Parental Postnatal Depressive Symptoms in NICU are strongly related to Demographic factors and Maternal as well as Neonatal Perinatal Clinical Outcome
Article Information
Ioanna Ioannou1*, Artemis Giotsa2
1PhD (c), Department of Early Childhood Education, University of Ioannina, Greece
2Professor of Social Psychology, Department of Early Childhood Education, University of Ioannina, Greece
*Corresponding Author: Ioanna Ioannou, PhD (c), Department of Early Childhood Education, University of Ioannina, Greece.
Received: 14 July 2023; Accepted: 21 July 2023; Published: xx July 2023
Citation: Ioanna Ioannou, Artemis Giotsa. Parental Postnatal Depressive Symptoms in NICU are strongly related to Demographic factors and Maternal as well as Neonatal Perinatal Clinical Outcome. Obstetrics and Gynecology Research. 6 (2023): 213-227.
Share at FacebookAbstract
Introduction:
Giving birth to a preterm infant is undoubtedly a stressful event on parents’ psychological wellbeing.
Aim:
To determine the depression levels and define the related demographic and clinical factors.
Material and Methods:
A prospective, follow up–cohort study, in “Helena Venizelou” Maternity Hospital’s NICU (Athens, Greece), between December 2019 to December 2022, with the sample of sixty couples, whose infants’ birth weight (BW) was <1750g and gestational age (GA) <34weeks. Data were collected using the Edinburg Postnatal Depression Scale in 3rd–4th, in 20th– 25th day of life and at NICU discharge.
Results:
Mothers experienced higher levels of depression than fathers did, at all assessments (1st: p<0.001, 2nd: p<0.001, 3rd: p<0.001). Our data concluded that the rate of maternal depression in the 1st & 2nd assessment [Mean (SD):14.9(5.9) and Mean (SD):12.4(6.2), respectively] was significantly higher than the 3rd [Mean (SD):10.3 (5.9)]. Moreover, the rate of fathers’ depression in the 1st assessment [Mean (SD):10.3(5.8)] was higher compared to the 3rd [Mean (SD):6.4(5.2)]. Other factors that contributed to higher levels of depression were maternal health problems & receiving in vitro fertilisation treatment for mothers as well as Apgar score 5’ for fathers. Maternal age, clinical course of pregnancy (primiparous & single pregnancies), neonatal BW & hospitalization duration as well as Apgar and CRIB II score were also found to correlate with levels of depression.
Conclusion:
NICU may be a stressful experience. Screening parents for postpartum depression during NICU stay is likely to result in improved identification of the parents at risk at this critical period, in order to plan early interventions.
Keywords
Prematurity; NICU; Parental depression; Postpartum depression; EPDS
Prematurity articles Prematurity Research articles Prematurity review articles Prematurity PubMed articles Prematurity PubMed Central articles Prematurity 2023 articles Prematurity 2024 articles Prematurity Scopus articles Prematurity impact factor journals Prematurity Scopus journals Prematurity PubMed journals Prematurity medical journals Prematurity free journals Prematurity best journals Prematurity top journals Prematurity free medical journals Prematurity famous journals Prematurity Google Scholar indexed journals NICU articles NICU Research articles NICU review articles NICU PubMed articles NICU PubMed Central articles NICU 2023 articles NICU 2024 articles NICU Scopus articles NICU impact factor journals NICU Scopus journals NICU PubMed journals NICU medical journals NICU free journals NICU best journals NICU top journals NICU free medical journals NICU famous journals NICU Google Scholar indexed journals Parental depression articles Parental depression Research articles Parental depression review articles Parental depression PubMed articles Parental depression PubMed Central articles Parental depression 2023 articles Parental depression 2024 articles Parental depression Scopus articles Parental depression impact factor journals Parental depression Scopus journals Parental depression PubMed journals Parental depression medical journals Parental depression free journals Parental depression best journals Parental depression top journals Parental depression free medical journals Parental depression famous journals Parental depression Google Scholar indexed journals Postpartum depression articles Postpartum depression Research articles Postpartum depression review articles Postpartum depression PubMed articles Postpartum depression PubMed Central articles Postpartum depression 2023 articles Postpartum depression 2024 articles Postpartum depression Scopus articles Postpartum depression impact factor journals Postpartum depression Scopus journals Postpartum depression PubMed journals Postpartum depression medical journals Postpartum depression free journals Postpartum depression best journals Postpartum depression top journals Postpartum depression free medical journals Postpartum depression famous journals Postpartum depression Google Scholar indexed journals EPDS articles EPDS Research articles EPDS review articles EPDS PubMed articles EPDS PubMed Central articles EPDS 2023 articles EPDS 2024 articles EPDS Scopus articles EPDS impact factor journals EPDS Scopus journals EPDS PubMed journals EPDS medical journals EPDS free journals EPDS best journals EPDS top journals EPDS free medical journals EPDS famous journals EPDS Google Scholar indexed journals premature babies articles premature babies Research articles premature babies review articles premature babies PubMed articles premature babies PubMed Central articles premature babies 2023 articles premature babies 2024 articles premature babies Scopus articles premature babies impact factor journals premature babies Scopus journals premature babies PubMed journals premature babies medical journals premature babies free journals premature babies best journals premature babies top journals premature babies free medical journals premature babies famous journals premature babies Google Scholar indexed journals preterm birth articles preterm birth Research articles preterm birth review articles preterm birth PubMed articles preterm birth PubMed Central articles preterm birth 2023 articles preterm birth 2024 articles preterm birth Scopus articles preterm birth impact factor journals preterm birth Scopus journals preterm birth PubMed journals preterm birth medical journals preterm birth free journals preterm birth best journals preterm birth top journals preterm birth free medical journals preterm birth famous journals preterm birth Google Scholar indexed journals Neonatal intensive Care Unit articles Neonatal intensive Care Unit Research articles Neonatal intensive Care Unit review articles Neonatal intensive Care Unit PubMed articles Neonatal intensive Care Unit PubMed Central articles Neonatal intensive Care Unit 2023 articles Neonatal intensive Care Unit 2024 articles Neonatal intensive Care Unit Scopus articles Neonatal intensive Care Unit impact factor journals Neonatal intensive Care Unit Scopus journals Neonatal intensive Care Unit PubMed journals Neonatal intensive Care Unit medical journals Neonatal intensive Care Unit free journals Neonatal intensive Care Unit best journals Neonatal intensive Care Unit top journals Neonatal intensive Care Unit free medical journals Neonatal intensive Care Unit famous journals Neonatal intensive Care Unit Google Scholar indexed journals infants articles infants Research articles infants review articles infants PubMed articles infants PubMed Central articles infants 2023 articles infants 2024 articles infants Scopus articles infants impact factor journals infants Scopus journals infants PubMed journals infants medical journals infants free journals infants best journals infants top journals infants free medical journals infants famous journals infants Google Scholar indexed journals low birth weight infant articles low birth weight infant Research articles low birth weight infant review articles low birth weight infant PubMed articles low birth weight infant PubMed Central articles low birth weight infant 2023 articles low birth weight infant 2024 articles low birth weight infant Scopus articles low birth weight infant impact factor journals low birth weight infant Scopus journals low birth weight infant PubMed journals low birth weight infant medical journals low birth weight infant free journals low birth weight infant best journals low birth weight infant top journals low birth weight infant free medical journals low birth weight infant famous journals low birth weight infant Google Scholar indexed journals
Article Details
Introduction
Annually almost 15 million premature babies are born worldwide comprising more than 10% of all infants born [1]. The World Health Organisation [2] defines preterm birth as any birth before 37 completed weeks of gestation, or fewer than 259 days since the first day of woman’s last menstrual period. This is further subdivided on the basis of gestational age (GA): i) extremely preterm (< 28 weeks), ii) very preterm (28 – <32 weeks), iii) moderate or preterm (32 – < 37 completed weeks of gestation), consisting the most extensively used and accepted definition of preterm birth [3]. Until the 90s, prematurity defined only by the BW, however, in recent years GA has been considered as the main indicator of physical and neurological maturation of preterm infants [4]. Preterm birth is a complicated event that presents two main outcomes: firstly, the medical and neurophysiological conditions that “put” the infant in danger (particularly infants with BW<1500g and GA<32w), and secondly, it could impose a negative impact both on parents’ relationship as well as on parents – infant interaction [5]. Giving birth to a premature infant requires early separation from its parents and long–term admission to the Neonatal intensive Care Unit (NICU). According to WHO, the average day of hospitalization of premature infants of less than 32 weeks is 35 days [6,7]. A study conducted by the Neocosur Network found that the average length of stay of a preterm and very low birth weight infant was 59 days [8].
Even though it has been widely known that preterm infants are at risk for developing deficit and delays, the underlying causes of these poorer developmental outcomes, and the role of parents, are still less understood. Specifically, as far as we know still few studies investigate parents’ initial experience and reaction the first days after the preterm birth of their baby [9,10,11,12,13,14]. Additionally, still few studies examine fathers’ experience of premature birth, even if they highlighted the importance of fathers’ experience [15,16,17]. Very few parents are prepared in advance for this possibility, which falls within the category of undesirable situation, riddled with excess tension that can trigger parental stress, anxiety and possibly depression [18,19].
Premature infant admission in the NICU is a psychological crisis for parents, that may be associated with multiple factors such as, adapting to have a “sick” infant, the stress of the NICU environment, the physical and emotional isolation from the infant, combined with the normal stress of parenthood [20,21,22,23]. The severity of mental health complications in mothers of preterm infants seems to depend on sociodemographic data, such as marital status & education level as well as infant characteristics, such as BW, GA and medical complications [24,25]. From the limited studies available for preterm infants’ fathers, as mentioned, it seemed that the depressive symptoms were associated with sociodemographic factors, as marital status and financial status [26,25]. In a specific study, almost 30 days after a preterm birth, parents were shocked by the physiological and psychological conditions of their infant [27,28]. Parents are asked to adapt to a different environment, a special language and sometimes even move away from home [29,30].
Feelings of depression consist a normal response to the suddenly disrupted pregnancy, but if these persist, they can negatively affect the parent–infant interaction, a fact that can even affect the growth of the preterm infant [31]. Several studies have reported that parental stress level and maternal depression have concluded to be significant risk factors for future social, behavioural and functional development of preterm infants [32]. In fact, maternal postpartum depression (PPD) and psychological distress is likely to increase a child’s risk for delayed or impaired cognitive, emotional and linguistic development as well as subsequent behavioural problems [33]. More recent research reveals that parental PPD detrimentally influences parenting and positive enrichment activities such as reading and telling stories [34]. Even while evidence supports the correlation between parental mental health, childhood development and the emotionally taxing NICU environment, the trajectory of maternal and parental depression symptoms from NICU admissions through the transition home isn’t well understood.
In view of these emotional circumstances, which may have short– and long–term impact, it is necessary to develop effective interventions to offer targeted support to parents of infants admitted in NICU. It’s important all health professionals who attending to an infant also deliver comprehensive care to the parents. Awareness in NICU could provide better preventive management for families and a springboard from which primary care physicians can further assess emotional risk factors during follow–up visits. Supporting parental mental health, both for parents themselves as well as for the infant’s development chances, is recommended as part of follow–up care in the Netherlands (2014) [35,36]. Evidence shows that within NICUs, parents’ unlimited access to their infants, a trustworthy staff–parent partnership and substantial emotional support are associated with a decreased level of depression in parents [37]. Parents of premature babies are called “preterm parents”, all negatives feelings and the uncertain future of their babies put them in a position of fragility that could influence their relationship with their babies [38,39]. Therefore, supporting parents during the NICU stay could protect the development of this “preterm family” [40].
The main objective of the present study was to determine the depression levels of parents immediately after the premature birth of their infants admitted in the NICU, during hospitalization and at discharge of the hospital, as well as to identify possible associated maternal and neonatal factors.
Method Sample
The present study is a prospective, follow up–cohort study conducted from December 2019 to December 2022. The participants in this study were parents whose infants were admitted in NICU of a tertiary maternity hospital in Athens (Greece) – the “Helena Venizelou” Maternity Hospital. Parents who participated in the study gave birth to preterm infants admitted in NICU. The sample was sixty (60) couples of parents, whose infants BW was under 1750g and GA under 34w. Exclusion criteria for this study were: i) Parents under the age of eighteen, ii) Non-Greek speakers, iii) Parents whose infant had died during the hospitalization period and iv) Parents whose infants were transferred to another NICU in Athens. The Hospital Ethics Committee and the University Ethics Committee of the University of Ioannina approved the study, and all participants gave written informed consent to participate in present study.
Rating Instruments
To assess parental symptoms of depression we used Edinburg Postpartum Depression Scale (1970) (EPDS). This scale has been translated in many languages and is a valuable tool of screening of postpartum depression [41,42]. The EPDS is a 10– item self–report scale with four response options for each of them. Responses are scored from 0 to 3 points. With a maximum score of 30, higher scores indicate greater symptoms of depression. The present study used the cut–off score 11/12 that is consistent to the screening of major depression to Greek population [43].
Among strategies that have been devised to address the global burden of neonatal mortality are early warning scores in maternity hospitals. One of the available scores is the Apgar score that provides a convenient and accepted instrument for reporting the status of the newborn infant immediately after birth and the response to resuscitation if needed. Apgar score evaluates the colour, heartbeat, reflexes, muscular tone and respiratory. Each indication is scored (0), (1) or (2). Scores of 7 to 10 are reassuring [44]. The Apgar score on its own doesn’t predict individual neonatal mortality or neurologic outcome and shouldn’t be used for that purpose [45]. The assessment of the severity of illness is very important to determine prognosis, including predicting mortality in infants hospitalized in NICU [46]. The Clinical Risk Index for Babies (CRIB II) score is a risk–adjustment instrument widely used in NICUs [47]. It was developed more than 20 years ago and is based upon six parameters. Recently, its validity has been questioned since mortality in NICUs has fallen because of improvement in therapy and monitoring [48]. In addition, CRIB incorporate two potentially questionable variables: a) the fraction of inspired oxygen (FiO2), which isn’t a true physiological measure because it is determined by the care team and b) data collected up to 12 hours after admission, thus potentially introducing early treatment bias. In order to overcome these issues, a new, simplified and recalibrated five–item CRIB II score was developed in 2003. CRIB II score is a validated measure of initial mortality risk and illness severity within one hour of admission. It takes into account the BW, GA, body temperature, base excess and sex of the infant to determine initial mortality risk [49]. The CRIB II score ranged from 0 to 27, with better prognosis with lower scores attained the best favourable results with score of one [93]. It is only applicable to preterm neonates with BW <1500g, as well as those with the GA <31w [50,51].
A standard survey questionnaire was constructed by the researchers to collect demographic and social factors such as: age, nationality, residency, educational level, family income, health insurance, employment and marital status. Also, a perinatal questionnaire was constructed to collect data on the maternal health status, as well as pregnancy and labor clinical course, such as: number of pregnancies, number of alive children born, type of delivery, medicines prescribed, smoking, coffee and/or alcohol consumption. A second perinatal questionnaire was also constructed to collect data on neonatal health status & clinical outcome: GA, BW, height, date of admission in NICU, morbidities, clinical risk index for babies and predicted death rate.
Procedures
The parents who met the inclusion criteria entered the study under informed consent. The demographic data were collected before the administration of the screening scale. They completed the EPDS in the Maternity Hospital at 3rd– 4th day of life. The EPDS were re–administered at 20th–25th day postpartum and the 3rd assessment was at infants’ discharge. Follow–up was conducted by self–report screening.
Statistical analysis
Variables were first tested for normality using the Kolmogorov–Smirnov criterion. Quantitative variables were expressed as mean (Standard Deviation) or as median (interquantile range). Qualitative variables were expressed as absolute and relative frequencies. Repeated measurements analysis of variance (ANOVA) was adopted to evaluate the changes observed in depression scores over the follow up period, between parents and across sample’s characteristics. Percentages of depression were compared between parents and between time points via McNemar test. In order to find independent factors that were associated with depression scale at each time point, linear regression analysis models were conducted with a stepwise method from which regression coefficients (β) and their standard errors (SE) were emerged. All reported p values are two– tailed. Statistical significance was set at p<0.05 and analyses were conducted using SPSS statistical software (version 26.0).
Results
Data from 60 labors were recorded. Sample’s characteristics are presented in table 1. Mean mother’s age was 33.6 years (SD=6.7 years), and mean father’s age was 37.2 years (SD=5.6 years). Primiparous were 73.3% of the women, while in 85% of the cases conception was spontaneous. In 90% of the cases, they gave birth with a caesarian section and 76.7% had a single pregnancy. Characteristics of newborns are presented in table 2. Mean GA was 29.5w (SD=2.3w) and mean BW was 1230g (SD=335g). Mean hospitalization duration was 63.8 days (SD=27.7 days).
Mothers’ depression score in total sample and by under study characteristics are presented in table 3. At week 1 (3rd– 4th day), month 1 (20th–25th day) and at discharge, depression scores were found to differ significantly only by type of conception, with women with spontaneous conception having significantly more depressive symptoms compared with women who conceived by IVF. In women who were under 33 years old, in cases where the newborn was at most 1235g, in cases with CRIP IIScore≥7, with Apgar score 1'≥7, with a hospitalization duration of at least 62 days as well as in women without any health problem, the depression score at discharge was significantly lower compared to week 1 (3rd–4th day), month 1 (20th–25th day). In women who were at least 33 years old, in single pregnancies, in primiparous women, in women with normal conception, in cases with CRIP II score≥7, with Apgar score 5'≥8 and with duration hospitalization less than 62 days, the depression score decreased significantly from week 1 (3rd–4th day), month 1 (20th–25th day), from month 1 (20th–25th day) to discharge, and overall from week 1 (3rd–4th day) to discharge. In non-singleton pregnancies and in cases with an Apgar score of 5'<8, depressive symptoms significantly decreased from week 1 (3rd–4th day) to discharge. Also, in cases where the newborn was at least 1235g, in cases with an Apgar score of 1'<7 and in women with a health problem, the symptoms of depression in the 1st week (3rd–4th day) significantly greater compared to 1 month (20th–25th day) after and at discharge. The degree of reduction in depressive symptoms differed significantly according to the presence of a maternal health problem. Specifically, in women with a health problem, depression symptoms significantly decreased in the 1st month (20th–25th day), while in women without a health problem, depression symptoms decreased significantly at discharge.
Fathers’ depression score in total sample and by under study characteristics are presented in table 4. Depression score at discharge was found to differ significantly only by maternal age, where in cases where the mother was over 33 years old, fathers had significantly fewer depressive symptoms. In cases where the newborn was less than 1235 g the depression score at discharge was significantly lower compared to 1 week (3rd–4th day) and 1 month (20th–25th day). In cases where mothers were at least 33 years old, in single pregnancies, in primiparous women, in women with spontaneous conception, in cases with CRIP II score≥7 , with CRIP II score<7, with Apgar score 1'<7, with Apgar score 1'≥7, with Apgar score 5'≥8, with hospitalization duration less than 62 days, with hospitalization duration at least 62 days as well as in the cases when mothers had no health problem, fathers' depression scores decreased significantly from week 1 (3rd–4th day) to month 1(20th–25th day), from month 1(20th–25th day) to discharge, and overall from week 1(3rd–4th day) to discharge. Also, in cases where the newborn was at least 1235g, in those with an Apgar score of 5'<8 and in the cases where mothers had a health problem, the symptoms of depression in the 1st week (3rd–4th day) were significantly greater compared to 1 month (20th–25th day) after and to discharge. In cases where mother was up to 33 years old and in those who were not primiparous, depressive symptoms decreased significantly from week 1 (3rd–4th day) to discharge. The degree of reduction of depressive symptoms in fathers differed significantly only according to Apgar score 5'. Specifically, in cases with Apgar score of 5'<8 the score decreased from the 1st week (3rd–4th day) to the 1st month (20th–25th day), where it remained at similar levels until discharge, while in cases with Apgar score of 5'>8 the score decreased continuously until discharge.
Mothers’ and fathers’ depression score at each time point are presented in figure 1. At all time points mothers’ score was significantly higher than fathers’. Also, the depression of both parents at 1st week (3rd–4th day) was significantly greater than at 1st month (20th–25th day) as well as at discharge. Also, the depression of both parents at 1st month (20th–25th day) was significantly greater than at discharge. Overall, the degree of depression score’s decrease was similar in both parents (p=0.618).
Maternal depression percentage decreased significantly from week 1 (3rd–4th day) to month 1(20th–25th day) (p=0.013), from month 1 (20th–25th day) to discharge (p<0.001), and overall, from week 1 to discharge (p=0.013) (Figure 2). Fathers' depression rate decreased significantly from week 1 (3rd–4th day) to month 1 (20th–25th day) (p=0.012) and overall, from week 1 (3rd–4th day) to discharge (p<0.001), while between month 1 (20th–25th day) and discharge there was no significant change (p>0.05). Comparing fathers' and mothers' depression percentages per time point, it was found that mothers' percentages were significantly higher during the 1st week (3rd–4th day) (p=0.001), during the 1st month (20th–25th day) (p=0.001) and at discharge (p=0.004).
Via linear regression it was found that only type of conception (IVF) was found to be significantly associated with mothers' depression score at discharge. Specifically, mothers who conceived by IVF had a 4.58 points lower score, i.e., significantly fewer depressive symptoms at discharge, compared to mothers who conceived spontaneously (β=-4.58; SE=2.06; p=.031). Only mother's age was found to be significantly associated with fathers' depression score at discharge. Specifically, when the newborn's mother was at least 33 years old, the fathers had a 2.84 points lower score, i.e., significantly fewer depressive symptoms at discharge (β=-2.84; SE=1.31; p=.034).
|
Mothers' characteristics |
N (%) |
|
Mothers’ age. mean (SD) |
33.6 (6.7) |
|
Educational level |
|
|
Primary |
0 (0.0) |
|
Secondary |
13 (21.7) |
|
University |
45 (75.0) |
|
MSc |
2 (3.3) |
|
Family income |
|
|
<12.000 euro |
14 (23.3) |
|
12.000 - 23.999 euro |
33 (55.0) |
|
24.000 - 35.999 euro |
12 (20.0) |
|
35.000 - 47.999 euro |
1 (1.7) |
|
Family status |
|
|
Married |
51 (85.0) |
|
Single |
3 (5.0) |
|
Cohabitation |
6 (10.0) |
|
Health problem |
17 (28.3) |
|
Smoking |
|
|
Νο |
48 (80) |
|
Yes |
11 (18.3) |
|
Vaping |
1 (1.7) |
|
Fathers' characteristics |
|
|
Age (years). mean (SD) |
37.2 (5.6) |
|
Educational level |
|
|
Primary |
2 (3.3) |
|
Secondary |
23 (38.3) |
|
University |
35 (58.3) |
|
Pregnancy and labor characteristics |
|
|
Primiparous |
44 (73.3) |
|
Conception |
|
|
Spontaneous |
51 (85.0) |
|
IVF |
9 (15.0) |
|
Labor |
|
|
Vaginal |
6 (10.0) |
|
Caesarian section |
54 (90.0) |
|
Pregnancy |
|
|
Single |
46 (76.7) |
|
Twin |
12 (20.0) |
|
Multiple |
2 (3.3) |
Table 1: Sample characteristics
|
Mean (SD) |
Median (IQR) |
|
|
Gestational age at labor (weeks) |
29.5 (2.3) |
29.7 (28.3 - 31.1) |
|
Head circumference (cm) |
26.8 (2.5) |
27 (25 - 28.3) |
|
Birth weight (gr) |
1230 (335) |
1280 (990 - 1459.5) |
|
Height (cm) |
35.6 (5.7) |
36 (32 - 40) |
|
CRIP 2 SCORE |
6.9 (3.7) |
6.5 (3 - 9.5) |
|
Predicted death rate |
8.7 (14.3) |
3.5 (0.6 - 10.1) |
|
Apgar Score (1min) |
6.2 (1.6) |
7 (5 - 7) |
|
Apgar Score (5min) |
7.8 (1.2) |
8 (7 - 9) |
|
Hospitalization duration (days) |
63.8 (27.7) |
60 (44 - 80) |
Table 2: Characteristics of infants
|
Mothers’ depression score |
P2 |
P3 |
||||||
|
1st week |
1st month |
At dischar ge |
||||||
|
Mean (SD) |
Mean (SD) |
Mean (SD) |
1st week vs 1st month |
1st week vs at discharge |
1st month vs at discharge |
|||
|
Total sample |
14.9 (5.9) |
12.4 (6.2) |
10.3 (5.9) |
0.004 |
<0.001 |
0.002 |
- |
|
|
<33 |
15.2 |
13.6 |
11.6 |
0.389 |
0.005 |
0.049 |
0.263 |
|
|
Mothers’ age |
years |
(5.4) |
(5.8) |
(5.9) |
||||
|
≥33 years |
14.6 (6.6) |
11 (6.4) |
8.9 (5.7) |
0.006 |
<0.001 |
0.036 |
||
|
P1 |
0.68 |
0.111 |
0.075 |
|||||
|
Νο |
15.7 |
14.5 |
12.1 |
>0.999 |
0.057 |
0.087 |
0.484 |
|
|
Primiparo us |
(6.8) |
(7.9) |
(7.2) |
|||||
|
Yes |
14.6 (5.7) |
11.6 (5.3) |
9.7 (5.3) |
0.003 |
<0.001 |
0.019 |
||
|
P1 |
0.532 |
0.104 |
0.173 |
|||||
|
Νο |
13.4 |
10.6 |
8.6 (6) |
0.25 |
0.013 |
0.274 |
0.965 |
|
|
Single prengancy |
(7) |
(6.2) |
||||||
|
Yes |
15.3 (5.6) |
12.9 (6.1) |
10.9 (5.8) |
0.02 |
<0.001 |
0.008 |
||
|
P1 |
0.277 |
0.221 |
0.204 |
|||||
|
Spontane |
15.7 |
13 (6.1) |
11 (5.8) |
0.005 |
<0.001 |
0.005 |
0.787 |
|
|
Conceptio n |
ous |
(5.7) |
||||||
|
IVF |
10.1 (5.3) |
8.6 (5.7) |
6.4 (5.4) |
>0.999 |
0.218 |
0.455 |
||
|
P1 |
0.008 |
0.044 |
0.031 |
|||||
|
Hospitaliz ation duration |
<62 days |
15.1 (6.9) |
12 (6.5) |
10 (5.9) |
0.019 |
<0.001 |
0.049 |
0.649 |
|
≥62 days |
14.7 (5) |
12.7 (5.9) |
10.6 (5.9) |
0.192 |
0.001 |
0.036 |
||
|
P1 |
0.784 |
0.675 |
0.676 |
|||||
|
Birth weight |
<1235gr |
14.6 (6) |
12.2 (6.3) |
10 (6) |
0.091 |
<0.001 |
0.023 |
0.951 |
|
≥1235gr |
15.1 (6) |
12.5 (6.2) |
10.6 (5.9) |
0.05 |
<0.001 |
0.074 |
||
|
P1 |
0.748 |
0.885 |
0.697 |
|||||
|
CRIP II SCORE |
<7 |
14 (6.6) |
11.7 (6.5) |
9.5 (6) |
0.145 |
0.001 |
0.039 |
0.939 |
|
≥7 |
15.6 (5.3) |
12.9 (5.9) |
11 (5.8) |
0.031 |
<0.001 |
0.044 |
||
|
P1 |
0.282 |
0.443 |
0.337 |
|||||
|
Apgar score 1' |
<7 |
15 (6.2) |
11.7 (6.1) |
10.4 (5) |
0.015 |
<0.001 |
0.304 |
0.551 |
|
≥7 |
14.8 (5.8) |
12.9 (6.2) |
10.3 (6.6) |
0.216 |
<0.001 |
0.004 |
||
|
P1 |
0.922 |
0.46 |
0.977 |
|||||
|
Apgar score 5' |
<8 |
14.5 (6.4) |
12 (6.9) |
11 (5.6) |
0.154 |
0.025 |
0.871 |
0.471 |
|
≥8 |
15.1 (5.8) |
12.6 (5.8) |
10 (6.1) |
0.031 |
<0.001 |
0.001 |
||
|
P1 |
0.734 |
0.718 |
0.555 |
|||||
|
Mother’s health problem |
Νο |
14.5 (5.7) |
13 (5.8) |
10.8 (6.1) |
0.26 |
<0.001 |
0.005 |
0.047 |
|
Yes |
15.9 (6.6) |
10.8 (7) |
9.2 (5.4) |
0.001 |
<0.001 |
0.458 |
||
|
P1 |
0.418 |
0.214 |
0.369 |
|||||
1p-value for group effect 2 p-value for time effect 3p-value from repeated measures ANOVA, regarding time*group effect
Table 3: Mothers’ depression score in total sample and according to demographics and clinical characteristics
|
Fathers’ depression score |
P2 |
P3 |
||||||
|
1st week |
1st month |
At dischar ge |
||||||
|
Mean (SD) |
Mean (SD) |
Mean (SD) |
1st week vs 1st month |
1st week vs At discharge |
1st month vs At discharge |
|||
|
Total sample |
10.3 (5.8) |
8.2 (5.1) |
6.4 (5.2) |
0.001 |
<0.001 |
<0.001 |
- |
|
|
Mothers’ age |
<33 years |
10.6 (5.4) |
9.2 (5.3) |
7.8 (5.2) |
0.191 |
0.002 |
0.06 |
0.096 |
|
≥33 years |
9.9 (6.2) |
7 (4.8) |
5 (4.9) |
0.001 |
<0.001 |
0.003 |
||
|
P1 |
0.668 |
0.105 |
0.034 |
|||||
|
Primiparo us |
Νο |
10.6 (6.1) |
8.4 (6.3) |
7.5 (6.2) |
0.136 |
0.024 |
0.742 |
0.556 |
|
Yes |
10.2 (5.7) |
8 (4.7) |
6 (4.8) |
0.004 |
<0.001 |
<0.001 |
||
|
P1 |
0.814 |
0.797 |
0.344 |
|||||
|
Single prengancy |
Νο |
8.6 (6) |
8.1 (6.1) |
6.4 (5.8) |
>0.999 |
0.191 |
0.158 |
0.136 |
|
Yes |
10.8 (5.7) |
8.2 (4.9) |
6.5 (5.1) |
<0.001 |
<0.001 |
0.002 |
||
|
P1 |
0.214 |
0.949 |
0.951 |
|||||
|
Conceptio n |
Spontane ous |
10.5 (5.8) |
8.3 (5.1) |
6.5 (5) |
0.001 |
<0.001 |
0.001 |
0.859 |
|
IVF |
9.1 (6.1) |
7.6 (5.6) |
5.8 (6.5) |
0.796 |
0.088 |
0.317 |
||
|
P1 |
0.521 |
0.71 |
0.686 |
|||||
|
Hospitaliz ation duration |
<62 days |
10 (5.5) |
7.7 (5.3) |
5.9 (5.4) |
0.009 |
<0.001 |
0.015 |
0.8 |
|
≥62 days |
10.5 (6.2) |
8.6 (5) |
6.9 (5.1) |
0.045 |
<0.001 |
0.017 |
||
|
P1 |
0.767 |
0.476 |
0.446 |
|||||
|
Birth weight |
<1235gr |
10.1 (5.5) |
8.7 (5.1) |
6.5 (4.9) |
0.181 |
<0.001 |
0.001 |
0.383 |
|
≥1235gr |
10.4 (6.2) |
7.6 (5.2) |
6.4 (5.6) |
0.001 |
<0.001 |
0.118 |
||
|
P1 |
0.826 |
0.441 |
0.961 |
|||||
|
CRIP II SCORE |
<7 |
10.1 (6.2) |
7.6 (5.3) |
5.9 (5.6) |
0.009 |
<0.001 |
0.029 |
0.76 |
|
≥7 |
10.4 (5.5) |
8.6 (5) |
6.8 (4.9) |
0.043 |
<0.001 |
0.009 |
||
|
P1 |
0.818 |
0.452 |
0.5 |
|||||
|
Apgar score 1' |
<7 |
10.4 (6) |
8.4 (5.5) |
6.8 (4.9) |
0.036 |
<0.001 |
0.039 |
0.897 |
|
≥7 |
10.2 (5.7) |
8 (4.9) |
6.1 (5.6) |
0.013 |
<0.001 |
0.006 |
||
|
P1 |
0.876 |
0.773 |
0.629 |
|||||
|
Apgar score 5' |
<8 |
10.3 (6.4) |
7.6 (5.2) |
7.7 (5.1) |
0.012 |
0.022 |
>0.999 |
0.043 |
|
≥8 |
10.2 (5.5) |
8.4 (5.2) |
5.8 (5.2) |
0.026 |
<0.001 |
<0.001 |
||
|
P1 |
0.949 |
0.562 |
0.181 |
|||||
|
Mother’s health problem |
Νο |
10.6 (5.9) |
8.9 (5) |
6.7 (5.4) |
0.031 |
<0.001 |
<0.001 |
0.23 |
|
Yes |
9.5 (5.5) |
6.2 (5.1) |
5.8 (4.8) |
0.005 |
0.003 |
>0.999 |
||
|
P1 |
0.54 |
0.069 |
0.537 |
|||||
1p-value for group effect 2 p-value for time effect 3p-value from repeated measures ANOVA, regarding time*group effect
Table 4: Fathers’ depression score in total sample and according to demographics and clinical characteristics
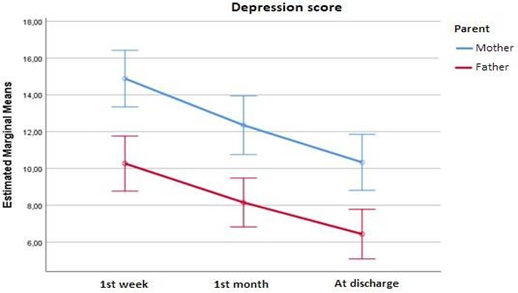
Figure 1: Mothers’ and fathers’ depression score by time point
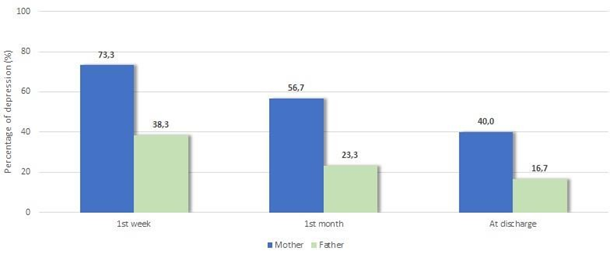
Figure 2: Proportion of mothers and fathers with depression during study follow up
Discussion
Preterm birth may be a stressful event for families. In particular, the unexpectedly preterm birth may cause negative feelings in both parents. These parents are often not mentally prepared for the experience of NICU, they become parents of preterm infants in an unfamiliar environment where they face chaotic, surreal and deeply depressing situations [52]. Much of caregiving in the NICU environment experienced by the infants is related to medical intervention. The NICU environment is often infant–centered instead of family–centered. In particular, since there is still a gap in literature about mothers’ and especially fathers’ experience and reaction to the premature birth and long hospitalization of their infants, this study explores parental depression immediately after infants’ birth, hospitalization and discharge of NICU.
Our findings suggested that both parents of preterm infants experienced elevated levels of depression symptoms shortly after birth [mothers: Mean (SD)=14.9(5.9) & fathers: Mean (SD)=10.3(5.8)]. This finding corroborated by the results of older studies [53,54,39], which also showed that parents of hospitalized preterm infants are at high risk to develop depressive symptoms. In particular, Alkozei A. et all (2014) in USA using EPDS to a sample of 85 mothers of preterm infants born between 25–34w GA, showed that 38% of mothers had significantly depressive symptoms (EPDS >10). In the same way, Ionio C. et all (2016) in Italy, examined 40 parents of preterm infants (GA=30.96±2.97) and 50 parents of full-term infants (GA=39.19±1.42), who filled out five scales; one of them was Profile of Mood States (POMS). Their findings showed that parents of preterm infants had more depressive symptoms and other feelings (hostility, anger) than parents of full–term infants. Also, an observational longitudinal study at north–eastern US, included 146 fathers with infants in NICU (GA=31.9w), using EPDS concluded that overall EPDS scores improved over time (p<.001) [81].
Another interesting finding in our study was that feelings of depression improved in the period just before infant’s discharge from NICU. However, they remained at significant levels. Specifically, mothers’ depression levels at 1st time point were increased [Mean (SD)=14.9(5.9)] while at discharge they were reduced [Mean (SD)=10.3(5.9)]. Similarly, fathers’ depression levels at 1st time point were increased [Mean (SD)=10.3(5.8)] while at discharge they were reduced [Mean (SD)=6.4(5.2)]. According to recent systematic reviews and meta–analysis, approximately 17% of mothers and 10% of fathers met criteria for perinatal depression after infants’ birth with an increasing prevalence during the first 3–6 months [55,56]. In agreement with our results, the findings of Pace C. C. et all (2016) in Australia suggested that parents (n=214) of preterm infants (GA<30w) had elevated rates of depression symptoms (assessed by Central for Epidemiological Studies Depression Scale – CESD) that declined over the time, although remaining above expected levels throughout the newborn period and at 6 months. Additionally, a study from Turkey included 110 couples and used EPDS to parents of 68 infants (mean GA=31.2±3.65w & mean BW=1542±743g), showed that parental depression was increased in 2nd week after birth compared with 6th week after birth. These results suggested that parental depressive symptoms improved during the NICU stay [57]. Also, a prospective longitudinal cohort study used EPDS to parents (n=431) at 3 assessments in NICU and one after discharge of NICU, concluded that parental depressive symptoms decreased over the time of postnatal period [58]. On the other hand, a prospective mixed method study was conducted in Australia, showed that fathers (n=22), who administered EPDS had high rates of depression, that can persist for months after the preterm birth [59]. Also, another study used the CESD–10, concluded that 45% of parents (n=300) reported depressive symptoms at NICU discharge [60].
Our study also highlighted gender differences: mothers tended to experience higher levels of depression than fathers. Comparing fathers' and mothers' depression percentages per time point, it was found that mothers' percentages were significantly higher during the 1st week (3rd–4th day) (p=0.001), during the 1st month (20th–25th day) (p=0.001) and at discharge (p=0.004). In previous studies on this topic, researchers have demonstrated that mothers, after their infants’ premature births and NICU stays, felt more depressed than their husbands/partners did. In particular [82], in Greece, studied 200 parents with preterm infants (GA<36 weeks) in the NICU and all parents completed Zung Self–Rating Depression Scale (SDS). They concluded that mothers experienced higher levels of depression than fathers (p=0.001). Our findings corroborated by the results of a recent research on this topic by [83], using the POMS scale to 43 mother and 38 fathers suggesting that mothers had higher levels of depression–dejection than fathers (p=0.011). Also, in a clinical study at
Chicago, using EPDS, studied postpartum depression among mothers and fathers (n=431) of preterm infants over the course of their admission in NICU, transition at a home and first 30 days at home, found that mothers were more likely to have a positive screen than fathers [58].
The transaction to motherhood is undoubtedly an important aspect in women’s life. The experience of being pregnant and giving birth is completely different than any other experience of men [61]. Pregnancy and childbirth are complex events, accompanied by biological, social and emotional changes [62]. Women by nature are more sensitive and psychologically weak than men and respond to postpartum depression differently [58]. Mothers mourn the loss of a healthy newborn, in contrast to the appearance and behaviour of their preterm baby, with it weak and naked between electrodes or attached to unknown mechanisms [29]. Mothers of preterm infants are characterised by a greater “fear of loss of the baby” (a dimension related to maternal perception of infant’s current condition) and more negative “first feelings towards the baby”. Additionally, they don’t feel ready for discharge and the narratives of their representation were disorganised [63]. Fathers don’t experience the NICU as mothers do [39]. Also, a number of possible explanations for the gender difference are provided by many researchers [64,20,52,47,65,39,58]. A possible explanation is that mothers use more escape coping, which is associated with negative mood and fathers use practical coping, which is associated with positive mood. A second explanation is that mothers left their daily routines and spent hours in the NICU, where they continue to experience infants’ fragility and mortality [30]. Also, they feel guiltier than fathers for their infants’ prematurity. In fact, fathers usually aren’t expected to be actively involved in their infant’s care as mothers, and their participation helps them to cope with negative feelings and adapt to their parental role.
Another interesting finding in our study was that the degree of reduction in depressive symptoms differed significantly according to the presence of a maternal health problem (perinatal complications, dysfunction of thyroid, preeclampsia, anaemia, gestational diabetes). Specifically, in women with a health problem, depression symptoms significantly decreased in the 1st month (20th–25th day) [1st time point: Mean (SD)=15.9(6.6) vs 2nd time point: Mean (SD)=10.8(7.0); p = 0.001], while in women without a health problem, depression symptoms decreased significantly at discharge [1st time point: Mean (SD)=14.5(5.7) vs 3rd time point: Mean (SD)=10.8(6.1); p<0.001]. Health problems during pregnancy are physically and emotionally taxing for parents, and especially for mothers. The literature review showed that various researchers have attributed mental disorders to the thyroid dysfunction [66,67]. A recent study in Greece aimed to investigate associations between perinatal complications and maternal mood at delivery and the early postpartum period as determinants of postpartum period, through the EPDS at 8 weeks after birth showed that gestational hypertension and/or preeclampsia was significantly associated with higher postpartum symptoms [68]. According to Bon E. A. et all (2010) in Sweden, the risk of postpartum depression increased with higher number of perinatal complications (p<0.001). A recent study by Konstantakou et all (2021) [84] in Greece, using EPDS & BDI scale, showed that there were positive associations between low–normal thyroid function at pregnancy and postpartum depression, anxiety, obsessive compulsive disorders (OCD) scores. Similarly, a study by Kanti V. et all (2022) [85], studied 28 pregnant women, showed that the thyroid dysfunction has positive correlation with postpartum depression (EPDS). In contrast to these studies, a study that conducted this year by Noshiro K., et all (2023) [84], at 99 pregnant women with EPDS, showed that weren’t significant correlations between thyroid hormones and postpartum depression. At the same page, a study of Simionescu A. et all (2019) [85] conducted in Romania, used EPDS for depression to 61 women during pregnancy and at least one year after giving birth, found that screening for thyroid disorders during pregnancy may not provide relevant information for detecting postpartum depression. Another health problem, consisting a risk factor for postpartum depression, is glucose metabolism disorders during pregnancy. A recent study in West China, showed that women with gestational diabetes mellitus (GDM) were more susceptible to postpartum depression than others without the condition, despite their depressive symptoms reducing over the time after childbirth [69]. In contrast, a retrospective cohort study in Canada, showed that GDM was associated with increased risk of depression in women particularly during pregnancy, but not during postnatal period [70]. Furthermore, anaemia is a common problem for women and specifically in the period of pregnancy [71]. According to WHO (2018) the prevalence of anaemia in this period is high (about 40% or more) and can continue at least 3 months postpartum, due to additional physiological demands. A prospective cohort study in Spain found that mothers (n=729) with low levels of iron marker, were more likely to have postpartum depression at 48 hours postpartum [72]. A population-based study of all singleton births (n = 511,938) in Finland concluded that anaemia is predictor of depression during pregnancy [73]. Also, another study in New South Wales (n=2.568), investigated the rates of hospitalization for anaemia and depression in women, showed that there was no relationship between maternal iron status and postnatal depression [74]. The association between pregnancy, perinatal, postpartum complications and postpartum depression is far from being elucidated. Future studies are needed to confirm the findings of our study and better understand the complex underlying processes.
Our study showed that mothers of infants who were conceived by IVF experienced low levels of depression during NICU stay. Specifically, mothers who conceived by IVF had a 4.58 points lower score, i.e., significantly fewer depressive symptoms at discharge, compared to mothers who conceived spontaneously (β=-4.58; SE=2.06; p=.031). Our finding corroborated of the case-control study of Vikstrom J. et all (2017) [86] in Sweden, which consisted of 3.532 pregnant women and diagnosed according to the International Classification of Diseases (ICD–10), concluded that women who received IVF treatment weren’t at risk of postnatal depression, the risk was increased among women with a history of mental illness. In the same page, a prospective cohort study of Mori E. et all (2018) [87] with participants (n=2.709 women) being recruited from 13 Japanese hospitals, observed that there wasn’t significant association between the mode of conception and depressive symptoms. Also, a prospective cohort study was conducted on postnatal mothers in 2019, showed that mode of conception wasn’t associated with an increase in postpartum depression among women who underwent infertility treatment [75]. Our results confirm above studies and lead to hypothesis that the transition to parenthood is a difficult process for parents who conceived by IVF. Mothers under infertility treatment experience more concerns, anxieties and fears during pregnancy and they are prepared for complications at birth such a preterm birth. In contrast, mothers who receive spontaneous aren’t prepared for any complication and when their infant has been hospitalized in the NICU, they are in “sock”. Many studies have made about the effect of IVF on postpartum depression. Their results are contradictory. According to Lee H. et all (2011), who used the Beck’s scale to 60 women, the prevalence of postpartum depression was 25%. Also, pearson correlation analysis showed that the frequency of receiving IVF treatment was positively correlated with postpartum depression. Also, a study of Alexopoulou P. et all (2018) to Greece, measured depression, among parents (n=200) of preterm infants (GA<36 weeks) hospitalized in NICU and all parents completed SDS showed that parents who were conceived by IVF experienced higher levels of depression compared with them who conceived spontaneously (p=0.055). In general, IVF treatment seems to have a complex and unique psychological influence, even if doesn’t increase the rate of postpartum depression [76].
Another interesting finding was that the degree of reduction of depressive symptoms in fathers differed significantly only according to Apgar score at 5'. Specifically, in cases with Apgar score of 5'<8 the score decreased from the 1st week to the 1st month, where it remained at similar levels until discharge, while in cases with Apgar score of 5'>8 the score decreased continuously until discharge. Our findings corroborated by older study of Ionio C. et all (2019), that also showed for fathers (n=38) of preterm infants, the Apgar score of 5' was significantly predicted depression, tension–anxiety and fatigue–inertia. In contrast, a newer descriptive cross-sectional study of Ünal N. et all (2023) [89] conducted in Turkey, having a sample of 60 parents with infants hospitalized in the NICU and using the PSS:NICU scale, showed that the Apgar score of 5' wasn’t significantly correlated with fathers’ stress scores. On the other hand, the Apgar score of 1' & 5' was significantly correlated with mothers’ stress scores (p<0.05). This is a finding that is inconsistent with our study that showed nonexistent correlation between Apgar score of 1'& 5' and mothers’ depression and anxiety. Usually, fathers are the ones who accompany their infant in NICU, because mothers are still recovering after birth. They have to make initial decisions, support the mother, find out information about infant’s condition and communicate to mother and other members of family, care for other children and sometimes have to return to work within few days of infant’s birth [77]. In this context they are often left alone, despite themselves being in state of concern and disorientation. Also, they are confronted with infant’s severity condition, a combination that can have a significant impact on their psychological state including affective disorders such as depression and anxiety. The Apgar score used in first five minutes as a screening instrument to assess the need of early intervention and in the fifth minute to assess how infants have reacted to previous care, remaining relevant for predicting neonatal survival. A low Apgar score of 5' and especially above 10 minutes of life is associated with sensor neural defects and additionally with long-term neurological morbidity, especially when both scores were low [78]. Still, in the fifth minute, the Apgar score is also relevant for the prediction of neonatal survival [44]. All of the above evidence demonstrates the complexity of the association of the Apgar score at 5' with parental depression, which requires further research.
Only mother's age was found to be significantly associated with fathers' depression score at discharge. Specifically, when the newborn's mother was at least 33 years old, the fathers had a 2.84 points lower score, i.e., significantly fewer depressive symptoms at discharge (β=-2.84; SE=1.31; p=.034). Other studies showed that after birth premature birth fathers have a supportive role [54,55]. Our study suggested that fathers with younger partners had increased the feeling of protection towards them, perhaps due to their inexperience which is consistent with their young age. Indeed, mothers with mean age 34(SD=6.3) in NICU reported that cope better when fathers are present. The fathers often tried to fit the role of strong postpartum partner, and they hid their own needs. In the cases where mothers were seriously ill postpartum, fathers were more concerned about the mother than their newborn infant, whereas the mothers were most concerned about their infant. Mothers experienced more stress when accompanying the baby in the NICU without having the father present [79]. At the same page, a multi–method longitudinal study of fathers (n=20) and mean maternal age 39.4(4.5) with preterm infants [Mean GA:30+2(3) & BW:1.389(.455) g] in level III-NICU in Italy showed that they put aside their own needs for support and relaxation to take care of their partner and infant. When mothers experienced postpartum complications, fathers divided the worries and attentions almost equally between partner and infant. These fathers mentioned the fear of losing both partner and infant(s) going back and forth between them in the first minutes, hours and days after birth [80].
Study limitations and need for further research
They are several limitations in this study that have to be mentioned. First of all, the sample consisted of parents with preterm infants. These infants needed specialise and specific care, as a result the equipment was often not available to cover their care. In these cases, the infants transferred to NICU of another hospital for treatment. Furthermore, more of these infants belonged to vulnerable groups and some of them didn’t survive. The above factors were contributed to influence the population of our study because we lost the communication with parents and consequently excluded of our study. Secondly, more factors that influenced the parental depression should examined, such as mental health problems, personality traits and relationships with the staff of the NICU. Also, limitation of this study was the use of self-reported questionnaire, which was a valuable resource for psychological research, but reliance only on them may lead to subjective bias report. Although the NICU hosted preterm infants of several regions of Greece, the results of this study can’t be generalised to the Greek population due the size of the sample.
Conclusions
This study investigated parents’ experience after the preterm birth of their infants and their hospitalization in the NICU. Our findings have suggested that parents of preterm infants, in particular mothers, are at risk of developing higher levels of depression. Furthermore, the severity of depression in parents of preterm infants hospitalized in the NICUs is influenced by various factors of parents themselves, as well as clinical characteristics of infants. The NICU environment can be stressful for most parents and influence their emotional state. The scientific community ought to run more studies about the factors that have a positive or negative impact on parents’ mental health and establish, as soon as possible, Interdisciplinary Perinatal Support Groups and Interventions to help parents through the experience of premature birth of their infants as well as begin immediately adaptive mode of care.
Declaration of interest
The authors declare no conflicts of interest with respect to the authorship, research and publication of the present article. The authors alone are responsible for the content and writing of the study.
References
- Blencowe H, Counsens S, Oestergaard M, et National, regional and worldwide estimates of preterm birth rate I the year 2010 with trends since 1990 for selected countries: A systematic analysis and implications. The Lancet 379 (2022): 2162-2172.
- WHO (2018). Pretermbirth. [sited 2021. Non. 14] available from: https://www.who.int/news-room/fact- sheets/detail/ Howson C P, Kinney M V, McDougall L, et al. Born Too Soon: preterm birth matters. Reprod. Health Suppl 1(Suppl 1) 10 (2013): S1.
- Sansavini AJ, & Chandler Lo sviluppo dei bambini nati pretermine: Aspetti neuropsychological, metodi di valutazione e intervini. Milano, Italy: Franco Angeli (2013): 1-10.
- Muller – Nix C, & Ansermet Prematurity, risk and prospective factors. In C. Zeanah Jr. (Ed). Handbook of infant mental health (3rd ed.). New York. USA: The Guildford Press 12 (2009): 604-621.
- Martin JA, Hamilton BE, Ventura SJ, et Births: Final data for 2010. National vital statistics reports 61 (2012): 1-72.
- Reihani T, Sekhavat pour Z, Heidarzadeh M, et Investigating the effect of spiritual self-care training on psychological stress of mothers with preterm infants admitted in neonatal intensive care unit (Persian). Iranian Journal of Obstetrics, Gynecology and Infertility 17 (2014): 18-28
- Marshall G, Luque MJ, Gonzalez AD, et Centre variability in risk of adjusted length of stay for very low birth weight infants in the Neocosur South American Network. J Pediatr (Rio J) (2012): 88(6):524-30
- Aagaard H, Hall Mothers’ experience of having a preterm infant in the neonatal care unit. A meta-synthesis. Journal of Pediatric Nursing 23 (2008): e26-e36.
- Arnold L, Sawyer A, Rabe H, et Parents’ first moments with their very preterm babies: A qualitative study. BMJ Open 3 (2013): e0002487.
- Jackson K, Ternetestedt BM, & Schollin From alienation to familiarity: Experience of mother and father of preterm infants. Journal of Advanced Nursing 43 (2003): 120-129.
- Mahoney Helping parents survive the emotional “roller coaster ride” in the newborn intensive care unit. Journal of Perinatology: Official Journal of the California Perinatal Association 14 (1994): 131-133.
- Orapiriyakul R, Jirapaet V, Rodcumdee Struggling to get connected: The process of maternal attachment to the preterm infant in the neonatal intensive care unit. The Journal of Nursing Research 11 (2007):251-263.
- Ionio C, Mascheroni E, Colombo C, et Stress and feelings in mothers and fathers in NICU: identifying risk for early interventions. Primary Health Care Research & Development 20 (2019): 1-7.
- Candelori C, Trymello C, Babore A, et The experience of premature birth for fathers: The application of the Clinical Interview for Parents of High-Risk Infants (CLIP) to an Italian sample. Frontiers in Psychology 6 (2015): 1444.
- Lundoqvist P, & Jakobson Swedish men’s experience of becoming fathers to their preterm infants. Neonatal Network 22 (2003): 25-31.
- Pohlman The primary of work and fathering preterm infants: Findings from an interpretive phenomenological study. Advance in Neonatal Care 5 (2005): 204-216.
- Arockiasamy V, Holsti L, Albersheim Fathers’ experience in the neonatal intensive care unit: a search for control. Pediatrics 121 (2008): e215-22.
- Carter JD, Mulder RT, Bertram AF, et Infants in a intensive care unit: parental response. Arch Dis Child Fetal Neonatal Ed 90 (2005): 109-113.
- Bell Adolescent mothers’ perception of the neonatal intensive care unit environment. J Perinatal & Neonatal Nursing 11 (1997): 77-84.
- Sullivan Development of father-infant attachment in father of preterm infants. Neonatal Netw 18 (1999): 33-39.
- Juliana Arantes Figueiredo de Paula Eduardo, Marcos Goncalves de Rezende, Paulo Rossi Menezes, et Preterm birth as a risk factor for postpartum depression: A systematic review and meta-analysis. Journal Affect Disord 259 (2019): 392-403.
- Persson C, Ericson J, Salari R, et NICU parents’ mental health: A comparative study with parents of term and healthy infants. Acta Paediatrica 1 (2023): 1-13
- Gondwe KW, White-Traut R, Brandon D, et The role of sociodemographic factors in maternal psychological distress and mothers-preterm infant interactions. Res Nurs Health 40 (2017): 528-540
- Winter L, Colditz PB, Sanders MR, et all. Depression, post-traumatic stress and relationship distress in parents of very preterm Arch Women’s Ment Health 21 (2018): 445-451
- Mackley AB, Locke RG, Spear ML, et Forgotten parent: NICU parental emotional response. Adv. Neonatal Care 10 (2010): 200-203
- Singer LT, Fulton SMA, Davillier MLSW, et al. Effects on infant risk status and maternal psychological distress on maternal-infant interactions during the first year of Journal of Developmental and Behavioural Pediatrics 24 (2003): 233-241.
- Hoffenkamp HN, Bracken J, Hall RA, et al. Parenting in complex conditions: Does preterm birth provide a context for the development of less optimal parental Journal of Pediatric Psychology 40 (2015): 559-571.
- Davis L, Edwards H, Mohay H, et The impact of very premature birth on the psychological health of mothers. Early Hum Dev 73 (2003): 61-70
- Clottey M, Dillard Post-traumatic stress disorder and neonatal intensive care. The International Journal of Children Education 28 (2013): 23-29
- Zelkowitz P, Bardin C, Papageorgiou Anxiety affects the relationship between parents and their very low birth weight infants. Infant Ment Health J 28 (2007): 296-313.
- Huhtala M, Korja R, Lehtonen L, et all. Associations between parental psychological well-being and socio-emotional development in 5-year-old preterm Early Hum Dev 90 (2014): 119-124.
- Santoro K, Peabody H. Identifying and treating maternal depression: strategies & considerations for health plans. Washington, DC: National institutes of Health care Management 1 (2010): 1-25.
- Davis RN, Davis MM, Freed GL, et al. Fathers’ depression related to positive and negative parenting behaviours with 1-old-year Paediatrics 127 (2011): 612-618.
- McGowan EC, & Vohr BR. Neurodevelopment follow-up of preterm infants: what’s new? Pediatri Clin North Am 66 (2019): 509-523.
- Jeukens-Visser M, Koldewijn K, van Wassenaer-Leemhuis AG, et al. Development and nationwide implementation of a postdischarge response parenting intervention program for very preterm born children: the TOP Infant Ment Health J 42 (2020): 423-437
- Axelin A, Feeley A, Campbell-Yeo M, et Symptoms of depression in parents after discharge from NICU associated with family-centered care. Journal of Advanced Nursing 78 (2021): 1676-1687
- Teti DM, Hess CR, O’Connell MA. Parental perceptions of infant vulnerability in a preterm sample: Prediction from maternal adaptation to parenthood during the neonatal Journal of Developmental and Behavioural Pediatrics 26 (2005): 283-292.
- Ionio C, Colombo C, Brazzoduro V, et Mothers and Fathers in NICU: The impact of Preterm Birth on Parental Distress. Europe’s Journal of Psychology 12 (2016): 604-621
- Korja R, Ahlqvist-Bojorkroth S, Savonlaht E, et Relations between maternal attachment representations and the quality of mother-infant interaction in preterm and full-term infants. Infant Behavior and Development 33 (2010): 330336.
- Cox JL, Holden JM, Sagovsky Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 150 (1987): 782-786
- Cox JL, Chapman G, Murray D, et Validation of the Edinburgh postnatal depression scale (EPDS) in non - postnatal women 39 (1996): 185-187.
- Leonardou AA, Zervas MI, Papageorgiou CC, et Validation of the Edinburg Postnatal Depression Scale in a sample of Greek mothers. Journal Reproductive Infant Psychol 27 (2009): 28-39.
- Simon VL, Hashmi FM, Bragg APGAR Score. StatPearls Publishing LLC. Bookshelf ID: NBK470569 (2022).
- Stark AR, Adamkin DH, Batton DG, et The apgar score. Pediatrics 117 (2006): 1444-1447.
- Thimoty J, Hilmanto D, Yuniati Score for Neonatal Acute Physiology Perinatal Extension II (SNAPPE II) as the predictor of neonatal mortality hospitalized in neonatal intensive care unit. Paediatr Indones 49 (2009): 155-159.
- International Neonatal The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. Lancet 342 (1993): 193-198.
- Mohkam M, Afjeii A, Payandeh P, et A comparison of CRIB, CRIB II, SNAP, SNAPII and SNAP-PE scores for prediction of mortality in critically ill neonates. MJIRI 24 (2011):193-199.
- Parry G, Tucker J, Tarnow-Mordi CRIB II: an update of the clinical risk index for babies score. Lancet 361 (2003): 1789-1791.
- Patrick SW, Schumacher RE, Davis Methods of mortality risk adjustment in the NICU: a 20-years review. Pediatrics 131 (2013): 68-74.
- Lee S M, Lee MH, Chang The clinical risk index for babies II of time-dependent mortality and short-term morbidities in very low birth weight infants. Neonatology 116 (2019): 244-251
- Franck L, Cox S, Allen A, et Measuring neonatal intensive care unit – related parental stress. Journal of advanced nursing 49 (2005): 608-615
- Obeidat HM, Bond EA, et The parental experience of having an infant in the newborn intensive care unit. The Journal of perinatal education 18 (2009): 3-10.
- Alkozei A, McMahon E, Lahaina Stress levels and depression symptoms in NICU mothers in the early postpartum period. The journal of maternal–fetal & neonatal medicine 27 (2014): 1738-1743.
- Cameron EE, Sedona ID, Tomfohr – Modsen Prevalence of depression in pregnancy and parental depression in pregnancy and postpartum: An updated mate-analysis. Journal Affect Disord 206 (2016): 189-203.
- Shorey S, Yin Ing Chee C, Debby Ng E, et Prevalence and incidence of postpartum depression among healthy mothers: A systematic review and meta-analysis. J Psychiatr Res 104 (2018): 235-248.
- Gonulal D, Yalaz M, Althun - Koraglu O, Kultursay Both parents of neonatal intensive care unit patients are at risk of depression. Turkey J Pediatr 56 (2014): 171-176.
- Garfield FC, Lee SY, Warner – Shifflett L, et Maternal and Parental Depression Symptoms During NICU Stay and Transition Home. Pediatrics 148 (2021): e2020042747
- Kothari A, Bruxner G, Dulhunty MJ, et Dads in Distress: symptoms of depression traumatic stress in fathers following poor fetal, neonatal, and maternal outcomes. BMC Pregnancy and Childbirth 22 (2022): 956.
- Soghier ML, Kritikos IK, Carty LC, et Parental Depression Symptoms at Neonatal Intensive Care Unit Discharge and Associated Risk Factors. J. Pediatr 227 (2020): 163-169.
- Wellon V Η σκοτεινη Πλεσρα της Μητρ?τητας. Αθηνα. Ελληνικα Γραμματα (1997).
- O’Hara WM, Swain Rates and risk of postnatal depression – a metanalysis. International Review of Psychiatry 8 (1996): 37-54.
- Trumbello C, Candelori C, Cofini M, et al. Mothers’ Depression, Anxiety, and Mental Representations After Preterm Birth: A Study During the Infant’s Hospitalization in a Neonatal Intensive Care Unit. Frontiers in Public Health 6 (2018): 359
- Affleck G, Tennen The effect of newborn intensive care on parents’ psychological well – being. Child Health Care 20 (1991): 6-14
- Pallas-Alonso RC, Losacco V, Maraschini A, et al. Parental involvement and kangaroo care in European neonatal intensive care units: a policy survey in eight Pediatr Crit Care Med 13 (2012): 568-577.
- Pedersen CA, Stern RA, Pate J, et Thyroid and adrenal measures during late pregnancy and puerperal in woman who have major depressed or who become dysphonic postpartum. Journal of Affective Disorders 29 (1993): 201-211.
- Keshavarzi F, Yazdchi K, Rahimi M, et al. Post Partum Depression and Thyroid Function. Iranian J Psychiatry 6 (2011): 117-120.
- Koutra K, Vassilaki M, Georgiou V, et al. Pregnancy, perinatal and postpartum complications as determinants of postpartum depression: the Rhea mother-child cohort in Crete, Greece. Epidemiology and Psychiatric Science 27 (2018): 244-255.
- Mak KLJ, Lee HA, Pham MN, et Gestational diabetes and postnatal depressive symptoms: A prospective cohort study in Western China. Women Birth 32 (2019): e427-e431.
- Pace R, Rahme E, Da Costa D, et al. Association between gestational diabetes mellitus and depression in parents: a retrospective cohort Clinical Epidemiology 10 (2018): 1827-1838
- Stevens AG, Finucane MM, De-Regil ML, et Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. The Lancet Glob Health 1 (2013): e16-25.
- Albacar G, Sans T, Martin-Santos R, et al. An association between plasma ferritin concentrations measured 48h after delivery and postpartum J Affect Disord 131 (2011): 136-142.
- Räisänen S, Lehto MS, Svarre Nielsen H, et Risk factors for and perinatal outcomes of major depression during pregnancy: a population-based analysis during 2002-2010 in Finland. BMJ Open 4 (2014): e004883.
- Armony-Sivan R, Eidelman AI, Lanir A, et al. Iron status and neurobehavioral development of premature infants. Perinatol 24 (2004): 757-762.
- Murugandam P, Shanmugam D, Ramachandran Does the Mode of Conception Influence Early Postpartum Depression? A Prospective Comparative Study from South India. Indian Journal of Psychological Medicine 42 (2020): 525-529
- Burns Parenting after infertility. In S.n. Covington & Burns L.H. (Eds), Infertility Counselling: A Comprehensive Handbook for Clinicians. Cambridge University Press 1 (2006): 493-507
- Logan RM, Dormine Finding My Way: A Phenomenology of Fathering in then NICU. Advances in Neonatal Care 18 (2018): 154-162.
- Leinonen E, Gissler M, Haataja L, et al. Low Apgar scores at both one and five minutes are associated with long- term neurological Acta paediatr 107 (2018): 942-951.
- Hagen IH, Iversen VC, Svindseth MF. Differences and similarities between mothers and fathers of premature children: a qualitative study of parents’ coping experiences in neonatal intensive care unit. BMC pediatrics 16 (2016): 92-
- Stefana A, Padovani ME, Biban P, et al. Fathers’ experiences with their preterm babies admitted to neonatal intensive care unit: A multi-method study. J Adv Nurs 74 (2018): 1090-1098.
- Cyr – Alves H, Lynn CM, Hyrkas K. Depression Screening Using the Edinburgh Postnatal Depression Scale for US Fathers of Critically III JOGNN 47 (2018): 146-157.
- Alexopoulou P, Evagelou E, Mpakoula – Tzoumaka C, et Assessing anxiety and depression in parents of preterm infants. Journal of Neonatal Nursing 24 (2018): 273-276
- Ionio C, Mascheroni E, Colombo C, et al. Stress and feelings in mothers and fathers in NICU: identifying risk for early Primary Health Care Research & Development 20 (2019): 1-7.
- Konstantakou P, Chalarakis N, Valsamakis G, et al. Associations of Thyroid Hormones Profile During Normal Pregnancy and Postpartum with Anxiety, Depression, and Obsessive/Compulsive Disorder Scores in Euthyroid Women. Frontiers in Neuroscience 15 (2021):
- Kanti V, Kumar S, Potturi G, et al. Correlation of third – trimester thyroid profile with postpartum depression in rural Journal of pharmaceutical and Allied Sciences 11 (2022): 3708-4711
- Noshiro K, Umazume T, Inubashiri M, et Association between Edinburg Postnatal Depression Scale and Serum Levels of Ketone Bodies and Vitamin D, Thyroid Function, and Iron Metabolism. Nutrients 15 (2023): 768-778.
- Simionescu AA, Marin Postpartum depression and thyroid dysfunction-should pregnant women be screened for thyroid disorders? J Mind Med Sci 6 (2019): 103-109.
- Vikström EJ, Vikström G, Hammar M, et Risk of postnatal depression or suicide after in vitro fertilisation treatment: a nationwide case-control study. BJOG 124 (2017): 435-442.
- Mori E, Iwata H, Maehara K, et Relationship between the mode of conception and depressive symptoms during the first 6 months postpartum in Japan. Reprod Med Biol 17 (2018): 275-282
- Ünal N, Küçükdag M, Sengun Evaluation of Stress Levels in Both Parents of Newborns Hospitalized in the Neonatal Intensive Care Unit. Mathews Journal of Pediatrics 8 (2023): 29-40.
- Philpott FL, Savage E, FitzGerald S, Leahy-Warren Anxiety in fathers in the perinatal period: Αsystematic review. Midwifery 76 (2019): 54-101.
- Gimenez EC, Sanchez-Luna Parents in the neonatal unit: how to prevent stress, anxiety and depression. Infant 11 (2015): 112-114.
- An Y, Wang Z, Ji H, et Pituitary-adrenal and sympathetic nervous system responses to psychiatric disorders in women undergoing in vitro fertilization treatment. Fertil Steril 96 (2011): 404-408.
- World Health Organisation (WHO). Recommendations on Antenatal Care for a Positive Pregnancy Experience: World Health Organisation 10 (2018): 1-10.
- Brito AS, Matsuo T, Gonzalez MR, Carvalho AB, Ferrari CRIB score, birth weight and gestational age in neonatal mortality risk evaluation. Rev Saude Publica 37 (2003): 597-602.
