Plasmodium falciparum Malaria Carriage Associates with Reduced γδ T-Cell and NK Cell Responses to Infected Red Blood Cells In Vitro
Article Information
Bourèma Kouriba*, #, 1, 5, Modibo Daou†, #, 1, 2, Charles Arama1, Nicolas Ouédraogo3, Karamoko Niaré1, 4, Yamoussa Keita1, Sibiri Sissoko1, Boucary Ouologuem1, Seydou Arama1, Ogobara K Doumbo†, 1, Robert W Sauerwein*, 2, Anja Scholzen*, 2
1Malaria Research and Training Centre, Department of Epidemiology of Parasitic Diseases, University of Science, Techniques and Technologies of Bamako, Bamako, Mali
2Department of Medical Microbiology, Radboud University Medical Centre, Nijmegen, The Netherlands
3Centre National de Recherche et de Formation sur le Paludisme (CNRFP), Ouagadougou, Burkina Faso
4Department of Pathology and Laboratory Medicine, Brown University, RI, USA.
5Centre d'Infectiologie Charles Mérieux-Mali, Bamako, Mali.
#These authors contributed equally to this work and share first authorship
†Deceased
*Corresponding author: Bourèma Kouriba, Centre d'Infectiologie Charles Mérieux-Mali, Bamako
Received: 07 December 2023 Accepted: 15 December 2023 Published: 29 December 2023
Citation: Bourèma Kouriba, Modibo Daou, Charles Arama, Nicolas Ouédraogo, Karamoko Niaré, Yamoussa Keita, Sibiri Sissoko, Boucary Ouologuem, Seydou Arama, Ogobara K Doumbo, Robert W Sauerwein, Anja Scholzen. Plasmodium falciparum Malaria Carriage Associates with Reduced γδ T-Cell and NK Cell Responses to Infected Red Blood Cells In Vitro. Archives of Microbiology and Immunology. 7 (2023): 513-527.
Share at FacebookAbstract
Background: Innate immune cells including γδ T-cells and NK cells are directly activated by Plasmodium falciparum parasites and contribute to the control of parasitaemia. The aim of this study was to determine whether parasite carriage affects innate immune cell responses in vitro to P. falciparum infected red blood cells (PfRBC).
Methods: Peripheral blood mononuclear cells were collected from 61 Malian children aged 5 to 15 years at the start of the transmission season. Parasite carriage at the start of the transmission season was assessed by PCR and microscopy for Malian children. Peripheral blood mononuclear cells were stimulated with PfRBC to assess cytokine production and degranulation of innate lymphocytes (γδ T-cells, CD3+CD56+ cells and NK cells) by flow cytometry.
Results: Granzyme B production in response to PfRBC was observed by all three innate cell subsets in Malian children, as were IFNγ production by γδ T-cells and NK cells and γδ T-cell degranulation. Children with ongoing P. falciparum infection showed significantly reduced PfRBC-specific IFNγ production by γδ T-cells and NK cells and degranulation by γδ T-cells as compared with those with undetectable parasitaemia by PCR and microscopy. Reduced IFNγ responses by NK cells were already observed for children with submicroscopic parasitaemia as compared to those with negative PCR. Children with high parasite densities showed a significant reduction in degranulating γδ T-cells relative to their low parasite density counterparts.
Conclusion: P. falciparum-specific responses by γδ T-cells and NK cells were negatively impacted by ongoing P. falciparum infection.
Keywords
Malaria, Plasmodium falciparum, degranulation, Granzyme B, IFNγ, γδ T-cells, NK cells
Malaria articles, Plasmodium falciparum articles, degranulation articles, Granzyme B articles, IFN? articles, ?? T-cells articles, NK cells.
Article Details
1. Introduction
Malaria caused by the protozoan parasite Plasmodium falciparum remains a major public health concern in sub-Saharan Africa, causing unacceptably high morbidity and mortality in children aged below 5 years and pregnant women [1]. Naturally acquired immunity to malaria develops with age after repeated exposure to infective mosquito bites and protects against clinical symptoms instead of inducing sterile protection from infection [2-6]. Protection from clinical symptoms requires both control of parasitaemia and a well-balanced immune response to avoid intense inflammation. The cellular mechanisms underlying acquired immunity from symptomatic disease are not yet fully understood, and studies have long focused on adaptive immune responses [2, 3, 6, 7]. Neutralization and opsonization by P. falciparum-specific antibodies seem to be the major adaptive immune mechanisms of blood-stage parasite clearance (6, 8-10). P. falciparum-specific T-cells mainly affect liver-stage infection, support humoral responses, and have been linked to protection from clinical malaria in some studies [6, 11-20]. Next to these adaptive responses, innate cellular responses to the parasite also play an important role in blood-stage infection control. Firstly, γδ T-cells and NK cells are predominant producers of IFNγ, a key cytokine in protection against malaria [21-26], in response to exposure to P. falciparum-infected red blood cells (PfRBC) [24, 27-31]. Accordingly, γδ T-cells producing proinflammatory cytokines in response to PfRBC have been linked to protection from parasitaemia [32]. Secondly, these innate immune cells directly mediate cellular cytotoxicity against PfRBC, involving release of granzymes and granulysin by degranulation upon exposure to merozoites and PfRBC [33-40]. γδ T-cells and NK are directly activated by PfRBC components [27, 29, 33, 34, 41-44]: via phosphoantigens recognized by vγ9vδ2T-cells [42, 43], which constitute the vast majority of γδ T-cells in circulation, via NK sensing of host Hsp70 expression on PfRBC [33], recognition of PfEMP-1 via the activating receptor NKp30 [45] and sensing of microvesicle-transported parasite RNA via the RIG-I-like receptor MDA5 [46]. Additionally, γδ T-cells and NK cells can get activated through engagement of CD16 (FcgRIIIA) and mediate antibody-dependent cytotoxicity of opsonized PfRBC [47-51], thus bridging innate and adaptive immunity. Already after a primary malaria infection, peripheral blood γδ T-cells are expanded and both NK and γδ T-cells show an enhanced responsiveness to PfRBC [31, 44, 52-56]. Specifically for NK cells this enhanced innate response appears to depend on cytokines like IL-2 provided by adaptive memory T-cells [52, 57]. While these innate immune cell activities likely help control parasitaemia, studies in a Ugandan child cohort have shown that proinflammatory cytokine production and degranulation by vδ2+ γδ T-cell in response to PfRBC is impaired by repeated exposure to parasites [32, 35, 58]. Reduced pro-inflammatory cytokine production by vδ2+ γδ T-cells is further associated with a reduced risk of clinical symptoms during subsequent P. falciparum infections [32, 58], indicating innate cell tolerance may play a role in anti-disease immunity. Moreover, acute malaria infection and cumulative exposure have been shown to phenotypically alter NK cells, resulting in increased expression of the inhibitory receptor PD-1 [59]. Such PD-1 expressing NK cells, when induced in vitro, associates with diminished natural cytotoxicity MHC class I negative target cells [59]. However, the impact of ongoing P. falciparum infection on the direct response of NK cells to PfRBC remains elusive. Here, we assessed the impact of ongoing P. falciparum infection at microscopic and submicroscopic parasite density on P. falciparum-specific cytokine and cytotoxic responses by innate immune cells including γδ T-cells and NK cells.
2. Materials and Methods
2.1 Study subjects
Peripheral blood mononuclear cells (PBMC) were collected from, malaria-exposed Malian children aged 5 to 15 years. Malian children were recruited in a longitudinal study in the village of Samako in the Sudanese savannah zone located in the Upper Niger valley 70 km southwest of Bamako (capital city of Mali). P. falciparum accounts for more than 95% of all malaria cases, with seasonal transmission from June to December [87]. From July to December of 2011, the overall incidence rate of clinical malaria was 1.46 episodes of malaria per person per season in the age category of 5 to 14-year-old children (Kone et al., unpublished data).
2.2 Parasitological and clinical follow-up
Children were followed up during one transmission season from July to December 2012 for malaria infection by active and passive case detection. Thick smears were performed, and axillary temperature was checked on a monthly basis. In case of malaria symptoms between scheduled visits, a rapid diagnostic test (OptiMAL, Flow Inc, Portland, OR, USA) and thick smear were performed, and axillary temperature was checked. Additionally, blood samples were collected on filter paper for PCR during two visits in July and October 2012, as described previously [88]. A clinical episode of malaria was defined as any parasite density ≥5,000 trophozoites/μL in combination with fever (≥37.5°C). Only children with exclusive P. falciparum infection were considered for this study, while children with co-infections with non-P. falciparum species were excluded.
Out of 170 children enrolled into this study, 61 children were selected based on age criteria (5 to 15 years) and parasite exposure (either by thick smear or PCR) at one or more time points during follow-up. Children were further sub-categorized based on parasite density at the time of blood collection for immunological analysis early in the transmission season (July 2012) or based on whether they experienced a clinical malaria episode during follow-up (Table 1).
Table 1: Demographic and parasitological parameters of Malian children
- In 61 Malian children at time of blood collection in July 2012 (early in the transmission season)
- Temperature data were missing for n = 1 child
2.3 Sample collection and Stimulation assay for immunological analysis
2-5 ml of ETDA anti-coagulated blood was collected from Malian children by venipuncture at the beginning of the transmission season (July 2012). PBMC were isolated using Ficoll isopaqueTM by density gradient centrifugation. PBMC were cryo-preserved and stored in liquid nitrogen. Stimulation and immunophenotyping assays were conducted in 2014. PBMC were thawed, washed, and re-suspended in RPMI 1640 culture medium containing 2mM glutamine, 1mM pyruvate, 50µg/mL gentamycin (Gibco) and 10% pooled human A+ serum (Sanquin, Nijmegen, NL). Pacific Blue-labeled anti-CD107a monoclonal antibody (mAb) (PacB; clone H4A3, Biolegend) was added during culture to evaluate cellular degranulation upon stimulation. PBMC (5x105 cells/well) were transferred in duplicate into 96-well round-bottom plates and stimulated with cryo-preserved P. falciparum (strain NF54) infected red blood cells (PfRBC; final concentration 5x106/ml, PBMC:PfRBC ratio 1:2), matched numbers of uninfected (u)RBC or RPMI only for 24 h at 37°C / 5%CO2. The final reaction volume was 200 µL. Four hours prior to harvest, 100 µL/well supernatant was collected and replaced with 10 µL/well fresh culture medium containing brefeldin A (final concentration 10µg/ml) and monensin (final 2µM) or brefeldin A with monensin and PMA (final 50ng/ml)/ionomycin (final 1µg/ml, all Sigma; positive control).
2.4 Immunostaining and flow cytometric analysis
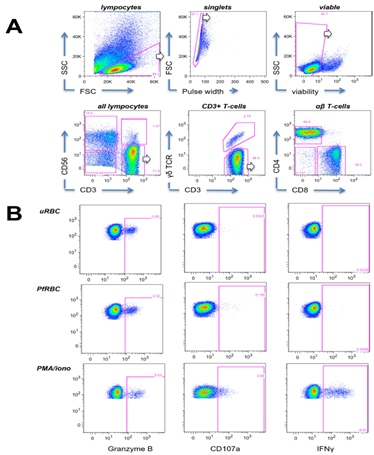
Following 24 hours of in vitro stimulation, PBMC were harvested, washed once with PBS and incubated in Life/Dead fixable Aqua dead cell stain (Invitrogen) for 30 min on ice. Cells were then washed with staining buffer (0.5% bovine serum albumin (BSA)/PBS) and incubated for 20 minutes with fluorescently labeled mAbs against the following cell surface markers at room temperature: Pan γδTCR PE (clone IMMU510), CD4 ECD (clone SFCI12T4D11; both Beckman Coulter), CD3 PerCP (clone UCHT-1), CD56 biotin (clone HCD56; both Biolegend) /Streptavidin eFluor660 (eBioscience), CD8 APC-H7 (clone SK1, BD Biosciences). PMA/ionomycin-stimulated cells were surface stained for CD3 only. Cells were then washed, incubated for 30 min on ice in Fix/Perm buffer (eBioscience), washed again and incubated for 30 min with the following mAbs against intracellular cytokines in permeabilization buffer (eBioscience): Granzyme B FITC (clone GB11) and IFNγ PECy7 (clone 4S.B3) (all Biolegend). After a final wash step, cells were resuspended in PBS containing 1% paraformaldehyde and read on a CyAn ADP 9-color flow cytometer (Dako/Beckman Coulter). The gating strategy for lymphocyte subsets and representative plots for cytokine staining are shown in Figure S1.
2.5 Enzyme-linked immune sorbent assay (ELISA)
Granzyme B concentrations in the supernatant after 20h of PBMC stimulation were determined by ELISA (Mabtech), according to the manufacturer’s recommendations, and standard curves included on each plate for quantification.
2.6 Statistical analysis
Statistical analysis was performed using GraphPad Prism v5 software. Inter-group comparisons were analyzed by non-parametric Mann-Whitney U test, while paired samples were compared by Wilcoxon matched-pairs signed rank test.
2.7 Ethics approval
This study was conducted according to the principles outlined in the Declaration of Helsinki. Ethical approval to conduct the field study in Malian children was obtained from the ethical committee of the Faculty of Medicine, Pharmacy and Odonto-Stomatology at the University of Science, Techniques and Technologies of Bamako (approval number 2011-58/FMPOS). Written informed consent was obtained from parents or legal guardians who consented on behalf of their children in the Malian cohort.
3. Results
3.1 Malian children show Th1 and cytotoxic innate responses to PfRBC
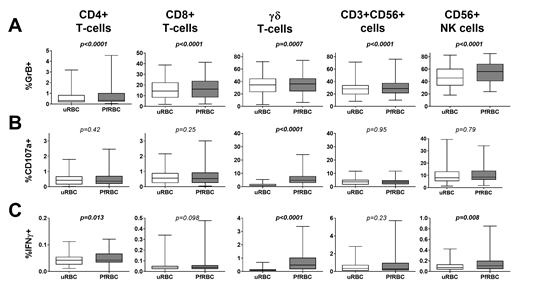
Cellular responses were assessed in 61 Malian children by flow cytometry following in vitro stimulation of PBMC with PfRBC for 24 hours. PfRBC stimulation significantly increased Granzyme B production by all adaptive and innate lymphocyte subsets compared to control uRBC-stimulated cultures (Figure 1A), in line with enhanced Granzyme B secretion into the supernatant (median with IQR: uRBC 542.3 pg/mL (344.1-748.1); PfRBC 579.9 pg/mL (369.7-1131); p<0.0001). Malian children further showed PfRBC-specific γδ T-cell degranulation (p<0.0001) (Figure 1B) and IFNγ production (p<0.0001) (Figure 1C).
Figure 1: Comparison of uRBC and PfRBC-induced cellular responses in Malian children. PBMCs were stimulated with uRBCs or PfRBC for 24h. Degranulation and cytokine production were assessed by flow cytometry. Data is presented for as whisker box plots, with boxes indicating the median and IQR, and whiskers the min and max responses for n=61 Malian children. PfRBC and uRBC responses were compared by Wilcoxon matched-pairs signed rank test.
3.2 P. falciparum infections at microscopic and submicroscopic parasite density affect innate cell responses to PfRBC
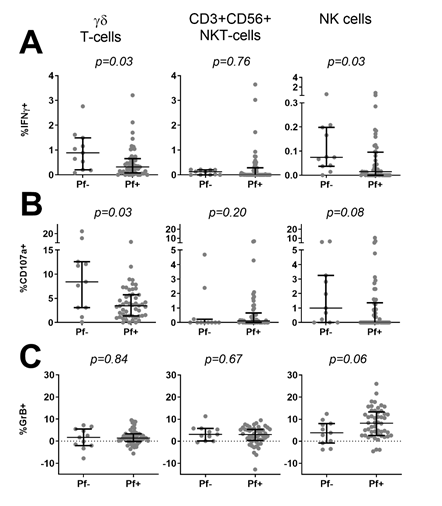
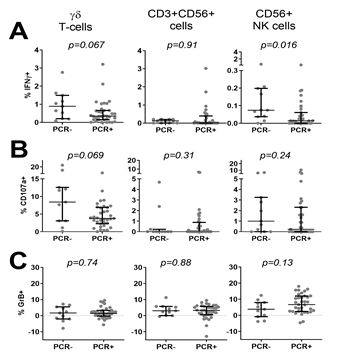
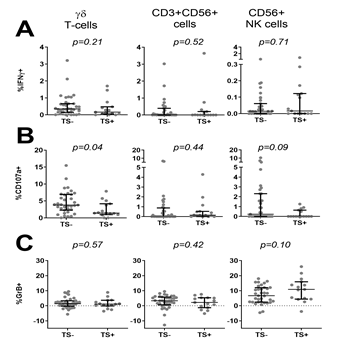
We next asked whether ongoing infection and parasite density at the time of PBMC collection affected innate responses to PfRBC. Children were divided into parasite-positive (n=51 Pf+) or negative (n=11 Pf-) based on PCR and thick smear data. Compared to Pf- children, Pf+ children showed significantly lower IFNγ production by γδ T-cells (p=0.03) and NK cells (p=0.03) in response to PfRBC (Figure 2A), a significantly lower frequency of degranulating CD107a+ γδ T-cells (p=0.03) and a similar trend for NK cells (p=0.08) (Figure 2B). On the other hand, Pf+ children showed a trend for increased Granzyme B content of NK cells (p=0.06) (Figure 2C). Responses by CD3+CD56+ cells did not differ between the two groups (Figure 2). Reduced lower IFNγ production by NK cells (p=0.016) (Figure 3A) as well as a trend for lower IFNγ production (p=0.067) and degranulation (p=0.069) by γδ T-cells (Figure 3B) in response to PfRBC was already evident when children only carried parasites at submicroscopic levels. To further investigate the effect of parasite density, PCR positive children were further subdivided based on thick smear (TS) results into low (submicroscopic infection only, TS-) and high parasite densities (patent infection, TS+). There was no significant difference in IFNγ production between TS+ and TS- children (Figure 4A). However, children with high parasite densities showed a significant reduction in degranulating CD107a+ γδ T-cells relative to their low parasite density counterparts (p=0.04), and a similar trend for NK cells (p=0.09) (Figure 4B). NK cell Granzyme B content on the other hand showed a trend for higher levels in children with a positive compared to negative PCR (p=0.13) (Figure 3B) and in TS+ compared to TS- children amongst those with PCR detectable parasitaemia (p=0.1) (Figure 4C).
Figure 1: Comparison of uRBC and PfRBC-induced cellular responses in Malian children. PBMCs were stimulated with uRBCs or PfRBC for 24h. Degranulation and cytokine production were assessed by flow cytometry. Data is presented for as whisker box plots, with boxes indicating the median and IQR, and whiskers the min and max responses for n=61 Malian children. PfRBC and uRBC responses were compared by Wilcoxon matched-pairs signed rank test.
Figure 3: Comparison of PfRBC-specific innate responses based on submicroscopic parasite prevalence at time of blood collection. PBMC from 46 thick smear negative Malian children were stimulated with either PfRBC or uRBC for 24h. (A) IFNγ production, (B) de-granulation assessed by CD107a expression and (C) Granzyme B content were assessed by flow cytometry. Parasite specific responses were calculated by subtraction of uRBC background responses. Data is presented for each individual donor (grey dots) and as median with IQR (black error bars) for n=11 children who were qPCR negative (PCR-) and n=35 children who were qPCR positive (PCR+) at the time of blood collection for immunological analysis. Groups were compared by Mann-Whitney U test.
Figure 4: Comparison of PfRBC-specific innate responses based on parasite density at time of blood collection. PBMC from 50 PCR+ Malian children were stimulated with either PfRBC or uRBC for 24h. (A) IFNγ production, (B) de-granulation assessed by CD107a expression and (C) Granzyme B content were assessed by flow cytometry. Parasite specific responses were calculated by subtraction of uRBC background responses. Data is presented for each individual donor (grey dots) and as median with IQR (black error bars) for n=35 children who were thick smear negative (TS-) and n=15 children who were thick smear positive (TS+) at the time of blood collection for immunological analysis. Groups were compared by Mann-Whitney U test.
3.3 Innate and adaptive responses to PfRBC do not correlate with incidence of clinical disease during follow-up
Finally, we assessed whether we could find any evidence to support prior findings by others that the level of cellular responses at the start of the transmission season associated with incidence of subsequent clinical disease. Children who developed asymptomatic or symptomatic infection during follow-up did not differ markedly in demographic or parasitological parameters at time of blood collection, except for a trend for higher parasite load in thick smear positive children amongst those developing symptomatic disease during follow-up compared to those that did not become symptomatic (p=0.066, Table 1). There was, however, no significant difference at the start of the transmission season between these two groups based on PfRBC-specific degranulation, IFNγ and Granzyme B production by any lymphocyte subset (Table S1).
4. Discussion
In this study, we assessed the impact of ongoing parasitaemia on innate immune cell responses to PfRBC. Ongoing parasitaemia in Malian children associated with reduced IFNγ and degranulation responses to PfRBC by γδ T-cells and reduced NK cell IFNγ responses. In a previous study, Tanzanian adults also showed much weaker IFNγ responses to PfRBC in a side-by-side comparison with Dutch adults both prior to and after a controlled human malaria infection [60]. This indicates that rather than age, prior repeated exposure to P. falciparum might be a driving factor for these impaired innate responses. Impaired pro-inflammatory cytokine production by innate cells during infection may be beneficial to the host by limiting the inflammatory response and thus promoting disease tolerance [61]. Indeed, lower levels of IFNγ and TNFα co-production by Vδ2 γδ T-cells are associated with a decreased likelihood to remain asymptomatic during an episode of P. falciparum infections in the following year in a Ugandan child cohort [32, 58]. In contrast, in the present study cytokine production or degranulation responses by γδ T-cells or other innate lymphocytes do not differ between children developing clinical symptoms or not during a malaria episode during follow-up. One contributing factor may be the definition of clinical immunity, which is more stringent in our study, as children were only considered asymptomatic if they did not experience any clinical episode during follow-up (as opposed to at least one asymptomatic episode in a previous study [58]). Another contributing factor may be the lower malaria incidence and hence prior malaria exposure in the area of the current study compared to the Ugandan child cohort [32, 58].
A small cohort study has previously reported that γδ T-cell cytokine production and proliferation are reduced during acute P. falciparum infection [62]. The four travelers examined in this study, however, had microscopically detectable parasites. Our study suggests that already submicroscopic parasitaemia can have a significant effect on innate lymphocyte function, namely γδ T-cell and NK cell IFNγ production and γδ T-cell degranulation. This finding contrasts with the findings in a Ugandan childhood cohort, where no differences between uninfected children and those with submicroscopic parasitaemia were found [32]. This is particularly relevant since a sizable part of the population carries parasites at submicroscopic levels, even in low transmission areas [63-69]. In our cohort, the proportion of children carrying submicroscopic parasites levels at the start of the transmission season was greater than 80%. Our findings on γδ T-cells are in line with previous studies reporting impaired degranulation and pro-inflammatory cytokine production in response to PfRBC in Ugandan children, which increased with cumulative episodes of malaria [32, 35, 58]. While evidence for impaired γδ T-cell directly to PfRBCs upon repeated parasite exposure is thus accumulating, the underlying mechanism remains unclear. Next to up-regulation of inhibitory receptors such as Tim-3 [32], another possibility is activation of the PPARα pathway, which has been linked to γδ T-cell desensitization upon repeated purified phosphoantigen exposure in macaques [70, 71].
As for NK cells, little is known thus far about any changes to their ability to directly respond to PfRBC following repeated exposure or ongoing infectious with P. falciparum except that they are functionally impaired in severe compared to uncomplicated malaria [59]. Even malaria-naïve individuals already show a great variability in their NK cell response to PfRBC, which could be linked to differences in receptors relevant for recognition of or interaction with PfRBC. Donors with NK cells capable of reducing PfRBC growth in vitro have been shown to express higher levels of the RNA sensor MDA5 [46]. The kinetics of NK activation (based on CD69 expression) have further been tentatively linked to NK cell expression of the activating receptor NKp30, with higher baseline expression correlating with NK activation at lower parasitaemia levels following controlled human malaria infection [72]. Additionally, NKp30 was upregulated at peak parasitaemia after controlled human malaria infection compared to baseline [72]. Finally, NK cells can be inhibited by PfRBC through interaction of P. falciparum RIFIN proteins with the inhibitory receptor LILRB1 on NK cells [73-75]. It remains to be investigated, however, whether expression of MDA5, NKp30 or LILRB1 is modulated by repeated parasite exposure and could be linked to the impaired response to PfRBC as reported herein. While reduced pro-inflammatory responses of innate lymphocytes to the malaria parasite might be beneficial in promoting disease tolerance [61], impaired cytotoxic effector function of innate immune cells may also negatively impact on disease control, since cellular cytotoxicity by both NK cells and γδ T-cells do contribute to control parasite growth [33, 37, 38, 47, 48]. In the current study, we specifically focus on direct responses of innate lymphocytes to PfRBC, using isolated PBMC and non-immune human serum, while antibody-dependent responses including antibody-dependent cytotoxicity were not investigated. As for γδ T-cells, expression of CD16 increases with repeated malaria exposure [32, 35, 58]. Notably, CD16 expression is associated with poor responsiveness of γδ T-cells to phosphoantigens [76] and instead mediates antibody-dependent cytotoxicity [50, 76]. Indeed, γδ T-cells have been shown to mediate cytotoxicity against PfRBC opsonized by hyperimmune IgG [77]. Therefore, γδ T-cells cytotoxicity may be retained in malaria exposed individuals but shifted from responses directly induced by PfRBC-inherent factors such as phosphoantigens to antibody-dependent mechanisms. Similarly, while NK cells show a trend for reduced degranulation activity induced by direct PfRBC recognition in our study, evidence is accumulating that their ability to mediate antibody-dependent cytotoxicity remains unaffected or is even enhanced [78]. This was shown previously for NK cells that up-regulated PD-1 expression upon exposure to PfRBC in vitro and selectively lost only their ability to kill MHC class I negative target cells, but not antibody-opsonized targets [59]. Notably, the ability of these PD-1 expressing NK cells to become activated by or kill PfRBC was not evaluated. Moreover, a subset of adaptive CD56 negative NK cells with potent antibody-dependent cytotoxic capacity have been shown to expand with repeated malaria exposure and associated with protection from clinical malaria [79, 80]. These specific NK cells have only recently been reported and were not subject of the current study. Unlike for γδ T-cells and NK cells, IFNγ and degranulation responses of CD3+CD56+ cells in Malian children were not affected by infection or parasite density status of Malian children. CD3+CD56+ cells are often referred to as NKT-like cells; however, recent studies have shown that this CD3+CD56+ T-cell population consists of multiple different subpopulations with varying polarization and cytotoxic potential [81]. CD1d restricted NKT cells expressing an invariant TCR are actually rarer and only a small subset of CD3+CD56+ cells [82, 83]. In as how far function specifically of CD1d restricted NKT cells is affected by parasite carriage has not been addressed in this present study. Finally, impaired innate responses may also inhibit induction of protective adaptive memory responses since specifically γδ T-cells are known for a variety of immune effector functions beyond cytokine production or target cell killing. These activities include the promotion of adaptive immune responses by antigen-presentation to CD4+ and CD8+ T-cells [84], which may also play a role in Plasmodium infection [85, 86]. Future studies are therefore needed to elucidate the influence of past or acute infections on a larger spectrum of γδ T-cell functions both in the presence and absence of parasite-specific antibodies, and the potential consequences for adaptive immunity.
5. Conclusion
In conclusion, ongoing P. falciparum-infection in Malian children impaired γδ T-cells and NK cell IFNγ production and γδ T-cells degranulation in direct response to PfRBC. For NK cells, these effects were already observed at submicroscopic parasite densities.
Abbreviations
IFN: Interferon
NK: Natural killer cell
PBMC: Peripheral blood mononuclear cell
Pf: Plasmodium falciparum
PfRBC: Plasmodium falciparum infected red blood cell
uRBC: uninfected red blood cell
Declarations
Consent for publication: Not applicable.
Availability of data and Materials: The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.
Competing Interests: The authors declare they have no competing interests.
Funding:
The field study and MD were funded by the APRIORI (African Poverty Related Infection Oriented Research Initiative, project number W.07.05.203.00) programme, subsidized by WOTRO Science for Global Development, a subdivision of The Netherlands Organization of Scientific Research (NWO). BK has received an EDCTP (European and Developing Countries Clinical Trial Partnership) senior fellowship. EDCTP project TA.2010.40200.007 enabled the establishment of the study site. The funders had no role in study design, data collection, analysis and interpretation, decision to publish, or writing of the manuscript.
Authors’ Contributions: MD, CA and NO conducted the experiments; MD, BK, CA and AS planned the experiments and analyzed the data; MD, BK, OD, and RS designed and supervised the field study; MD, BK, CA, KN, YK, SS, BO and SA performed the field studies and collected samples and clinical data; MD, BK, OD, RS, and AS interpreted the data and wrote the manuscript. All authors reviewed and approved the manuscript.
Acknowledgements
We acknowledge all Malian children and their families who made this study possible.
References
- World malaria report 2022. World Health Organization (2022).
- Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev 22 (2009): 13-36.
- Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol 9 (2008): 725-32.
- Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol 28 (2006): 51-60.
- Tran TM, Li S, Doumbo S, Doumtabe D, Huang CY, Dia S, et al. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis 57 (2013): 40-7.
- Gonzales SJ, Reyes RA, Braddom AE, Batugedara G, Bol S, Bunnik EM. Naturally Acquired Humoral Immunity Against Plasmodium falciparum Malaria. Front Immunol 11 (2020): 594653.
- Struik SS, Riley EM. Does malaria suffer from lack of memory? Immunol Rev 201 (2004): 268-90.
- Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med 172 (1990): 1633-41.
- Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature 192 (1961): 733-7.
- Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg 45 (1991): 297-308.
- Hoffman SL, Oster CN, Mason C, Beier JC, Sherwood JA, Ballou WR, et al. Human lymphocyte proliferative response to a sporozoite T cell epitope correlates with resistance to falciparum malaria. J Immunol 142 (1989): 1299-303.
- Todryk SM, Bejon P, Mwangi T, Plebanski M, Urban B, Marsh K, et al. Correlation of memory T cell responses against TRAP with protection from clinical malaria, and CD4 CD25 high T cells with susceptibility in Kenyans. PLoS One 3 (2008): e2027.
- Kurtis JD, Hollingdale MR, Luty AJ, Lanar DE, Krzych U, Duffy PE. Pre-erythrocytic immunity to Plasmodium falciparum: the case for an LSA-1 vaccine. Trends Parasitol 17 (2001): 219-23.
- Luty AJ, Lell B, Schmidt-Ott R, Lehman LG, Luckner D, Greve B, et al. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis 179 (1999): 980-8.
- Moormann AM, Sumba PO, Chelimo K, Fang H, Tisch DJ, Dent AE, et al. Humoral and cellular immunity to Plasmodium falciparum merozoite surface protein 1 and protection from infection with blood-stage parasites. J Infect Dis 208 (2013): 149-58.
- Olotu A, Moris P, Mwacharo J, Vekemans J, Kimani D, Janssens M, et al. Circumsporozoite-specific T cell responses in children vaccinated with RTS, S/AS01E and protection against P falciparum clinical malaria. PLoS One 6 (2011): e25786.
- Ndungu FM, Mwacharo J, Kimani D, Kai O, Moris P, Jongert E, et al. A statistical interaction between circumsporozoite protein-specific T cell and antibody responses and risk of clinical malaria episodes following vaccination with RTS,S/AS01E. PLoS One 7 (2012): e52870.
- Bergmann ES, Ballou RW, Krzych U. Detection of CD4+CD45RO+ T lymphocytes producing IL-4 in response to antigens on Plasmodium falciparum erythrocytes: an in vitro correlate of protective immunity induced with attenuated Plasmodium falciparum sporozoites. Cell Immunol 180 (1997): 143-52.
- Gitau EN, Tuju J, Karanja H, Stevenson L, Requena P, Kimani E, et al. CD4+ T cell responses to the Plasmodium falciparum erythrocyte membrane protein 1 in children with mild malaria. J Immunol 192 (2014): 1753-61.
- Moris P, Jongert E, van der Most RG. Characterization of T-cell immune responses in clinical trials of the candidate RTS, S malaria vaccine. Hum Vaccin Immunother 14 (2018): 17-27.
- McCall MB, Hopman J, Daou M, Maiga B, Dara V, Ploemen I, et al. Early interferon-gamma response against Plasmodium falciparum correlates with interethnic differences in susceptibility to parasitemia between sympatric Fulani and Dogon in Mali. J Infect Dis 201 (2010): 142-52.
- Deloron P, Chougnet C, Lepers JP, Tallet S, Coulanges P. Protective value of elevated levels of gamma interferon in serum against exoerythrocytic stages of Plasmodium falciparum. J Clin Microbiol 29 (1991): 1757-60.
- McCall MB, Sauerwein RW. Interferon-gamma--central mediator of protective immune responses against the pre-erythrocytic and blood stage of malaria. J Leukoc Biol 88 (2010): 1131-43.
- D' Ombrain MC, Robinson LJ, Stanisic DI, Taraika J, Bernard N, Michon P, et al. Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis 47 (2008): 1380-7.
- Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis 185 (2002): 971-9.
- Robinson LJ, D'Ombrain MC, Stanisic DI, Taraika J, Bernard N, Richards JS, et al. Cellular tumor necrosis factor, gamma interferon, and interleukin-6 responses as correlates of immunity and risk of clinical Plasmodium falciparum malaria in children from Papua New Guinea. Infect Immun 77 (2009): 3033-43.
- D'Ombrain MC, Hansen DS, Simpson KM, Schofield L. gammadelta-T cells expressing NK receptors predominate over NK cells and conventional T cells in the innate IFN-gamma response to Plasmodium falciparum malaria. Eur J Immunol 37 (2007): 1864-73.
- Newman KC, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol 7 (2007): 279-91.
- Artavanis-Tsakonas K, Eleme K, McQueen KL, Cheng NW, Parham P, Davis DM, et al. Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J Immunol 171 (2003): 5396-405.
- Artavanis-Tsakonas K, Riley EM. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol 169 (2002): 2956-63.
- Teirlinck AC, McCall MB, Roestenberg M, Scholzen A, Woestenenk R, de Mast Q, et al. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog 7 (2011): e1002389.
- Jagannathan P, Lutwama F, Boyle MJ, Nankya F, Farrington LA, McIntyre TI, et al. Vδ2+ T cell response to malaria correlates with protection from infection but is attenuated with repeated exposure. Sci Rep 7 (2017): 11487.
- Bottger E, Multhoff G, Kun JF, Esen M. Plasmodium falciparum-infected erythrocytes induce granzyme B by NK cells through expression of host-Hsp70. PLoS One 7 (2012): e33774.
- Korbel DS, Newman KC, Almeida CR, Davis DM, Riley EM. Heterogeneous human NK cell responses to Plasmodium falciparum-infected erythrocytes. J Immunol 175 (2005): 7466-73.
- Farrington LA, Jagannathan P, McIntyre TI, Vance HM, Bowen K, Boyle MJ, et al. Frequent malaria drives progressive Vdelta2 T cell loss, dysfunction, and CD16 upregulation during early childhood. J Infect Dis (2015).
- Costa G, Loizon S, Guenot M, Mocan I, Halary F, de Saint-Basile G, et al. Control of Plasmodium falciparum erythrocytic cycle: gammadelta T cells target the red blood cell-invasive merozoites. Blood 118 (2011): 6952-62.
- Farouk SE, Mincheva-Nilsson L, Krensky AM, Dieli F, Troye-Blomberg M. Gamma delta T cells inhibit in vitro growth of the asexual blood stages of Plasmodium falciparum by a granule exocytosis-dependent cytotoxic pathway that requires granulysin. Eur J Immunol 34 (2004): 2248-56.
- Troye-Blomberg M, Worku S, Tangteerawatana P, Jamshaid R, Soderstrom K, Elghazali G, et al. Human gamma delta T cells that inhibit the in vitro growth of the asexual blood stages of the Plasmodium falciparum parasite express cytolytic and proinflammatory molecules. Scand J Immunol 50 (1999): 642-50.
- Hernández-Castañeda MA, Happ K, Cattalani F, Wallimann A, Blanchard M, Fellay I, et al. γδ T Cells Kill Plasmodium falciparum in a Granzyme- and Granulysin-Dependent Mechanism during the Late Blood Stage. J Immunol 204 (2020): 1798-809.
- Odera DO, Tuju J, Mwai K, Nkumama IN, Fürle K, Chege T, et al. Anti-merozoite antibodies induce natural killer cell effector function and are associated with immunity against malaria. Sci Transl Med 15 (2023): eabn5993.
- Behr C, Dubois P. Preferential expansion of V gamma 9 V delta 2 T cells following stimulation of peripheral blood lymphocytes with extracts of Plasmodium falciparum. Int Immunol 4 (1992): 361-6.
- Behr C, Poupot R, Peyrat MA, Poquet Y, Constant P, Dubois P, et al. Plasmodium falciparum stimuli for human gammadelta T cells are related to phosphorylated antigens of mycobacteria. Infect Immun 64 (1996): 2892-6.
- Guenot M, Loizon S, Howard J, Costa G, Baker DA, Mohabeer SY, et al. Phosphoantigen Burst upon Plasmodium falciparum Schizont Rupture Can Distantly Activate Vgamma9Vdelta2 T Cells. Infect Immun 83 (2015): 3816-24.
- Ho M, Tongtawe P, Kriangkum J, Wimonwattrawatee T, Pattanapanyasat K, Bryant L, et al. Polyclonal expansion of peripheral gamma delta T cells in human Plasmodium falciparum malaria. Infect Immun 62 (1994): 855-62.
- Mavoungou E, Held J, Mewono L, Kremsner PG. A Duffy binding-like domain is involved in the NKp30-mediated recognition of Plasmodium falciparum-parasitized erythrocytes by natural killer cells. J Infect Dis 195 (2007): 1521-31.
- Ye W, Chew M, Hou J, Lai F, Leopold SJ, Loo HL, et al. Microvesicles from malaria-infected red blood cells activate natural killer cells via MDA5 pathway. PLOS Pathogens 14 (2018): e1007298.
- Arora G, Hart GT, Manzella-Lapeira J, Doritchamou JY, Narum DL, Thomas LM, et al. NK cells inhibit Plasmodium falciparum growth in red blood cells via antibody-dependent cellular cytotoxicity. Elife (2018): 7.
- Hart GT, Tran TM, Theorell J, Schlums H, Arora G, Rajagopalan S, et al. Adaptive NK cells in people exposed to Plasmodium falciparum correlate with protection from malaria. J Exp Med 216 (2019): 1280-90.
- Damelang T, Aitken EH, Hasang W, Lopez E, Killian M, Unger HW, et al. Antibody mediated activation of natural killer cells in malaria exposed pregnant women. Scientific Reports 11 (2021): 4130.
- He X, Liang H, Hong K, Li H, Peng H, Zhao Y, et al. The potential role of CD16+ Vgamma2Vdelta2 T cell-mediated antibody-dependent cell-mediated cytotoxicity in control of HIV type 1 disease. AIDS Res Hum Retroviruses 29 (2013): 1562-70.
- Lafont V, Liautard J, Liautard JP, Favero J. Production of TNF-alpha by human V gamma 9V delta 2 T cells via engagement of Fc gamma RIIIA, the low affinity type 3 receptor for the Fc portion of IgG, expressed upon TCR activation by nonpeptidic antigen. J Immunol 166 (2001): 7190-9.
- McCall MB, Roestenberg M, Ploemen I, Teirlinck A, Hopman J, de Mast Q, et al. Memory-like IFN-gamma response by NK cells following malaria infection reveals the crucial role of T cells in NK cell activation by P. falciparum. Eur J Immunol 40 (2010): 3472-7.
- Roussilhon C, Agrapart M, Ballet JJ, Bensussan A. T lymphocytes bearing the gamma delta T cell receptor in patients with acute Plasmodium falciparum malaria. J Infect Dis 162 (1990): 283-5.
- Roussilhon C, Agrapart M, Guglielmi P, Bensussan A, Brasseur P, Ballet JJ. Human TcR gamma delta+ lymphocyte response on primary exposure to Plasmodium falciparum. Clin Exp Immunol 95 (1994): 91-7.
- Hviid L, Kurtzhals JA, Dodoo D, Rodrigues O, Ronn A, Commey JO, et al. The gamma/delta T-cell response to Plasmodium falciparum malaria in a population in which malaria is endemic. Infect Immun 64 (1996): 4359-62.
- Cairo C, Longinaker N, Cappelli G, Leke RGF, Ondo MM, Djokam R, et al. Cord blood Vγ2Vδ2 T cells provide a molecular marker for the influence of pregnancy-associated malaria on neonatal immunity. The Journal of infectious diseases 209 (2014): 1653-62.
- Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol 184 (2010): 6043-52.
- Jagannathan P, Kim CC, Greenhouse B, Nankya F, Bowen K, Eccles-James I, et al. Loss and dysfunction of Vdelta2 (+) gammadelta T cells are associated with clinical tolerance to malaria. Sci Transl Med 6 (2014): 251ra117.
- Moebius J, Guha R, Peterson M, Abdi K, Skinner J, Li S, et al. PD-1 Expression on NK Cells in Malaria-Exposed Individuals Is Associated with Diminished Natural Cytotoxicity and Enhanced Antibody-Dependent Cellular Cytotoxicity. Infect Immun 88 (2020).
- Obiero JM, Shekalaghe S, Hermsen CC, Mpina M, Bijker EM, Roestenberg M, et al. Impact of malaria preexposure on antiparasite cellular and humoral immune responses after controlled human malaria infection. Infect Immun 83 (2015): 2185-96.
- Wolf AS, Sherratt S, Riley EM. NK Cells: Uncertain Allies against Malaria. Front Immunol 8 (2017): 212.
- Martini F, Paglia MG, Montesano C, Enders PJ, Gentile M, Pauza CD, et al. V gamma 9V delta 2 T-cell anergy and complementarity-determining region 3-specific depletion during paroxysm of nonendemic malaria infection. Infect Immun 71 (2003): 2945-9.
- Vallejo AF, Chaparro PE, Benavides Y, Alvarez A, Quintero JP, Padilla J, et al. High prevalence of sub-microscopic infections in Colombia. Malar J 14 (2015): 201.
- Golassa L, Enweji N, Erko B, Aseffa A, Swedberg G. Detection of a substantial number of sub-microscopic Plasmodium falciparum infections by polymerase chain reaction: a potential threat to malaria control and diagnosis in Ethiopia. Malar J 12 (2013): 352.
- Imwong M, Nguyen TN, Tripura R, Peto TJ, Lee SJ, Lwin KM, et al. The epidemiology of subclinical malaria infections in South-East Asia: findings from cross-sectional surveys in Thailand-Myanmar border areas, Cambodia, and Vietnam. Malar J 14 (2015): 381.
- Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 12 (2014): 833-40.
- Okell LC, Bousema T, Griffin JT, Ouedraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun 3 (2012): 1237.
- Tadesse FG, van den Hoogen L, Lanke K, Schildkraut J, Tetteh K, Aseffa A, et al. The shape of the iceberg: quantification of submicroscopic Plasmodium falciparum and Plasmodium vivax parasitaemia and gametocytaemia in five low endemic settings in Ethiopia. Malaria Journal 16 (2017): 99.
- Rek J, Katrak S, Obasi H, Nayebare P, Katureebe A, Kakande E, et al. Characterizing microscopic and submicroscopic malaria parasitaemia at three sites with varied transmission intensity in Uganda. Malar J 15 (2016): 470.
- Sicard H, Ingoure S, Luciani B, Serraz C, Fournié JJ, Bonneville M, et al. In vivo immunomanipulation of V gamma 9V delta 2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol 175 (2005): 5471-80.
- Poupot M, Boissard F, Betous D, Bardouillet L, Fruchon S, L'Faqihi-Olive F, et al. The PPARα pathway in Vγ9Vδ2 T cell anergy. Cell Mol Biol Lett 19 (2014): 649-58.
- Walk J, Sauerwein RW. Activatory Receptor NKp30 Predicts NK Cell Activation During Controlled Human Malaria Infection. Front Immunol 10 (2019): 2864.
- Harrison TE, Mørch AM, Felce JH, Sakoguchi A, Reid AJ, Arase H, et al. Structural basis for RIFIN-mediated activation of LILRB1 in malaria. Nature 587 (2020): 309-12.
- Chew M, Ye W, Omelianczyk RI, Pasaje CF, Hoo R, Chen Q, et al. Selective expression of variant surface antigens enables Plasmodium falciparum to evade immune clearance in vivo. Nat Commun 13 (2022): 4067.
- Saito F, Hirayasu K, Satoh T, Wang CW, Lusingu J, Arimori T, et al. Immune evasion of Plasmodium falciparum by RIFIN via inhibitory receptors. Nature 552 (2017): 101-5.
- Angelini DF, Borsellino G, Poupot M, Diamantini A, Poupot R, Bernardi G, et al. FcgammaRIII discriminates between 2 subsets of Vgamma9Vdelta2 effector cells with different responses and activation pathways. Blood 104 (2004): 1801-7.
- Farrington LA, Callaway PC, Vance HM, Baskevitch K, Lutz E, Warrier L, et al. Opsonized antigen activates Vδ2+ T cells via CD16/FCγRIIIa in individuals with chronic malaria exposure. PLOS Pathogens 16 (2020): e1008997.
- Goodier MR, Wolf AS, Riley EM. Differentiation and adaptation of natural killer cells for anti-malarial immunity. Immunol Rev 293 (2020): 25-37.
- Ty M, Sun S, Callaway PC, Rek J, Press KD, van der Ploeg K, et al. Malaria-driven expansion of adaptive-like functional CD56-negative NK cells correlates with clinical immunity to malaria. Sci Transl Med 15 (2023): eadd9012.
- Forconi CS, Oduor CI, Oluoch PO, Ong'echa JM, Münz C, Bailey JA, et al. A New Hope for CD56(neg)CD16(pos) NK Cells as Unconventional Cytotoxic Mediators: An Adaptation to Chronic Diseases. Front Cell Infect Microbiol 10 (2020): 162.
- Romero-Olmedo AJ, Schulz AR, Huber M, Brehm CU, Chang H-D, Chiarolla CM, et al. Deep phenotypical characterization of human CD3+CD56+ T cells by mass cytometry. European Journal of Immunology 51 (2021): 672-81.
- Lenart M, Pyrc K, Siedlar M. Can we define CD3(+)CD56(+) cells as NKT cells with impunity? Clin Immunol 226 (2021): 108708.
- Le Dieu R, Taussig D, MacDougal F, Lister A, Gribben JG. CD3+/CD56+ Cells, but Not Natural Killer T Cells, Are Increased in Peripheral Blood of Untreated Patients with Leukemia. Blood 110 (2007): 1815.
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol 13 (2013): 88-100.
- Inoue S, Niikura M, Takeo S, Mineo S, Kawakami Y, Uchida A, et al. Enhancement of dendritic cell activation via CD40 ligand-expressing gammadelta T cells is responsible for protective immunity to Plasmodium parasites. Proc Natl Acad Sci USA 109 (2012): 12129-34.
- Howard J, Loizon S, Tyler CJ, Duluc D, Moser B, Mechain M, et al. The Antigen-Presenting Potential of Vγ9Vδ2 T Cells During Plasmodium falciparum Blood-Stage Infection. The Journal of infectious diseases 215 (2017): 1569-79.
- Sagara I, Rulisa S, Mbacham W, Adam I, Sissoko K, Maiga H, et al. Efficacy and safety of a fixed dose artesunate-sulphamethoxypyrazine-pyrimethamine compared to artemether-lumefantrine for the treatment of uncomplicated falciparum malaria across Africa: a randomized multi-centre trial. Malar J 8 (2009): 63.
- Daou M, Kouriba B, Ouedraogo N, Diarra I, Arama C, Keita Y, et al. Protection of Malian children from clinical malaria is associated with recognition of multiple antigens. Malar J 14 (2015): 56.
Supplementary Informations
Table S1: Relationship between PfRBC re-stimulated responses and clinical disease during follow-up
|
Cellular |
CD4+ |
CD8+ |
γδT |
CD3+CD56+ |
CD56+ NK |
|
|
responsesa |
||||||
|
IFNγd |
Symptb |
0.008 (0.0-0.02) |
0.004 (0.0-0.015) |
0.39 (0.09-0.96) |
0.0007 (0.0-0.15) |
0.024 (0.0-0.09) |
|
Asymptc |
0.007 (0.0-0.017) |
0.008 (0.0-0.022) |
0.31 (0.15-0.72) |
0.017 (0.0-0.39) |
0.029 (0.0-0.18) |
|
|
p= 0.74 |
p= 0.33 |
p= 0.91 |
p= 0.43 |
p= 0.47 |
||
|
CD107ad |
Sympt |
0.052 (0.0-0.14) |
0.05 (0.0-0.23) |
3.77 (1.42-7.28) |
0.02 (0.0-0.54) |
0.23 (0.0-1.59) |
|
Asympt |
0.012 (0.0-0.07) |
0.015 (0.0-0.16) |
3.33 (1.37-6.50) |
0.05 (0.0-0.81) |
0.11 (0.0-2.11) |
|
|
p= 0.35 |
p= 0.44 |
p= 0.70 |
p= 0.80 |
p= 0.71 |
||
|
GrzBd |
Sympt |
0.12 (-0.024-0.19) |
1.2 (0.30-1.95) |
2.06 (-0.22-4.05) |
4.7 (0.35-6.2) |
8.5 (2.9-12.8) |
|
Asympt |
0.04 (-0.015-0.15) |
0.9 (0.09 -2.85) |
1.2 (-1.38-3.35) |
2.2 (0.13-4.9) |
4.9 (2.6-10.8) |
|
|
p= 0.13 |
p= 0.87 |
p= 0.49 |
p= 0.095 |
P= 0.30 |
- In 61 Malian children early in the transmission season (July 2012)
- n=33 children becoming symptomatic after exposure confirmed by thick smear or PCR
- n=28 children remaining asymptomatic despite exposure confirmed by thick smear or PCR
- PfRBC-specific responses (shown as median percentage responding cells with IQR) were calculated by individual subtraction of uRBC background responses and analyzed by Mann-Whitney U test
Figure S1: Flow cytometry gating strategy. (A) PBMCs were sequentially gated to remove debris, doublets and dead cells. Viable lymphocytes were then distinguished into CD3-CD56+ NK cells, CD3+CD56+ cells and CD3+CD56- T-cells. CD3+CD56- T-cells were further subdivided based on presence or absence of Pan-γδTCR expression. Non-γδ T-cells were divided into CD4+ and CD8+ T-cells. (B) Representative plots are shown for cytokine production (Granzyme B, IFNγ) and degranulation (CD107a) by total CD3+ T-cells during 24h stimulation with uRBC or PfRBC, or 4h stimulation with PMA/ionomycin.