Pharmacokinetic Evaluation of Lansoprazole/sodium Bicarbonate capsule compared with Lansoprazole capsule in Healthy Chinese Adults
Article Information
Meihua Lin1,2, Yunliang Zheng1,2, You Zhai1,2, Chang Xu1,2, Minglan Wu1,2, Qian Huang1,2, Guolan Wu1,2, Jianzhong Shentu1,2, Jian Liu1,2*, Lihua Wu1,2*
1Research Center of Clinical Pharmacy, First Affliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
2Zhejiang Provincial Key Laboratory for Drug Evaluation and Clinical Research, China
*Corresponding Author(s): Jian Liu, Lihua Wu, Zhejiang Provincial Key Laboratory for Drug Evaluation and Clinical Research, Research Center of Clinical Pharmacy, First Affiliated Hospital, College of Medicine, Zhejiang University, #79 Qingchun Road, Hangzhou, 310003, China
Received: 21 October 2020; Accepted: 02 November 2020; Published: 27 November 2020
Citation: Meihua Lin, Yunliang Zheng, You Zhai, Chang Xu, Minglan Wu, Qian Huang, Guolan Wu, Jianzhong Shentu, Jian Liu, Lihua Wu. Pharmacokinetic Evaluation of Lansoprazole/sodium Bicarbonate capsule compared with Lansoprazole capsule in Healthy Chinese Adults. Journal of Pharmacy and Pharmacology Research 4 (2020): 139-148.
Share at FacebookAbstract
Abstract
Objective: This study was conducted to characterize the pharmacokinetic (PK) and safety profiles of the investigational immediate-released (IR) formulation containing lansoprazole/sodium bicarbonate (30/1100 mg) in healthy Chinese adult volunteers, as compared with the commercially available enteric-coated formulation of lansoprazole.
Methods: A single-dose, two-treatment, three-period, partial-replicate, cross-over study was conducted. Thirty qualified subjects were randomized to one of the following dosing sequences: TRR, RTR or RRT (T refers to the test drug and R is the reference) in equal numbers. A single dose of the T/R was administrated orally in fasted condition and the washout period was scheduled as 6 days. Pharmacokinetic and safety were assessed.
Results: It was observed that the PK parameter Tmax was shortened while the Cmax heightened (p<0.001) in the test product compared with reference formulation. The geometric mean ratio (GMR) of AUC0-t and AUC0-∞ was 1.1205 and 1.1118, respectively. 90% CIs for the GMR all fell within the range of 80% to 125%. Both formulations were well tolerated.
Conclusions: The two products were bioequivalent as to systemic exposure based on AUC0-t and AUC0-∞, while the IR formulation had a faster absorption and probably prompt onset of action.
Keywords
Pharmacokinetics; Safety; Immediate-released formulation; Lansoprazole/sodium bicarbonate
Pharmacokinetics articles; Safety articles; Immediate-released formulation articles; Lansoprazole/sodium bicarbonate articles
Article Details
Introduction
The proton pump inhibitors (PPIs) targeted the gastric H+, K+-ATPase represented a milestone in the treatment of gastro-oesophageal reflux disease (GERD), peptic ulcers as well as stress-related erosive syndrome. Since PPIs are acid-labile, they are generally administered orally in the form of capsules containing enteric-coated granules. The different enteric coatings are considered to be necessary to prevent the drugs from degradation in the acidic conditions within the stomach and increase the bioavailability, while the absorption of the PPIs into the systemic circulation is delayed [1].
In 2004, an immediate-released (IR) formulation containing omeprazole 20 mg and sodium bicarbonate 1100 mg (Zegerid), manufactured by Santarus Inc, USA, was approved by U.S. Food and Drug Administration for treatment of patients with various acid-related diseases as on-demand therapy. As to this formulation, the composition of sodium bicarbonate not only protects omeprazole from gastric acid degradation, but also acts as a neutralizer of gastric acid [2]. Therefore, it provided an improved, rapid symptom relief regardless of the accelerated absorption of omeprazole [3,4]. Referring to such development strategies an investigational drug that combines 30 mg of lansoprazole with 1100 mg of sodium bicarbonate in a gelatin capsule as an IR formulation has been developed, anticipating the potential clinical advantages in quick relief of the symptoms, convenience and better compliance, especially for long-term oral administration in acid-related disease patients. In accordance with NMPA guidelines, the bioavailability of any new product with the original market standard must be compared. Thus, a randomized, open-label, single-dose, two-treatment, three-period, three-sequence, partial-replicate, cross-over study was performed to identify the investigational drug's PK and safety profiles in healthy Chinese adult volunteers under fasting conditions. The objective was to show whether the bioavailability of the IR formulation was equivalent to that of the same doses of commercially available enteric-coated formulation of oral lansoprazole capsule, whereas the onset of action was quicker due to the shorter time to achieve maximum plasma concentration (Tmax) and higher maximum plasma concentration (Cmax).
Subjects and Methods
Study Drugs and Administration
The investigational IR capsule of lansoprazole (containing 30 mg of lansoprazole and 1100 mg of sodium bicarbonate) was manufactured by Beijing Xuze Pharmaceutical Co., Ltd., China (batch number 17100301, expiry date October, 2019). The active comparator lansoprazole enteric-coated capsule (containing 30 mg of lansoprazole) was a commercial product from Takeda Pharmaceutical Company Limited, Japan (batch number A104, expiry date September, 2018).
Subjects
Suitable Chinese healthy adults with a body mass index between 19 and26 kg/m2 were recruited at the Phase I Clinical Research Center of the first Affiliated Hospital of Zhejiang University in accordance with the Declaration of Helsinki and the Good Clinical Practice (GCP) guideline in April, 2018. Written informed consent forms were obtained before screening. Subjects were determined to be in good health based on medical history, vital signs, physical examination, laboratory tests, 12-lead electrocardiography (ECG) and chest X-ray. Inclusion and exclusion criteria are detailed at the service of the U.S. National Institutes of Health (https://www.clinicaltrials.gov), No. NCT03488173.
Study Design and Treatment
This study was a single center, randomized, open-label, single-dose, two-treatment, three-period, partial replicate pharmacokinetic study. The protocol was reviewed and approved by the ethics committee of the first Affiliated Hospital of Zhejiang University (approval No. 2017-EC-81). In this randomized open-label study, the bioavailability of IR capsule of lansoprazole (containing 30 mg of lansoprazole and 1100 mg of sodium bicarbonate) was compared with the bioavailability of commercially available enteric-coated oral formulation of lansoprazole (reference product). The dose of lansoprazole was selected at 30 mg according to the recent guidance for bioequivalence studies of oral lansoprazole.
Previous bioequivalence studies of oral lansoprazole formulations indicated that lansoprazole is a highly variable drug [5]. Thus, the reference-scaled average bioequivalence (RSABE) method was applied, and a partial-replicate, three-sequence, three-period, cross-over design was used, in which participants received the reference product twice [6,7]. Based on the randomization schedule, which was generated by using SAS version 9.4 (SAS Institute Inc, Cary, NC), subjects were assigned to one of three dosing sequences (TRR, RTR, or RRT, where T is the test drug and R is the reference) in equal numbers (10 cases). The dose in each of the three dosing periods was separated by a 6-day washout period.
The subjects were administrated orally with a single dose of either the T or R drug in a fasted condition. A total of 240 mL warm water was delivered to make sure the capsule was swallowed. Subjects were required to avoid lying down and keep fasted for the following 4 hours. Besidewater was prohibited for at least 2 hours after the drug was administered. The participants were ambulatory, and instructed to abstain from strenuous activity, smoking and alcohol, grape juice, caffeine or poppy-containing foods consumption, taking other medications during the whole study. The post-study safety assessment was performed for all of the subjects at the end of the third dosing (24h after the last dose).
Blood Collection and Bioanalytical Analysis
A total of 17 blood samples were collected during each period. Briefly, blood samples (4 mL) at predetermined intervals of 0, 0.17, 0.33, 0.5, 0.75, 1, 1.33, 1.67, 2, 2.5, 3, 3.5, 4, 6, 8, 12 and 16 h post dosing were drawn into coded, K2-EDTA tubes and centrifuged. Following, the plasma samples were separated and kept at -70±10 °C pending analysis. The whole procedure including blood samples collecting, processing and storage are light-proof.
Plasma concentration of lansoprazole was measured by a validated liquid chromatography with tandem mass spectrometry (LC–MS/MS) method, which was performed by Shanghai Xihua Scientific CO., Ltd. The sample analysts were blinded to the randomization. The validated concentration range for lansoprazole was 2.0 to 2000.0 ng/mL. The intra-assay and inter-assay coefficients of variation for precision and accuracy were 1.5%~4.3% and -9.8%~13.3%, respectively.
Safety Assessment
Safety assessment was performed as scheduled in the protocol. Any adverse events (AEs) would be collected and closely followed to satisfactory resolution by physicians. Severity of AEs was evaluated according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, and the relationship with study treatment was also assessed.
Pharmacokinetic and Statistical Analysis
PK parameters of lansoprazole were determined through a non-compartmental model analysis with Phoenix WinNonlin software, version 8.0 (Pharsight, Inc., CA, USA). The Cmax and Tmax were obtained directly from the observed data. AUC0–t was calculated using the linear trapezoidal linear interpolation. AUC0–∞ was calculated as the sum of AUC0–t and the extrapolated area determined by dividing the last quantifiable concentration (Ct) by the slope of the terminal log linear phase (Ke). The apparent total clearance of the drug from plasma after oral administration (Cl/F) was calculated as dose/AUC0–∞. The apparent volume of distribution (Vd/F) was based on the terminal elimination phase (Cl/F/Ke). The elimination half-life (t1/2) was calculated from the slope of the terminal log linear phase as Ln (2)/Ke. The relative bioavailability (F) of the tested formulation was calculated as follows: F= AUC0-t (test)/AUC0-t (reference)*100%.
In this study, AUC0-t and AUC0-∞ were considered primary variables for bioequivalence determination. Analysis of variance (ANOVA) was used to assess the effect of formulation, sequence, period, and subjects nested in sequence on natural logarithm (ln)-transformed pharmacokinetic parameters (AUC0-∞ and AUC0-t). Parametric 90% confidence intervals (CIs) for the geometric mean ratio (GMR) between the two formulations (test-reference) were determined. The RSABE method was used to compare IR capsule with the reference product in case of the intra-individual coefficient of variation for the reference product being 30% or more, otherwise, the unscaled average bioequivalence method was used. Bioequivalence was identified when the point estimate values of GMR fell within the range of 80% to 125%, and the 90% CIs fell into the bioequivalence range that scaled based on intra-individual variance (confirmed by the upper bound of 95% confidence interval of less or equal 0).
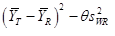
Statistical analysis was performed with the statistical software package SAS analysis system (V9.4) (SAS Institute Inc, Cary, North Carolina). The evaluation of bioequivalence was based on General Linear Model (GLM) and Howe first order approximation for PK Parameter with intra-subject coefficient of variation greater or equal 30%, mixed linear model (MIXED) for PK Parameter with intra-subject coefficient of variation less than 30%; the comparison of Tmax was based on paired non-parametric test.
Results
Baseline Characteristics of Subjects
As shown in figure 1, a total of 79 healthy Chinese adults were screened in April, 2018. Ultimately, 30 cases (18 males and 12 females) were enrolled and randomly assigned as described in “Methods”. One participant did not report to our center for the third treatment period due to traffic accident. The other 29 subjects completed all three study periods. Participants had a mean (SD) age of 26.0 (5.5) years (range, 20-43 y) and a mean (SD) body mass index of 22.4 (2.1) kg/m2 (range, 19.1-25.9 kg/m2). The mean (SD) height was 166.1 (7.0) cm (range, 154.1-180.1cm), and the mean (SD) weight was 62.0 (9.4) kg (range, 46.0-81.4 kg).
Pharmacokinetic Properties
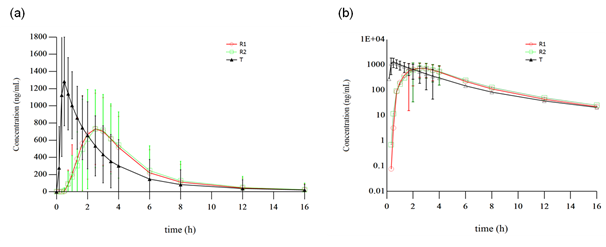
The mean plasma concentration–time profile of lansoprazole was shown in figure 2. One subject was found about her past history of bile reflux gastritis and finally was considered to be enrolled by protocol-violation, another subject withdrew due to the traffic accident before the third period of administration, there by 28 subjects were included in the pharmacokinetic analyses. Major pharmacokinetic parameters of lansoprazole are summarized in Table 1. Compared to the reference formulation, a significantly shortened Tmax with a higher Cmax (p<0.001) was observed in the test product.
AUC0-t and AUC0-∞ were the primary PK parameters for bioequivalence evaluation. The reference lansoprazole showed an intra-individual coefficient of variation of 24.64% and 23.68%, respectively for AUC0-t and AUC0-∞ in this study. Thus, the unscaled average bioequivalence method was used. Bioequivalence data are presented in Table 2. The GMR of AUC0-t and AUC0-∞ was 1.1205 and 1.1118, respectively. 90% CIs for the GMR were all within the range of 80% to 125%. Therefore, the two products were bioequivalent as to the extent of drug absorption or exposure.
Safety and Tolerability
A total of 31 AEs occurred in 18 subjects were recorded during the study. The vast majority of AEs (27/31, 87.1%) were identified through laboratory investigations, and were considered mild (grade 1), and spontaneously recovered without intervention. One subject reported transient diarrhea and abdominal distention as grade 1 AE, which was considered probably related to the drug and resolved without any medicine. But finally she was found to hide her past history of bile reflux gastritis. One subject experienced transient and mild dizzy when venous indwelling before the first treatment period, and it was unrelated to the drug. The incidence of AEs is summarized in Table 3. A serious adverse event was reported in one participant who happened to a traffic accident on the day just before the third treatment period, which was considered not related to the drug. He fully recovered after 1 month treatment in local hospital.
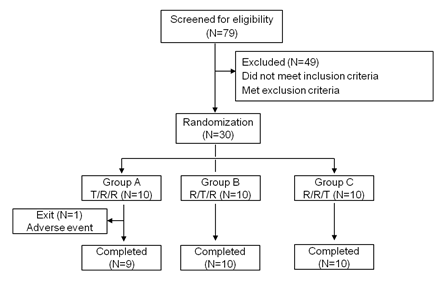
figure 1: Study design and disposition of subjects
figure 2: Mean plasma concentration-time profiles of lansoprazole obtained after single oral administration of the test (T) and reference (R) products in 30 healthy Chinese volunteers. (a) Linear scale, (b) Semilogarithmic scale. R1 refers to the first time the R product was received (N=30). R2 refers to the second time the R product was received (N=29).
Table 1: Pharmacokinetic parameters of lansoprazole
|
Parameter |
T (N=28) |
R1a (N=28) |
R2b (N=28) |
R |
|
Tmax, hc |
0.495 (0.167,1.66) |
2.49 (0.992,4.00) |
2.50 (0.999,4.00) |
2.49 (0.992,4.00) |
|
Cmax, ng/mL |
1510±489 |
972±431 |
1010±492 |
989±459 |
|
AUC0–t, ng·h/mL |
3710±3100 |
3350±2900 |
3460±2970 |
3410±2910 |
|
AUC0-∞, ng·h/mL |
3880±3580 |
3620±3380 |
3640±3440 |
3630±3380 |
|
t½, h |
1.71±1.28 |
1.60±1.11 |
1.60±1.11 |
1.60±1.11 |
|
Vd/F, L |
21.7±6.88 |
23.2±10.7 |
23.7±12.4 |
23.5±11.5 |
|
CL/F, L/h |
11.8±7.19 |
13.1±8.14 |
13.7±10.0 |
13.5±9.06 |
|
F (%) |
113±23.5 |
Data are presented as mean ±SD unless otherwise stated. aR1 refers to the first time the reference product was received. bR2 refers to the second time the reference product was received. cTmax are presented as median (minimum, maximum).
Table 2: GMR and the corresponding 90% CIs for the primary PK parameters of lansoprazole
|
Parameter |
Geometric mean (T) |
Geometric mean (R) |
GMR |
90% CI |
Intra-individual variationa |
|
|
CV (%) |
SWR |
|||||
|
AUC0-t, ng·h/mL |
2990 |
2670 |
1.1205 |
(1.0409, 1.2061) |
24.64 |
0.2428 |
|
AUC0-∞, ng·h/mL |
3140 |
2820 |
1.1118 |
(1.0299, 1.2003) |
23.68 |
0.2336 |
aThe intra-individual variation for receiving the reference product
Table 3: Total number of adverse events and percentage of healthy subjects experiencing adverse events in the study
|
Parameter |
T (N=30) |
R (N=30) |
|
Any adverse event |
6 (20.0%) |
12 (40.0%) |
|
Adverse event may relate to drug |
||
|
Serum triglyceride increased |
0 |
3 (10.0%) |
|
Bile acid increased |
5 (16.7%) |
4 (13.3%) |
|
Uric acid increased |
1 (3.3%) |
2 (6.6%) |
|
Leukocyte count increased |
1 (3.3%) |
1 (3.3%) |
|
Neutrophil count increased |
1 (3.3%) |
1 (3.3%) |
|
Positive urine leukocyte |
2 (6.6%) |
4 (13.3%) |
|
Positive urine protein |
0 |
1 (3.3%) |
|
Positive urine erythrocyte |
0 |
1 (3.3%) |
|
Diarrhea |
0 |
1 (3.3%) |
|
Abdominal distention |
0 |
1 (3.3%) |
|
Adverse event not relate to drug |
||
|
Dizzy |
0 |
1 (3.3%) |
|
Traffic accident |
0 |
1 (3.3%) |
Values were given as No (%).
Discussion
Although the conventional enteric-coated preparations of PPIs have been widely prescribed for GERD and other acid-related diseases, most patients did not achieve complete relief of symptoms after the first dose, partly owing to the delayed absorption. Efforts have been made to develop new formulations for accelerating absorption of PPIs and to potentially improve patient satisfaction and clinical outcomes with PPIs treatment.
In this study, pharmacokinetic characteristics of a new developed IR formulation and the reference enteric-coated formulation of oral lansoprazole capsule were compared under fasting condition. The PK parameters of the reference capsules from our study were similar to those previously reported in the literature with the half-life of lansoprazole being about 2 hours [8,9]. It was shown that the median of Tmax decreased from about 2.5 h after receiving the reference, to about 0.5h after administration of test IR formulation, which indicated the immediate release and rapid absorption properties of the investigational product. Meanwhile, mean of Cmax increased from 989 to 1510 ng/mL between two formulations, while AUC did not change significantly. Bioequivalence was established based on AUC0-t and AUC0-∞ values of lansoprazole. Therefore, the new introduced IR formulation resulted in a faster absorption and probably prompt onset of action without impairment of systemic exposure.
When the IR formulation was administered, Cmax increased by about 50% as compared to the conventional enteric-coated delay-released formulation, there might be some concerns about the safety of lansoprazole. To our knowledge, lansoprazole has a very wide range of dosage with good safety. Twice daily intravenous dosing of lansoprazole 30 mg may result in Cmax of around 2000 ng/mL, whereas no drug-related moderate or serious AE was reported [10]. In addition, we previously found that a single intravenously administration of 90 mg dexlansoprazole, which is R-(+)-enantiomer of lansoprazole, was also safe and well tolerated. In the present study, both formulations were well tolerated, with AEs of low incidence and mild severity.
Conclusion
In conclusion, the present study has clearly demonstrated that compared to the conventional enteric-coated lansoprazole formulation, the investigational IR formulation had a significantly faster absorption, whereas the two products were bioequivalent as to systemic exposure. Both formulations were safe and well tolerated.
Acknowledgements
The authors would like to thank all study participants, as well as clinical investigators, nurses, and study coordinators for their contribution to this study. We would also thank Shanghai Xihua Scientific CO., Ltd. for bioanalytical work and Junhe Technology CO., Ltd. for the pharmacokinetic and statistical analysis.
Author contributions
WLH and LJ were responsible for the study design, data acquisition, and data analysis, as well as writing and revising the article. LMH and ZYL participated in conducting the study as a sub-investigator, and LMH contributed to manuscript drafting. ZY, HQ, XC, WML conducted the study as investigators. STJZ contributed to study design and data analysis. WGL contributed to the quality control through the study. All authors have approved the final version of the text and agreed to be accountable for the work.
Compliance with Ethical Standards
This study was conducted in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study. Subjects were covered under the commercial insurance provided by the sponsor.
Conflict of interest
The authors have indicated that they have no conflicts of interest with regard to the content of this article.
Reference
- Fock KM, Ang TL, Bee LC, et al. Proton pump inhibitors: do differences in pharmacokinetics translate into differences in clinical outcomes?. Clinical Pharmacokinetics 47 (2008): 1-7.
- Howden CW. immediate-release proton-pump inhibitor therapy–potential advantages. Alimentary Pharmacology & Therapeutics 22 (2005): 25-30.
- Castell D, Bagin R, Goldlust B, et al. Comparison of the effects of immediate-release omeprazole powder for oral suspension and pantoprazole delayed-release tablets on nocturnal acid breakthrough in patients with symptomatic gastro-oesophageal reflux disease. Alimentary Pharmacology & Therapeutics 21 (2005): 1467-1474.
- Shin JM, Kim N. Pharmacokinetics and Pharmacodynamics of the Proton Pump Inhibitors. Journal of Neurogastroenterology and Motility 19 (2013): 25-35.
- Thota S, Khan SM, Tippabhotla SK, et al. Bioequivalence of two lansoprazole delayed release capsules 30 mg in healthy male volunteers under fasting, fed and fasting-applesauce conditions: a partial replicate crossover study design to estimate the pharmacokinetics of highly variable drugs. Drug Research 63 (2013): 551-557.
- Endrenyi L, Tothfalusi L. Bioequivalence for highly variable drugs: regulatory agreements, disagreements, and harmonization. Journal of Pharmacokinetics and Pharmacodynamics 46 (2019): 117-126.
- US Food and Drug Administration. Guidance for industry. Bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an Abbreviated New Drug Application. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM377465.pdf.
- Landes BD, Petite JP, Flouvat B. Clinical pharmacokinetics of lansoprazole. Clinical Pharmacokinetics 28 (1995): 458-470.
- Song M, Gao X, Hang TJ, et al. Pharmacokinetic properties of lansoprazole (30-mg enteric-coated capsules) and its metabolites: a single-dose, open-label study in healthy Chinese male subjects. Current Therapeutic Research 70 (2009): 228-239.
- Wu L, Liu J, Zheng Y, et al. Pharmacokinetic/Pharmacodynamic Evaluation of Dexlansoprazole Infusion Injection Compared with Lansoprazole in Healthy Chinese Adults. Clinical Drug Investigation 39 (2019): 953-965.