Polypectomy of a Pedunculated Sigmoid Gastrointestinal Stromal Tumor : is that Feasible ? About a Case Report
Article Information
Sabrine Soua1*, Ghanem Mohamed1, Olfa Hallara2, Nidham Eddine Kchir3, Mohamed Riadh Bouali1
1Military Hospital of Tunis, Tunisia
2Fattouma Bourguiba Hospital, Monastir, Tunisia
3La Rabta Hospital, Tunis, Tunisia
*Corresponding author: Sabrine Soua, Department of Gastroenterology, Military Hospital of Tunis, Faculty of Medecine of Tunis, Tunisia.
Received: 30 August 2023; Accepted: 06 September 2023; Published: 14 November 2023.
Citation: Sabrine Soua, Ghanem Mohamed, Olfa Hallara, Nidham Eddine Kchir, Mohamed Riadh Bouali. Polypectomy of a Pedunculated Sigmoid Gastrointestinal Stromal Tumor : is that Feasible ? About a Case Report. Archives of Clinical and Biomedical Research. 7 (2023): 556-559.
Share at FacebookAbstract
Gastrointestinal stromal tumors (GIST) are rare neoplasms that represent approximately 1–2% of gastrointestinal cancers. Colonic localization is very rare (5%). Treatment is based on surgery. In this case, we report a successful endoscopic resection of a small sigmoid GIST with conventional polypectomy in a 72-year-old woman.
Keywords
Gastrointestinal stromal tumors; colonic GIST; sigmoid GIST; endoscopic resection; polypectomy
Gastrointestinal stromal tumors articles; colonic GIST articles; sigmoid GIST articles; endoscopic resection articles; polypectomy articles.
Article Details
1. Introduction
Gastrointestinal stromal tumors (GIST) are rare neoplasms that represent approximately 1–2% of gastrointestinal cancers [1). Despite their rarity, GISTs represent the main mesenchymal tumors of the gastrointestinal tract. They are derived from Cajal cells or from one of their precursors. They mainly develop in the stomach and the small intestine. Colonic localization is very rare (5%) [2]. Treatment is based on surgery for localized GISTs and tyrosine kinase inhibitors for specific indications [3]. Endoscopic resection is a novel treatment for colonic GISTs, but it is rarely reported. This article reports the successful endoscopic resection of a small sigmoid GIST with polypectomy.
2. Case presentation
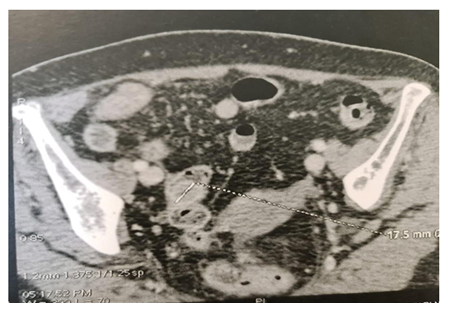
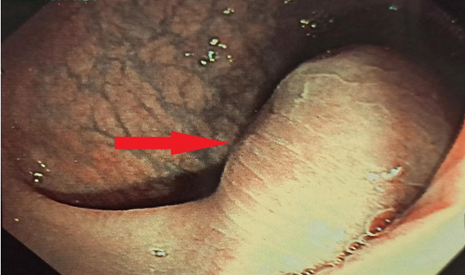
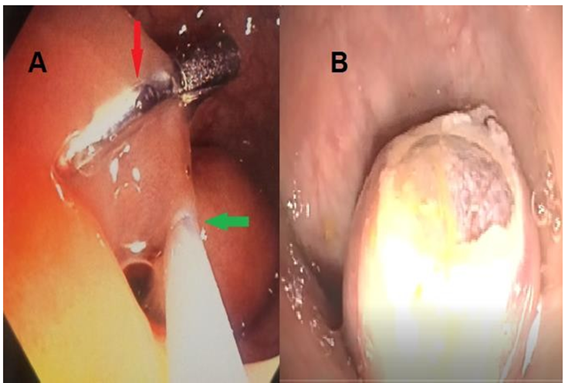
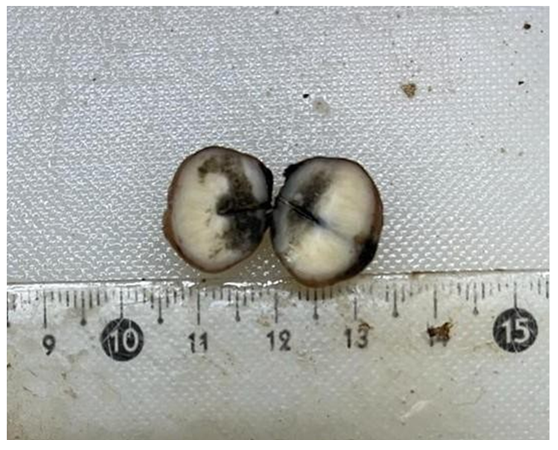
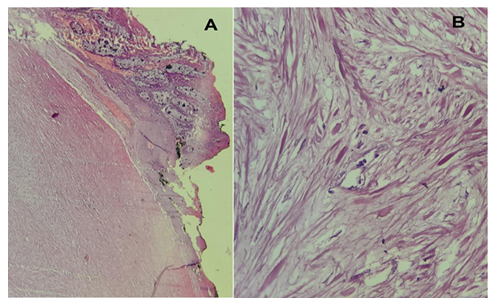
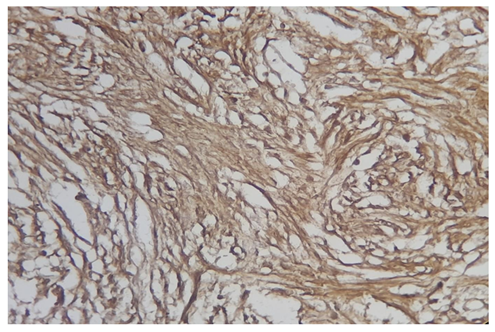
A 72-year-old woman presented with vague abdominal pain. An abdominopelvic CT scan revealed a 17-mm sigmoid lesion that has the same characteristics as the muscle (Figure 1). She underwent a colonoscopy, which showed an 18-mm pedunculated lesion covered by normal mucosa in the sigmoid colon (Figure 2). We performed a hot snare polypectomy after prophylactically placing a hemostatic clip of 20 mm (Lockado) on the pedicle (Figure 3). The tumor was retrieved using a retrieval net and sent to the pathologist. The specimen's histology revealed that the formation pushes the mucosa on the surface and sits at the level of the submucosa (Figures 4 and 5). Under microscopy, a spindle cell neoplasm was found. The resection base was healthy. Immunohistochemical examination showed that the tumor cells were positive for c-KIT (CD117) and negative for Dog-1 (Figure 6). The mitotic index was very low, at less than one mitosis per 50 high-power fields. The diagnosis of GIST derived from the superficial layer of the sigmoid colon wall was then made. Given the complete endoscopic resection, additional surgical resection was not indicated. At 3 months' follow-up, the patient was symptom-free.
Discussion
GISTs are most often found in the stomach (in 60 to 70% of cases) and the small intestine (in 20 to 30% of cases). Those located in the rectocolon are relatively rare, representing approximately 5% of all GISTs. Colonic localization is rarer and represents 1–2% of all GISTs [4]. Studies on colonic GISTs are limited by their rarity. GISTs can occur in adults at any age, rarely before the age of 40, with a peak frequency around the age of 50–60 and a sex ratio nearing one [5]. The clinical presentation depends on the location, the size of the tumor, and the presence or absence of metastases. Up to 21% of tumors are found incidentally during radiological examinations performed for other reasons [6]. Intestinal occlusion and bleeding are the main discovery circumstances. In the colon, these tumors are more frequently found in the left and transverse colons (71%) [7]. The most common tests used to diagnose colorectal GISTs are computed tomography and endoscopy or endoscopic ultrasound. On a CT scan, small tumors usually appear as homogeneous, well-limited masses with a density similar to that of muscle [8]. The puncture biopsy (by echoendoscopic or percutaneous route) carries a risk of hemorrhage and potentially of peritoneal dissemination of tumor cells on the route of the puncture. For these reasons, the preoperative biopsy is not part of the pretherapeutic assessment if a resection is planned [9]. However, a biopsy should be performed in the case of unresectable GIST to establish the diagnosis and justify the preoperative administration of imatinib [3]. More than 95% of GISTs express CD117 (also called c-Kit) in the immunohistochemical study [10]. There are rare GISTs (<5% of cases) that do not express CD117. The diagnosis of these CD117-negative GISTs is aided by the identification of DOG-1 expression. Exceptionally, like in our case, stromal tumors may arise from the superficial layer of the colonic wall and present as a pedunculated polyp [11]. For patients with localized colonic GISTs, surgery is the treatment of choice. In these cases, a segmental colectomy with no lymph node dissection is indicated [12]. With the development of endoscopy, endoscopic resection of GISTs has become possible. The main endoscopic techniques reported were endoscopic full-thickness resection and submucosal tunneling endoscopic resection. While endoscopic resection of gastric GISTs is relatively common [13], colorectal GISTs have rarely been reported. The reason for this is the relatively thin colon wall, which increases the risk of perforation [14]. For our patient, a hot snare polypectomy was done without complications. Conventional polypectomy was feasible in our case because of the tumor's small size, pedunculated aspect, and development from the superficial layer of the colonic wall. Advanced age and comorbidities were other reasons for the choice of endoscopic treatment. The main prognostic factors are tumor size and mitotic activity [10]. The classifications AFIP of Miettinen and modified NIH are the most commonly used to assess the risk of recurrence after resection [9]. The risk of recurrence for our patient is zero according to the Miettinen classification and very low according to the NIH classification, while his whole colon is preserved. Therefore, an additional treatment with Tyrosine Kinase inhibitors was not prescribed.
Conclusion:
Surgical resection is the main treatment for localized colonic GISTs. However, with the development of endoscopy, an endoscopic resection can be possible in special cases.
Acknowledgments:
None
Funding:
None
Conflicts of interest:
The authors declare no conflicts of interest regarding the publication of this paper.
Data availability statement:
The data that support the findings of this article are available from the corresponding author on reasonable request.
Ethics statement:
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
References:
- Miettinen M, Lasota J. Gastrointestinal stromal tumors - definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 15 janv 438 (2001): 1-12.
- Kukar M, Kapil A, Papenfuss W, et al. Gastrointestinal stromal tumors (GISTs) at uncommon locations: A large population based analysis: GIST’s at Uncommon Locations. J Surg Oncol. mai 111 (2015): 696-701.
- Blay JY, Kang YK, Nishida T, et al. Gastrointestinal stromal tumours. Nat Rev Dis Primer. déc 7 (2021): 22.
- Feng F, Tian Y, Liu Z, et al. Clinicopathological features and prognosis of colonic gastrointestinal stromal tumors: evaluation of a pooled case series. Oncotarget. 28 juin 7 (2016): 40735-40745.
- Søreide K, Sandvik OM, Søreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. févr 40 (2016): 39-46.
- Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era: A population-based study in Western Sweden. Cancer. 15 févr 103 (2005): 821-829.
- Niazi AK, Kaley K, Saif MW. Gastrointestinal stromal tumor of colon: a case report and review of literature. Anticancer Res. mai 34 (2014): 2547-2550.
- Chourmouzi D, Sinakos E, Papalavrentios L, et al. Gastrointestinal stromal tumors: a pictorial review. J Gastrointest Liver Dis JGLD 18 (2009): 379-383.
- Landi B, Blay JY, Bonvalot S, et al. Gastrointestinal stromal tumours (GISTs): French Intergroup Clinical Practice Guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis 51 (2019): 1223-1231.
- Casali PG, Abecassis N, Bauer S, et al. Gastrointestinal stromal tumours: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29 (2018): 68-78.
- Deeb L, Hindy P, Gress F, et al. A Rare Case of GIST Arising from Mucsularis Mucosa Masquerading as a Gastric Hyperplastic Polyp: 1039. Am J Gastroenterol 106 (2011): 387.
- Roberts PJ, Eisenberg B. Clinical presentation of gastrointestinal stromal tumors and treatment of operable disease. Eur J Cancer38 (2002): 37-38.
- Tan Y, Tan L, Lu J, et al. Endoscopic resection of gastric gastrointestinal stromal tumors. Transl Gastroenterol Hepatol 2 (2017): 115.
- Colombo M, Spadaccini M, Maselli R. Endoscopic resection of GIST: feasible or fairytale? Laparosc Surg. avr 6 (2022): 12.