Pathophysiology of Hodgkin’s Lymphoma and Role of Immune System
Article Information
Kalsoom Abidi Malik, Zunaira Hameed*, Marvah Qiass
Institute of Microbiology and Molecular Genetics University of Punjab Lahore
*Corresponding author: Zunaira Hameed, Institute of Microbiology and Molecular Genetics University of Punjab Lahore, Pakistan
Received: 22 March 2022; Accepted: 05 April 2022; Published: 01 June 2022
Citation: Kalsoom Abidi Malik, Zunaira Hameed, Marvah Qiass. Pathophysiology of Hodgkin’s Lymphoma and Role of Immune System. Archives of Microbiology and Immunology 6 (2022): 188-204
Share at FacebookAbstract
Hodgkin’s lymphoma is a disease which originates from white blood cells that are known as lymphocytes. It is primarily mutation of B lymphocytes, it has a special ability to cause deficiency of immune system and also give immune escape process to avoid from self-destruction. In this article we will discuss Hodgkin’s disease and its relation with immune system and also evaluate the function of regulatory B cells in Hodgkin’s disease, origin of HRS and HP cells, role of stains and down regulation of B cells. Epstein-Barr disease (EBV) is the vitally powerful expert that dependably has been connected with HD, EBV-encoded RNA is recognized in the HRS cells more than in 40% of cases.
Keywords
Lymphoma, lymphocytes, cytokines, tumor cells, HRS and HP cells, EBV, chemokines, interleukins
Article Details
1. Introduction
1.1 Hodgkin’s lymphoma:
It is the cancer of immune system that is identified by cell type called the Reed-Sternberg cell.
1.2 Lymphoma:
The major types of Lymphoma are given below
- Hodgkin Lymphoma
- Non Hodgkin Lymphoma
Hodgkin’s lymphoma was first discovered by Thomas Hodgkin in 1832 perhaps earlier reference was provided by Marcello Malpighi in 1666. It is discovered as disease of lymph nodes and spleen that was fatal. Lymphoma begins when lymphatic system’s healthy cells change and grow uncontrolled. This uncontrolled growth involves many parts of lymphatic system and spread in various parts of body.
Hodgkin’s lymphoma mostly attacks the lymph nodes of neck and area of lungs. It also affects the lymph nodes of arms, groin and pelvis or abdomen.
If Hodgkin’s lymphoma spreads in body it effects bone marrow, lungs, spleen or bones but this is not usual.
It effect mostly people ranging in any age but it is mostly seen in those between 20 and 40 years old and mostly those over 55 [1].
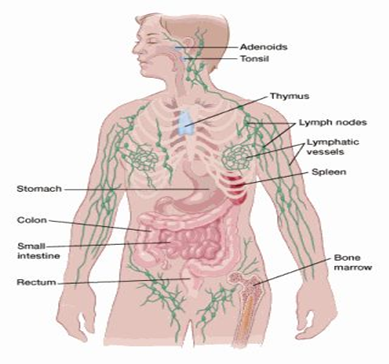
1.3 The lymphatic system:
To know the Hodgkin’s disease it is important to be aware of lymph framework that is otherwise called lymphatic framework. The lymph framework is significant component of defense systems, which help to battle against illnesses and diseases. This framework additionally controls the progression of liquids through the body. The lymphatic framework is basically made of cells called lymphocytes. There are two fundamental kinds of lymphocytes:
1.4 B lymphocytes (B cells):
B cells made antibodies which protect the body against germs.
1.5 T lymphocytes (T cells):
T cells protect body from all types of germs that harm the body and also protect from mutant cells. T-cells also help the body to enhance activity of immune. This is major role of T-lymphocytes. Usually HL start in B lymphocytes [2].
2. Start and Spread of HL:
Lymph tissues are available in many parts of the body for that reason HL may start from any part of the body.
Significant destinations where lymphoid tissues are available:
2.1 Lymph nodes:
Lymph nodes that are present everywhere in the body. They are bean size collection of immune cells and lymphocytes. They are found in chest, abdomen, groin,neck . They are connected with each other through lymphatic vessels.
2.2 Lymphatic vessels:
This is network if small tubules that connect lymph nodes and carry lymph. They collect lymph from body parts and put it into the blood stream.
2.3 Spleen:
Spleen is part of our immune system and is organ present under lower ribs. It makes cells of immune system and also filters germs and those bacteria that are harmful to body and also generate new healthy cells.
2.4 Bone marrow:
Bone marrow are spongy tissues present inside the bone where blood cells are made.
2.5 Thymus:
Thymus is small organ present infront of heart. Thymus is necessary to develop T lymphocytes.
2.6 Tonsils and adenoids:
Adenoids are small collection of lymph tissues present throat’s back side. It make antibodies which are necessary to kill germs.
2.7 Digestive tract:
Digestive system has various organs which contain lymph nodes such as stomach and intestines. That is why Hodgkin’s lymphoma start from every where usually start from upper segment of the body i.e under arms, neck and chest.
Hodgkin’s lymphoma usually start and spread from nodes to nodes by lymph vessels. It reach the blood stream and invade to other parts of the body [3].
3. Types of Hodgkin lymphoma:
Some major and different types of HL that spread in different ways and treated in different ways are:
- Classic HL
- Nodular lymphocyte-predominant HL
HL is classified in to more than 9 to 10 classes.
3.1 Classic Hodgkin lymphoma
The cancer cells of cHL are called Reed-Sternberg cells. They are type of mutant B lymphocytes. Those people who got cHL have small number of Reed-Sternberg and have many numbers of immune cells. These immune cells cause Protuberance in lymph nodes.
cHL has futher four major types:
- Nodular sclerosis Hodgkin lymphoma or NSCHL:
Most common type in developed countries of HL is NSCHL. 7 out of 10 cases are of NPHL. It started from chest and neck mostly and most common in teen age but it may also occur in people of any age.
- Mixed cellularity Hodgkin Lymphoma or MCCHL:
This type is considered as second most common type of HL. This type has 4 out of 10 cases. It is seen in mostly those peolpy who are already infected with HIV infection. It is seen in adults as well as elders. It can start from lymph nodes and invade in . It also occur in upper half of body.
- Lymphocytes rich Hodgkin:
This subtype is not very common, that is why it is rare in people. It usually occur in upper half portion of body. This type is seldom found as compare to others.
- Lymphocytes- depleted HL:
This form is very rare and seen mostly in old age people and those that are infected with Human immuno virus. It is most belligerent form of Hodgkin lymphoma and it is now advanced from being discovered. It is found in spleen, abdomen, and Liver.
3.2 Nodular predominant Hodgkin Lymphoma:
Lymphocyte-predominant Hodgkin Lymphoma has mostly about 5% cases, and the cancer cells of this type are known as pop corn cells because their shape is like pop corn.
These cells are also known as histolytic or lymphocytic.
It usually occur in lymph nodes and in neck. This type is most common in men then women and this type grow more slowly and is treated differently [4].
4. Epidemiology:
- Lymphoma account for 0.53% of cancer diagnosed each year and 0.23% of deaths due to lymphoma each year in U.S.
- Incidence of Lymphoma case is less than 3 per 100,000.
- It is male predominance disease(1:1:1).
- It has peak of bidominance occur in age from 25-30 and > 55.
- This disease is rare in children attack usually those less than 10 years [5].
5. Symptoms of Lymphoma:
Some common symptoms of Hodgkin lymphoma are:
- Enlarged lymph
- Chills
- Weigh loss
- Chest pain or pressure
- Shortness of breath or cough
- Feeling full after small amount of food
- Fatigue
- Swollen abdomen
- Severe itching
- Night sweats
6. Reasons of lymphoma:
The major cause of HL is change in make up of DNA of white blood cells called B lymphocytes.
The proper reason of HL is not known . DNA gives cell a basic commands for its survival. Any change in DNA instruction keep the cell growing uncontrollably. Due to uncontrolled division of cell abnormal lymphocytes begin to produce and got multiply in one or more lymph nodes in particular body parts. Many types of hodgkin lymphoma exists but disgnosis is based on the type of cell involved in cell. When cell type is diagnosed it determine the treatment option [6].
7. Cure of Hodgkin Lymphoma:
Chemotherapy and radiotherapy are major treatment of hodgkin lymphoma. Depending on the case of HL treatment should be given to patient. Cancer patients mmayb treated with immunotherapy or transplansment of stem cell. Surgery is very rarely used for HL and biopsy and staging is not used.
8. Risk Factors:
Risk factors that increase the risk of Hodgkin lymphoma include:
- Family History:
Any history in Family or blood relative with Hodgkin lymphoma increase risk factor of having Hodgkin lymphoma .
- Past Epstein- Barr infection:
People who had been ill by Epstian barr virus are more prone to have Hodgkin lymphoma. These infections are mononucleosis and more likely to cause disease.
- Being male:
Males are slightly more prone to Hodgkin Lymphoma as compare to women.
The other factors that are included, one of them is age HL attack ar certain age.(7)
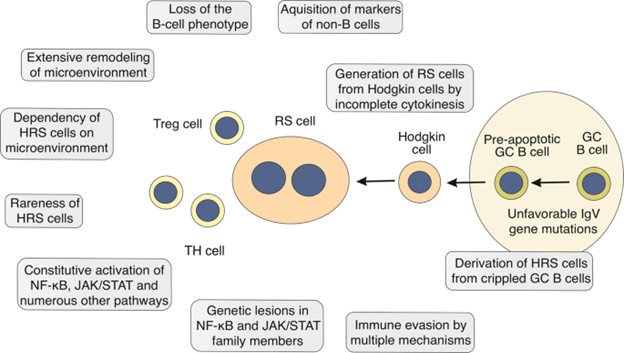
9. Cellular origin of HRS and HP cells:
Two Main components of Hodgkin lymphoma are first one is Hodgkin and Reed Sternberg cells HRS and the second one is lymphoma predominant HP cells. Cellular origins of Hodgkin Reed-Sternberg cells were not clear for many years. The reason the HRS cells have phenotype in immunological aspects that did not match with the phenotype of any normal cell of immune system. The complicated expression of markers of different type of cells of hematopoietic system was shown by the HRS cells.[8] Altered B cells were confirmed in HRS cells when isolated HRS cells are genetically analyzed, as they contain V gene having rearranged immunoglobulin Ig heavy and light chain specifically for B cells.[9] In IgV gene, the somatic changes have observed that verified their origin from the GC-experienced B cells, so hyper somatic variations have mechanism which produce variations occurring in GC B cells. Secondary lymphoid organs have germinal centre that are histological structures where humoral immune response depending on T cells occur. The production of mutations in gene IgV in HL demonstrate HRS cells which are produced by the early apoptosis in GC B cells. As variations in GC B cell normally perform apoptosis [10].
As HRS cells are huge in size, having many nuclei (multi nuclei) with two identical shaped nuclei that are present inside the reactive background of cell. For CHL, RS cells are considered pathognomonic. Most of the isolated Reed Sternberg cells have rearranged Ig gene.
10. Immunohistochemistry stains:
RS cells have immunohistochemistry stains that have positive values for CD15 and CD30 and they have negative values for CD20 and CD45, which have positive value just in NLP-HL cells. Moreover these stains are also positive for PAX5, CD25, CD95, CD86, CD40, HLA-DR. Contrarily, RS cells are not preset in NLP-HL but they have lymphocytic cells that are large in size, have many lobes in their nuclei and their nucleus contain nucleoli which are basophilic and their size is smaller than those observed in RS cells. When single isolated LP cell is observed, rearranged immunoglobulin genes are appeared. These cells have positive value for CO20, CD45, CD75, EMA, OCT2, BCL6 and J chain [11].
The steps included in the process in which transfer of malignant GC B cells (pre apoptotic) to the HRS cells is not known properly however, get away from caspase-mediated cell death appears to be the initial and important step. Well in this situation, it is interesting perception, that all the instances with threatening variations that inhibit BCR to express were observed Epstein-Barr virus (EBV) positive. [12] So most cases almost 30% are occurred because of this EBV viral infection with HRS cells. In cases that are positive for EBV, some viral genes involved such as membrane proteins LMP1 and LMP2a are produced. As LMP1 imitate a functioning receptor that is CD40 and LMP2a copy the signaling of BCR. These were primary endurance signs for GC B cells. Without a doubt, LMP2a can protect BCR-disabled GC B cells against programmed cell death. In this way, in some of the cHL instances, EBV seems to assume a crucial part in initial steps of the change cycle toward HRS cells [13, 14].
11. Down regulation in B cells:
In HRS Cells, the B cell down regulation process is not clearly known. Although the quantity of those factors that are taking part is clear. This incorporates down regulation of transcription element for the genes of B-cell (such as OCT2, PU.1, BOB1), up regulation of transcription element which represses the appearance of B-cell (such as ID2, ABF1), and genetic quieting of the genes of B-cell [15].
In NLPHL, phenotypic appearance of LP Cells demonstrated their beginning. The B cell indicators such as CD19, CD20, CD79 are produced by them. Ig V genes that have somatic variations are carried by LP Cells. Respective BCR are produced by LP cells and the change design shows choice for articulation of a useful BCR [16].
12. Outlook of hereditary lesions in HRS and LP cells:
CHL is the lymphoid abnormality having biggest degree of irregularities in chromosomes. Aneuploidy observed practically in every instance, underlying distortions, acquires and misfortunes are additionally normal. These occasions can likewise be sub clonal, demonstrating that HRS cells have great hereditary unsteadiness [17].
Movements influencing the Ig position are observed in around 20% of instances. Yet the movement accomplices are assorted, and Ig positions were quieted in cells of Reed stern berg, part performed by these movements to set up lymphomal clone remains generally hazy. Explanations behind the continuous mathematical and underlying chromosomal deviations were for the most part obscure. Telomere malfunctioning is associated with these mechanisms [18].
The quest to study about the somatic variations in specified HRS cell genes was hindered by the requirement to get single separated uncommon HRS cell for histological examination by the dissection of histo segments or stream cytometric cell arranging in solution containing living cells [19].
Subsequently, moderately couple of genes were read up for variations, and earlier the principal entire study of exome sequencing started to give extreme top to bottom image how hereditary sores in HRS cells observed. A principle discovery of various examinations is that a transformation in individuals from the NF-κB pathway is a primary element of HRS cells. This incorporates gains and intensifications of the genes encoding the NF-κB factor REL and BCL3 [20].
Less recurring are hereditary sores in other negative controllers of NF-κB, in particular in operating changes or excision of CYLD and TRAF3. Prominently, TNFAIP3 and NFKBIA transformations have greater repetition in EBV-uninfected cHL, showing that in instances in which EBV cause infection the viral LMP1, a solid NF-κB activator, can restore the requirement to in operate TNFAIP3 or NFKBIA. The altered NF-κB pathway elements catalogue showed that the sanctioned and the non-accepted NF-κB pathways also impacted by transformations in HRS cells. Chiefly found from investigations of HL cell lines, it appears to be frequently different of such elements producing changes in similar HRS-cell clones. This recommends that more than one sore is frequently required to dysregulate the NF-κB pathway adequately [21].
13. Identification of genetic lesions:
Additional frequent hereditary lesions will be recognized in additional examinations, various decisions have already been taken. First of all, no hereditary sore is viewed as in basically the cases as a whole, so an illness characterizing injury is absent. Secondly, as it is obvious that these are not specified genes which are fundamental for HL pathogenesis, however the down regulation of specific mechanism, that could happen via transformations influencing different elements of mechanism. The hereditary sores in individuals from the NF-κB and JAK/STAT mechanism are the extreme noteworthy models in such manner, possible influencing essentially all instances. Thirdly, broadening earlier perception of TNFAIP3 and NFKBIA changes generally in EBV-negative instances, two exome sequencing investigations demonstrate that the clones of HRS-cell are contaminated with EBV, have a considerably lesser variation than EBV-negative instances. This shows that the appearance of genes of virus replace the requirement of numerous oncogene and variation in cancer repressor gene. Fourthly, up to this point, no continuous hereditary injury has been recognized which could clarify deficiency of the B-cell gene articulation program of HRS cells. Fifth, none of hereditary lesions have recognized which are explicit of HRS cells. Practically all the genes often changed in the cells of Reed stern berg have likewise appeared transformed in further B-cell. May be it is the mixture of hereditary changes that are explicit for cHL. Additionally, one might hypothesize that the personality of HRS cells and the peculiarity of cHL isn't exclusively characterized by the scene of its hereditary injuries, however by the way that the changing occasions (mostly) happened in an exceptional cell, in particular a pre-apoptotic GC B cell [22].
14. Hodgkin's disease and Immune System:
All the patients throughout the world exhibit a defect in their cellular immunity persistently when suffer with this disease. Those patients who left untreated show depressed natural killer cell mediated cytotoxicity. Because of the increased sensitivity to the suppressor monocytes, T-suppressor cells, and the abnormal production of interleukin-2, the defects in cellular immunity appear. In advanced disease, patients show defect in inherent T-lymphocyte [23].
14.1 Hodgkin's Disease and EBV:
EBV stands for Epstein-Barr virus which is the main irresistible entity that is related with Hodgkin’s disease. In about 40% of cases, Hodgkin and Reed Sternberg cells were found to have RNA encoded by Epstein-Barr [24-26]. An enormous number of people suffering from Hodgkin disease have high EBV neutralizer titers, proposing that EBV contamination might go before the advancement of HD, and clonally the studies showed that EBV disease went before the extension of growth cell population [27]. The rate of HD that is related to EBV fluctuates by age, gender, nationality, histological subtype, monetary level and also by genetics [28, 29].
The host’s hereditary helplessness has brought about a faulty insusceptible reaction permitting expansion and harmful change of EBV-tainted cells.
14.2 LMP-1 and NF-kappa B (Nuclear factor kappa-B):
The Epstein-Barr virusal gene expression of Epstein-Barr nuclear antigen 1 (EBNA-1), latent membrane protein 1 (LMP1) and latent membrane protein 2 (LMP2) - (type II inactivity) is the sign of EBV-positive Hodgkin’s disease. LMP1 which is the inactive one is a protein of epstein-barr (a viral protein) and an individual from the TNFR (Tumor Necrosis Factor Receptor) super family that enacts NF-kappa B and balances developmental and apoptotic pathways [30]. The initiation of nuclear factor kappa-B and the related record bring about directing numerous exercises including the resistant framework, cell expansion, cancer metastasis, irritation and viral replication [31]. Various examinations showed the abnormal expression of p50/p65 (RelA) heterodimer, a type of nuclear factor kappa-B, by threatening Hodgkin and Reed Sternberg cells, which is basic for cell endurance [32-35].
14.3 Tumor Immune Escape Mechanisms in Hodgkin Disease:
The tumor cells secrete cytokine and chemokine and contribute its part to get away from the immune system. For instance, the transforming growth factor (TGF-β) is secreted which represses the development of cytotoxic T-lymphocyte (CTLs), as well as the T cells, while interleukin-13 (IL-13) straightforwardly advances the development and let the HD cell lines to survive. Thymus and activation regulated chemokine (TARC) and IL-10 contrarily influence CTL and the action of antigen presenting cell, prompting the deregulated development of cancer cells of Hodgkin’s disease. HRS cells shed CD30 meddles between the working of antigen presenting cells, lymphocytes, and the cytokines. The FasL pathway’s activation by the tumor cells is a component by which they show FasL getting away from the destruction of FasL by the immune system. PGE2 (prostaglandin E2- a molecule responsible for the development and production of cytokine of immune cells that have a role in the innate immunity [36]) secretion by monocytes overexpress IDO (Indoleamine 2,3 dioxygenase - enzyme which is responsible for the essential amino acid’s degradation such as of tryptophan in either dendritic cells or other cells of immune system [37]), prompting the depletion of tryptophan, which is viewed as a defensive mechanism instigated by IFN-γ, and in result suppressing the division of T-cells [38].
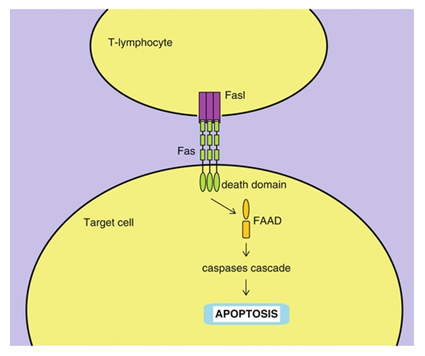
14.4 FasL pathway:
For the regulated death of the cells (apoptosis), there are two molecules that are Fas and the Fas ligand, the members of TNF family. Their proper ligation results in the caspases cascade leading to the apoptosis [39] as shown in the figure [40] below.
What is the Caspases cascade? So basically caspases stands for the class of proteases mainly of cysteine which transfers the signals for apoptosis in the form of cascade (proteolytic) and caspases cleave and activate other caspases to carry out the degradation process that leads to the apoptosis [41].
14.5 Confined Pattern of Ags:
HD due to EBV is a perfect model in which the person's immunity fails to destroy the HD growth cells (tumor) even after the tumor cells have expressed the antigens of virus. The limited exhibition or expression of viral antigens could be a reason for that. The expression of the viral genes on HRS cells is restricted to the immunodominant LMP 1 and 2, EBNA 1 (it have gly-ala repeating units hat restrain HLA class I antigen’s working), and little non-polyadenylated RNAs named EBV early RNA (EBER) 1 and 2, that can be transcribed however not translated [42-44]. A recommendation is that the peptides of LMP1 and LMP2 are not a match for other EBV LMPs for binding to the person’s HLA antigens; accordingly this brings about a poor signal to CTLs which is LMP specific. This little gene expression that is quite a weak target for the activity of CTL thus allows the cancerous cells to avoid the immune response [45].
15. Contribution of cytokines:
15.1 Changing Growth factor- Beta:
Many malignant cells release cytokines as their safety mechanism to avoid the immune response of the host. The cells that are engaged with HD include T lymphocytes and the cytokines that are responsible for the proliferation of the tumor cells. [46] TGF-β is the potent immunosuppressive cytokine that has an anti-proliferative and anti-cytotoxic result on Tc cells [47]. Therefore, the release of this cytokine by the tumors can affect the immune response.
15.2 Interleukins:
Reports from the past have showed that few cytokines are showed in biopsy material by the cell lines of Hodgkin disease and by Hodgkin and Reed Sternberg cells. These cytokines include interleukins (1, 5, 6, 7, 9 and 10), TGF-β and others [48]. Due the imbalanced production of these cytokines, the symptoms of HD appear [49]. Moreover, IL-10 can repress the growth rate of T-lymphocytes [50]. IL-10 is found in HRS cells (EBV-positive) [51].
15.3 Interleukin-13:
Interleukin-13 is a Th2 cytokine which is similar to interleukin-4. Combined they show a significant part in the humoral immunity by means of their exercises on B cells [52]. Interleukin-13, because of its affects on macrophages and B-cells, show anti-inflammatory effects [53-57]. IL-13 also acts as an autocrine development factor in Hodgkin’s disease and has a major contribution in the enactment of STAT6 ( driving force for the differentiation of Th2 cells [stat 6 immunology] ), that has an association with the multiplication of typical T & B-cells [58].
16. Contribution of chemokines:
Chemokines are same as cytokines and differ only at one point that is the initial one has properties of attracting specific substances by releasing chemicals. Chemokines that are released by HRS cells show an essential part in the trafficking of leukocytes. This provides the favorable environment inside the cells (HRS). It was proposed that in HD, the maximum makeup of the tumor cells is by the infiltrate that is reactive. This infiltrate is produced due to the chemokinal and cytokinal secretions by the malignant cells [59-61]. Studies have shown the presence of IL-8 in the sera of patients suffering from HD [62, 63]. Interferon-inducible protein-10 (IP-10) is a chemoattractant for enacted T-cells communicating the CXCR3 chemokine receptor [64]. Past investigations of the disease have also revealed a relationship between this IP-10 and HD [65]. Additionally, these examinations likewise have revealed that the expression of IP-10 in patients that are positive for EBV is far stronger than those that are negative for EBV in HD [66-68]. Thus, it is reasonable to think that the expression of LMP1 can play a part in the production of IP-10 in the HRS cells.
17. Down regulation of Fas-ligand:
Fas ligand (FasL) is activated by T-lymphocytes, natural killer cells [69, 70] and also by some non-lymphoid cells. FasL which is bound to the membrane incites cell death of the target after engaging its receptor that is Fas and thus shows an important role in the down regulation of immune system as well as in the cell-mediated immunity [71]. Therefore, the activation of Fas/FasL pathway leads to the fact that those tumors that express this FasL can easily avoid the host’s immunity.
18. Indoleamine 2, 3-dioxygenase's secretion:
One more way by which malignant cells can avoid immune response is by the production of PGE2 and IDO. The dysfunctional of monocytes in HD patients is due to the over production of PGE2 which in turn increases IDO production by monocytes, tumor and dendritic cells. The activation of IDO restricts the accessibility of tryptophan, a basic amino acid for the synthesis of protein. These decreased levels of tryptophan make T cells to be captured in the G1 phase of the cell cycle. This shows an escape mechanism of malignant cells. It was suggested that increased release of IFN-γ during the host's survival mechanism i.e. anti-tumor response by immune system prompts the IDO's activation in macrophages and dendritic cells that leads to the suppression in multiplication of T-cells and then act as a suppressant of immunity [72-80].
References
- Hodgkin Lymphoma: Diagnosis and Treatment. Mayo Clin Proc 90 (2015): 1574-1583.
- Bartlett NL, Foyil KV. Chapter 105: Hodgkin lymphoma. In: Niederhuber JE, Armitage JO, Dorshow JH, Kastan MB, Tepper JE, eds. Abeloff’s Clinical Oncology. 5th Philadelphia, Pa Elsevier (2014).
- National Comprehensive Cancer Network, Clinical Practice Guidelines in Oncology (NCCN Guidelines®), Hodgkin Lymphoma, Version I.2018 - December 20, 2017.
- Shanbhag S, Ambinder RF. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J Clin 68 (2018): 116-132.
- Younes A, Carbone A, Johnson P, Dabaja B, Ansell S, Kuruvilla J. Chapter 102: Hodgkin’s lymphoma. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 10th Philadelphia, Pa: Lippincott Williams & Wilkins (2015).
- National Cancer Institute. Adult Hodgkin Lymphoma Treatment (PDQ®)-Health Professional Version. March 1, (2018).
- Pinnix CC, Osborne EM, Chihara D, et al. Maternal and Fetal Outcomes After Therapy for Hodgkin or Non-Hodgkin Lymphoma Diagnosed During Pregnancy. JAMA Oncol 2 (2016): 1065-1069.
- Foss HD, Reusch R, Demel G, Lenz G, Anagnostopoulos I, Hummel M, et al. Frequent expression of the B-cell-specific activator protein in Reed-Sternberg cells of classical Hodgkin’s disease provides further evidence for its B-cell origin. Blood 94 (1999): 3108-13.
- Bräuninger A, Wacker HH, Rajewsky K, Küppers R, Hansmann ML. Typing the histogenetic origin of the tumor cells of lymphocyte-rich classical Hodgkin’s lymphoma in relation to tumor cells of classical and lymphocyte-predominance Hodgkin’s lymphoma. Cancer Res 63 (2003): 1644-51.
- Kanzler H, Küppers R, Hansmann ML. Rajewsky K. Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells J Exp Med 184 (1996): 1495-505.
- Justiz Vaillant AA, Stang CM.StatPearls [Internet].StatPearls Publishing; Treasure Island (FL): Aug 30, (2021).
- Bräuninger A, Schmitz R, Bechtel D, Renne C, Hansmann ML, Küppers R. Molecular biology of Hodgkin’s and Reed/Sternberg cells in Hodgkin’s lymphoma Int J Cancer 118 (2006): 1853-61.
- Kapatai G, Murray P. Contribution of the Epstein Barr virus to the molecular pathogenesis of Hodgkin lymphoma J Clin Pathol 60 (2007): 1342-9.
- Mancao C, Hammerschmidt W. Epstein-Barr virus latent membrane protein 2A is a B-cell receptor mimic and essential for B-cell survival Blood 110 (2007): 3715-21.
- Müschen M, Rajewsky K, Bräuninger A, Baur AS, Oudejans JJ, Roers A, et al. Rare occurrence of classical Hodgkin’s disease as a T cell lymphoma J Exp Med 191 (2000): 387-94.
- Braeuninger A, Küppers R, Strickler JG, Wacker HH, Rajewsky K, Hansmann ML. Hodgkin and Reed-Sternberg cells in lymphocyte predominant Hodgkin disease represent clonal populations of germinal center-derived tumor B cells. Proc Natl Acad Sci USA 94 (1997): 9337-42.
- Weber-Matthiesen K, Deerberg J, Poetsch M, Grote W, Schlegelberger B. Numerical chromosome aberrations are present within the CD30+ Hodgkin and Reed-Sternberg cells in 100% of analyzed cases of Hodgkin’s disease. Blood 86 (1995): 1464-8.
- Cuceu C, Hempel WM, Sabatier L, Bosq J, Carde P, M’Kacher R. Chromosomal instability in Hodgkin lymphoma: an in-depth review and perspectives. Cancers (Basel) 10 (2018): 91.
- Joos S, Menz CK, Wrobel G, Siebert R, Gesk S, Ohl S, et al. Classical Hodgkin lymphoma is characterized by recurrent copy number gains of the short arm of chromosome 2 Blood 99 (2002): 1381-7.
- Lake A, Shield LA, Cordano P, Chui DT, Osborne J, Crae S, et al. Mutations of NFKBIA, encoding IkappaB alpha, are a recurrent finding in classical Hodgkin lymphoma but are not a unifying feature of non-EBV-associated cases. Int J Cancer 125 (2009): 1334-42.
- Weniger MA, Küppers R. NF-kappaB deregulation in Hodgkin lymphoma. Semin Cancer Biol 39 (2016): 32-9.
- Wienand K, Chapuy B, Stewart C, Dunford AJ, Wu D, Kim J, et al. Genomic analyses of flow-sorted Hodgkin Reed-Sternberg cells reveal complementary mechanisms of immune evasion Blood Adv 3 (2019): 4065-80.
- Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, et al. Epstein-Barr virus-associated Hodgkin's disease: epidemiologic characteristics in international dataInt J Cancer70 (1997): 375-382.
- Brauninger A, Schmitz R, Bechtel D, Renne C, Hansmann ML, Kuppers R. Molecular biology of Hodgkin's and Reed/Sternberg cells in Hodgkin's lymphomaInt J Cancer118 (2006): 1853-1861.
- Weiss LM. Epstein-Barr virus and Hodgkin's diseaseCurr Oncol Rep2 (2000): 199-204.
- Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, et al. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death.Cell65 (1991): 1107-1115.
- Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, et al. Epstein-Barr virus-associated Hodgkin's disease: epidemiologic characteristics in international dataInt J Cancer70 (1997): 375-382.
- Weinreb M, Day PJ, Niggli F, Powell JE, Raafat F, Hesseling PB, et al. The role of Epstein-Barr virus in Hodgkin's disease from different geographical areas.Arch Dis Child74 (1996): 27-31.
- Chang KL, Albujar PF, Chen YY, Johnson RM, Weiss LM. High prevalence of Epstein-Barr virus in the Reed-Sternberg cells of Hodgkin's disease occurring in PeruBlood81 (1993): 496-501.
- Ambinder RF, Browning PJ, Lorenzana I, Leventhal BG, Cosenza H, Mann RB, et al. Epstein-Barr virus and childhood Hodgkin's disease in Honduras and the United StatesBlood81 (1993): 462-467.
- Gulley ML, Eagan PA, Quintanilla-Martinez L, Picado AL, Smir BN, Childs C, et al. Epstein-Barr virus DNA is abundant and monoclonal in the Reed-Sternberg cells of Hodgkin's disease: association with mixed cellularity subtype and Hispanic American ethnicity.Blood83 (1994): 1595-1602.
- Alexander FE, Lawrence DJ, Freeland J, Krajewski AS, Angus B, Taylor GM, et al. An epidemiologic study of index and family infectious mononucleosis and adult Hodgkin's disease (HD): evidence for a specific association with EBV+ve HD in young adults.Int J Cancer107 (2003): 298-302.
- Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, et al. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death.Cell65 (1991): 1107-1115.
- Karin M, Lin A. NF-kappaB at the crossroads of life and death.Nat Immunol3 (2002): 221-227.
- Younes A, Garg A, Aggarwal BB. Nuclear transcription factor-kappaB in Hodgkin's disease.Leuk Lymphoma44 (2003): 929-935.
- Staudt LM. The molecular and cellular origins of Hodgkin's diseaseJ Exp Med191 (2000): 207-212.
- Bargou RC, Emmerich F, Krappmann D, Bommert K, Mapara MY, Arnold W, et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells.J Clin Invest100 (1997): 2961-2969.
- Bargou RC, Leng C, Krappmann D, Emmerich F, Mapara MY, Bommert K, et al. High-level nuclear NF-kappa B and Oct-2is a common feature of cultured Hodgkin/Reed-Sternberg cellsBlood87 (1996): 4340-4347.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3168532/
- Qu L, Rowe DT. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes.J Virol66 (1992): 3715-3724.
- Tierney RJ, Steven N, Young LS, Rickinson AB. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state.J Virol68 (1994): 7374-7385.
- Tierney RJ, Steven N, Young LS, Rickinson AB. Epstein-Barr virus latency in blood mononuclear cells: Analysis of viral gene transcription during primary infection and in the carrier state.J Virol68 (1994): 7374-7385.
- Deacon EM, Pallesen G, Niedobitek G, Crocker J, Brooks L, Rickinson AB, et al. Epstein-Barr virus and Hodgkin's disease: transcriptional analysis of virus latency in the malignant cells.J Exp Med177 (1993): 339-349.
- Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells.Cell Mol Life Sci66 (2009): 2603-2622.
- McHugh RS, Shevach EM. The role of suppressor T cells in regulation of immune responses.J Allergy Clin Immunol110 (2002): 693-702.
- Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma.Blood103 (2004): 1755-1762.
- Shevach EM. Regulatory T cells in autoimmmunity*.Annu Rev Immunol18 (2000): 423-449.
- Skinnider BF, Elia AJ, Gascoyne RD, Trumper LH, von Bonin F, Kapp U, et al. Interleukin 13 and interleukin 13 receptor are frequently expressed by Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma.Blood97 (2001): 250-255.
- Gruss HJ, Pinto A, Duyster J, Poppema S, Herrmann F. Hodgkin's disease: a tumor with disturbed immunological pathways.Immunol Today18 (1997): 156-163.
- Maggio E, van den BA, Diepstra A, Kluiver J, Visser L, Poppema S. Chemokines, cytokines and their receptors in Hodgkin's lymphoma cell lines and tissues.Ann Oncol13 (2002): 52-56.
- Kanegane H, Wakiguchi H, Kanegane C, Kurashige T, Tosato G. Viral interleukin-10 in chronic active Epstein-Barr virus infection.J Infect Dis176 (1997): 254-257.
- Bohlen H, Kessler M, Sextro M, Diehl V, Tesch H. Poor clinical outcome of patients with Hodgkin's disease and elevated interleukin-10 serum levels. Clinical significance of interleukin-10 serum levels for Hodgkin's disease.Ann Hematol79 (2000): 110-113.
- Sarris AH, Kliche KO, Pethambaram P, Preti A, Tucker S, Jackow C, et al. Interleukin-10 levels are often elevated in serum of adults with Hodgkin's disease and are associated with inferior failure-free survival.Ann Oncol10 (1999): 433-440.
- Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells.Immunol Today15 (1994): 19-26.
- McKenzie AN, Culpepper JA, de Waal MR, Briere F, Punnonen J, Aversa G, et al. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function.Proc Natl Acad Sci U S A90 (1993): 3735-3739.
- Defrance T, Carayon P, Billian G, Guillemot JC, Minty A, Caput D, et al. Interleukin 13 is a B cell stimulating factorJ Exp Med179 (1994): 135-143.
- Lomo J, Blomhoff HK, Jacobsen SE, Krajewski S, Reed JC, Smeland EB. Interleukin-13 in combination with CD40 ligand potently inhibits apoptosis in human B lymphocytes: upregulation of Bcl-xL and Mcl-1.Blood89 (1997): 4415-4424.
- Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses.Nature362 (1993): 248-250.
- de Waal MR, Figdor CG, Huijbens R, Mohan-Peterson S, Bennett B, Culpepper J, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10.J Immunol151 (1993): 6370-6381.
- Skinnider BF, Kapp U, Mak TW. Interleukin 13: a growth factor in hodgkin lymphoma.Int Arch Allergy Immunol126 (2001): 267-276.
- Teruya-Feldstein J, Jaffe ES, Burd PR, Kingma DW, Setsuda JE, Tosato G. Differential chemokine expression in tissues involved by Hodgkin's disease: direct correlation of eotaxin expression and tissue eosinophilia.Blood93 (1999): 2463-2470.
- van den BA, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin's lymphoma.Am J Pathol154 (1999): 1685-1691.
- Teruya-Feldstein J, Tosato G, Jaffe ES. The role of chemokines in Hodgkin's disease.Leuk Lymphoma38 (2000): 363-371.
- Trumper L, Jung W, Dahl G, Diehl V, Gause A, Pfreundschuh M. Interleukin-7, interleukin-8, soluble TNF receptor, and p53 protein levels are elevated in the serum of patients with Hodgkin's disease.Ann Oncol5 (1994): 93-96.
- Luciani MG, Stoppacciaro A, Peri G, Mantovani A, Ruco LP. The monocyte chemotactic protein a (MCP-1) and interleukin 8 (IL-8) in Hodgkin's disease and in solid tumours.Mol Pathol51 (1998): 273-276.
- Moser B, Loetscher P. Lymphocyte traffic control by chemokines.Nat Immunol2 (2001): 123-128.
- Teruya-Feldstein J, Jaffe ES, Burd PR, Kingma DW, Setsuda JE, Tosato G. Differential chemokine expression in tissues involved by Hodgkin's disease: direct correlation of eotaxin expression and tissue eosinophilia.Blood93 (1999): 2463-2470.
- Ohshima K, Tutiya T, Yamaguchi T, Suzuki K, Suzumiya J, Kawasaki C, et al. Infiltration of Th1 and Th2 lymphocytes around Hodgkin and Reed-Sternberg (H&RS) cells in Hodgkin disease: Relation with expression of CXC and CC chemokines on H&RS cells.Int J Cancer98 (2002): 567-572.
- Ohshima K, Karube K, Hamasaki M, Suefuji H, Tutiya T, Yamaguchi T, et al. Imbalances of chemokines, chemokine receptors and cytokines in Hodgkin lymphoma: classical Hodgkin lymphoma vs. Hodgkin-like ATLL.Int J Cancer106 (2003): 706-712.
- Teichmann M, Meyer B, Beck A, Niedobitek G. Expression of the interferon-inducible chemokine IP-10 (CXCL10), a chemokine with proposed anti-neoplastic functions, in Hodgkin lymphoma and nasopharyngeal carcinoma.J Pathol206 (2005): 68-75.
- Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, et al. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity.J Exp Med187 (1998): 1205-1213.
- Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding.Nat Med4 (1998): 31-36.
- Montel AH, Bochan MR, Hobbs JA, Lynch DH, Brahmi Z. Fas involvement in cytotoxicity mediated by human NK cells.Cell Immunol166 (1995): 236-246.
- Suda T, Okazaki T, Naito Y, Yokota T, Arai N, Ozaki S, et al. Expression of the Fas ligand in cells of T cell lineage.J Immunol154 (1995): 3806-3813.
- Nagata S, Golstein P. The Fas death factor.Science267 (1995): 1449-1456.
- Nagata S, Golstein P. The Fas death factor.Science267 (1995): 1449-1456.
- Bonfoco E, Stuart PM, Brunner T, Lin T, Griffith TS, Gao Y, et al. Inducible nonlymphoid expression of Fas ligand is responsible for superantigen-induced peripheral deletion of T cells.Immunity9 (1998): 711-720.
- O'Connell J, O'Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand.J Exp Med184 (1996): 1075-1082.
- Strand S, Hofmann WJ, Hug H, Muller M, Otto G, Strand D, et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells--a mechanism of immune evasion?Nat Med2 (1996): 1361-1366.
- Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE, et al. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape.Science274 (1996): 1363-1366.
- Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase.J Exp Med196 (2002): 459-468.
- Yoshida R, Imanishi J, Oku T, Kishida T, Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon.Proc Natl Acad Sci USA78 (1981): 129-132.
- Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism.FASEB J5 (1991): 2516-2522.
- Werner ER, Bitterlich G, Fuchs D, Hausen A, Reibnegger G, Szabo G, et al. Human macrophages degrade tryptophan upon induction by interferon-gamma.Life Sci41 (1987): 273-280.
- Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2, 3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation.J Immunol164 (2000): 3596-3599.
- Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase.Nat Med9 (2003): 1269-1274.
- Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase.Nat Med9 (2003): 1269-1274.
- Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation.Clin Chim Acta(2005).
- Schroecksnadel K, Winkler C, Fuith LC, Fuchs D. Tryptophan degradation in patients with gynecological cancer correlates with immune activation.Cancer Lett223 (2005): 323-329.