early detection and regular follow up for developmental abnormalities in these patients is important to initiate early intervention measures for better developmental outcome.
So aim of this study is to see the predictors of neurodevelopmental outcome in hyperbilirubinemic term and late preterm infants. A total of 90 neonates with hyper-bilirubinemia, admitted to NICU and fulfilling the inclusion/exclusion criteria were included in the study and developmental evaluation was done at 3 and 12 months by BSID III method. Out of 90 hyperbilirubinemic neonates enrolled in the study, 72 neonates have completed 1st follow up at 3 months of age and 67 infants have completed 2nd follow up at 12 months of age. Among 72 infants 41.7% were males while 52.3% were females; 26.4% were preterm (gestational age <37 weeks) while the rest 73.6% were term (gestational age >37 weeks) deliveries; 69 (95.8%) babies were inborn whereas 3 (94.2%) were outborn. As our centre is a tertiary referral hospital so most of the referred cases are complicated and mechanical ventilated patient. That’s why outborn fresh neonatal hyperbiliru-binemic patients were low. Birth weight of these hyper-bilirubinemic neonates were between 1800 g to 2499 g for 18.1% and 2500 g to 4000 g for 81.9%. Peak serum bilirubin level of 87.5% neonates was below 20 mg/dl while 12.5 % cases were having peak level equal to or higher than 20 mg/dl. In our study, 23.6 % cases had onset of jaundice within 24 hours of birth while in 76.4 % cases, time of onset of jaundice was more than 24 hours of birth. 50% of patients were treated by phototherapy for less than 48 hours and another 50% were treated for more than 48 hours. Etiology for hyper-bilirubinemia was hemo-lytic in 31.9 % cases (ABO, Rh, ABO+Rh, DCT positive) and non- hemolytic in the rest 68.1%. Overall 84.7% patients were treated by phototherapy and 15.3% patients by exchange transfusion. Percen-tage of rebound hyperbilirubinemia was 12.5%.
In our study, the prevalence of abnormal neuro-developmental outcome (composite score <70) according to BSID III method was 12.5 % at 3 months which decreased to 3% on follow up at 12 months suggesting that some component of neuro-developmental abnormalities due to hyperbiliru-binemia could be transient or reversible. Similar decrease in prevalence has been reported by Monika Sharma et al.[12] was 10.42% at 3 months which decreased to 6.25% and Chen et al. [19] (10.42% in hemolytic group and 2% in non-hemolytic group were neurodevelopmental abnormal at initial evaluation and returned to normal on follow up at 3 years). Yilmaz et al. [20] reported neurodevelo-pmental abnormalities in 11.5% cases in their study using DDST which is quite similar to the prevalence of neurodevelopmental abnormalities in our study (12.5%). Hymen et al. [21] reported that the prevalence of neurodevelopmental abnormalities was higher in hyper-bilirubinemic neonates with >20 mg/dl bilirubin where evaluation was done at 4 years of age. But in our study, there was no significant neurodevelopmental abnormalities found when bilirubin level > 20 mg/dl and also by Thomas and Mark et al. [22]. Similar picture was found by Thomas et al. [23] where cut off values of serum bilirubin was 25 mg/dl and by Lunsing et al. [10] where cutoff value of serum bilirubin was 17.6 mg/dl. But Monika Sharma et al [12] found that when serum bilirubin level ≥ 25 mg/dl having significant abnormalities in neurodevelopment and Ozgurhan and Comert found abnormal neurodevelopment when PSB > 22 mg/dl. Composite score in male neonates was also not statistically different to composite score observed in female neonates and also neurodevel-opmental outcome was similar observed in term and preterm (35 – 36 weeks) neonates in our study. Similar result was found in relation of gestational age and gender to neurodevelopment done by Monika Sharma et al. [12].
In contrast to these, other studies like Scheidt et al.[24] reported significant relationship of gestational age and neurodevelopmental abnormalities which were more in babies with low gestational age, Wolf et al. [25] reported that neurodevelopmental abnormalities were more in those hyper-bilirubinemic neonates who were having lower gestational age. The significant association between time of onset of jaundice within 24 hours of age and abnormal neurodevelopmental outcome has also been reported by Monika Sharma et al. [12] and Babu et al. [26] Babu et al. [11] Agarwal et al. [27] and Monika Sharma et al. [12] noticed that significantly higher prevalence of neurodevelopmental abnormalities in those who required exchange transfusion as compared to those who received phototherapy. Prevalence of abnormal neurodevelopmental out-come was statistically significant those were treated with exchange transfusion than phototherapy on evaluation done by Monika Sharma et al. [12] at 3months (p=0.0001) and 12 months (p=0.001). The hyperbilirubinemic neonates who were having hemolytic etiology, the prevalence of abnormal neurodevelopmental outcome (DQ ≤70) was higher (16.33% at 3 month, 10.20% at 12 months) as compared to those neonates who were having non hemolytic etiology (4.26% at 3 month, 2.13% at 12 months) in study done by Monika Sharma et al.[12] and similar result was found by Babu et al. [26] But in our study early onset of jaundice, hyperbilir-ubinemia due to hemolytic disease and those were treated by exchange transfusion were not signif-icantly associated with abnormal neurodevelopment. All cases of early onset jaundice were due to hemolytic diseases and all of them were inborn. As all early onset hyperbilirubinemia neonates were booked cases so early intervention were taken by exchange transfusion and phototherapy. That’s why bilirubin level were not high to cause abnormal neurodevelopment.
4.1 Limitations
- Small sample size due to Covid-19 pandemic situation. Due to lock down in covid-19 pandemic, infants from outside Dhaka did not come for follow up. Moreover few mothers denied to come for follow up due to risk of SARS- CoV-2 transmission.
- Single centre study.
- Long term follow up could not be done.
- BSID III was not done by single clinical psychologist.
5. Conclusion
Among 72 neonates, 9 (12.5%) had abnormal neurodevelopment results at 3 months, whereas 2 (3%) had neurodevelopmental abnormalities at 12 months suggesting reversibility of adverse neuro-development outcome. When treated with photo-therapy or exchange transfusion, either hemolytic or non-hemolytic neonatal jaundice, onset of jaundice within 24 hours of age, peak serum bilirubin levels and duration of phototherapy were not associated with adverse neurodevelopmental outcomes in infants born at or near term.
Recommendations
- Patients with neonatal jaundice should be referred for early and long term neuro-developmental follow up. If they have developmental delay, early intervention with developmental therapy is needed to reduce the prevalence of neurodevelopmental abn-ormalities in hyperbilirubinemic neonates.
- Further research with good sample size and with long term follow up will give better insight to the predictors of adverse neurodevelopmental outcome in patients with neonatal jaundice.
References
- Maisels, M.J., Watchko, J.F., Neonatal hyperbilirubinemia. In:J. M. Fanaroff, A.A. Fanaroff, ed. Care of the High Risk Neonate, 6thed, Philadelphia: Saunders, An Imprint of Elsevier, 2012, 310-345.
- American Academy of Pediatrics. Practice parameter: management of Hyperbiliru-binemia in the healthy term newborn. Provisional Committee for Quality Improve-ment and Subcommittee on hyperbiliru-binemia. Pediatrics 1994; 94: 558–565
- Mallick PK, Alam B. Aetiological study of neonatal hyperbilirubinaemia-A hospital based prospective study. Medicine Today. 2012; 24(2):73-4.
- Gomella, T. L., Cunningham, M. D., Eyal, F. G., & Tuttle, D. J. (Eds.). Neonatology: management, procedures, on-call problems, diseases, and drugs New York: McGraw-Hill Education Medical. (2013); (pp. 565-570).
- Newman, T. B., Liljestrand, P., Jeremy, R. J., Ferriero, D. M., Wu, Y. W., Hudes, E. S., & Escobar, G. J. Outcomes among newborns with total serum bilirubin levels of 25 mg per deciliter or more. New England Journal of Medicine. (2006); 354(18), 1889-1900.
- American Academy of Pediatrics Subcom-mittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. . (2004); 114(1), 297-316.
- Harris, M.C., Roth, P. Neonatology. In: A. Richard, R.A. Polin, M.F. Ditmer, eds. Pediatrics Secret, Philadelphia, Hanley and Belfus, 1989, 261-264.
- Morris, B. H., Oh, W., Tyson, J. E., Stevenson, D. K., Phelps, D. L., O'Shea, T. M. & Higgins, R. D... Aggressive vs. conservative phototherapy for infants with extremely low birth weight. New England Journal of Medicine. (2008); 359(18), 1885-1896.
- De Carvalho, Manoel. "Treatment of neonatal hyperbilirubinemia." J Pediatr 77.Suppl 1 (2001): S71-S80.
- Lunsing RJ, Pardoen WF, Hadders-Algra M. Neurodevelopment after moderate hyperbili-rubinemia at term. Pediatric research. 2013 May; 73(5):655-60.
- Babu TA, Bhat BV, Joseph NM. Neurobehavior of term neonates with neonatal hyperbilirubinemia. Journal of pediatric neurosciences. 2013 Jan; 8(1):11.
- Sharma, M., Sengar, G.S., Nagaraj, N., Khandelwal, S. and Yadav, V. A study of predictors & prevalence of neurodeve-lopmental outcome in hyperbilirubinemic neonates admitted in NICU. Indian Journal of Neurosciences, 2016, 2(4), pp.108-112.
- Steiner TJ, Birbeck GL, Jensen R, Katsarava Z, Martelletti P, Stovner LJ. The global campaign, world health organization and lifting the burden: collaboration in action. The journal of headache and pain. 2011 Jun; 12(3):273-4.
- Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J pediatr. 1967 Aug 1; 71(2):159-63.
- Power ME, Tilman D, Estes JA, Menge BA, Bond WJ, Mills LS, Daily G, Castilla JC, Lubchenco J, Paine RT. Challenges in the quest for keystones: identifying keystone species is difficult—but essential to understanding how loss of species will affect ecosystems. BioScience. 1996 Sep 1; 46(8):609-20.
- Gomella TL, Cunningham MD, Eyal FG, Tuttle DJ, editors. Neonatology: manage-ment, procedures, on-call problems, diseases, and drugs. New York: McGraw-Hill Education Medical; 2013.
- DeSilva M, Munoz FM, Mcmillan M, Kawai AT, Marshall H, Macartney KK, Joshi J, Oneko M, Rose AE, Dolk H, Trotta F. Congenital anomalies: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2016 Dec 1; 34(49):6015.
- Gomella TL, Cunningham MD, Eyal FG, Tuttle DJ, editors. Neonatology: manage-ment, procedures, on-call problems, diseases, and drugs. New York: McGraw-Hill Education Medical; 2013.
- Wang L. Chen. Lixin (The Second Construction Engineering Corp. of Benxi Iron & Steel Corp., 117100). 2000:1997-0.
- Yilmaz Ö. Seismic data analysis: Proces-sing, inversion, and interpretation of seismic data. Society of exploration geophysicists; 2001 Jan 1.
- Basaran M, Usal D, Aydemir C. Hymen sparing surgery for imperforate hymen: case reports and review of literature. Journal of pediatric and adolescent gynecology. 2009 Aug 1; 22(4):e61-4.
- Thomas ME, Schreiner GF. Contribution of proteinuria to progressive renal injury: consequences of tubular uptake of fatty acid bearing albumin. American journal of nephrology. 1993; 13(5):385-98.
- Thomas DR. A general inductive approach for analyzing qualitative evaluation data. American journal of evaluation. 2006 Jun; 27(2):237-46.
- Lee J, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT. Metal–organic framework materials as catalysts. Chemical Society Reviews. 2009; 38(5):1450-9.
- Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001 Jul; 32(7):1635-9.
- Babu A, Bhat V. Predictors of abnormal neurodevelopment at 6 months in term babies with early neonatal hyperbilir-ubinemia. A prospective cohort study from South India. Birth. 2011 Jul 1; 34(21):82-95.
- Agrawal A, Pandya S, Shrivastava J. Neurodevelopmental outcome at 6 months of age in full-term healthy newborns with neonatal hyperbilirubinemia. Journal of Clinical Neonatology. 2020 Apr 1; 9(2):138.