Organizational Impact of an ID NOW™ COVID-19 Point-Of-Care Testing for SARS-Cov2 Detection in A Maternity Ward
Article Information
Jean-Claude Nguyen Van1*, Benoît Pilmis2, Amir Khaterchi1, Olivier Billuart3, Gauthier Péan De Ponfilly1, Alban Le Monnier1, Elie Azria4-5, Assaf Mizrahi1
1Service de Microbiologie Clinique, Groupe Hospitalier Paris Saint-Joseph - Paris (France)
2Unité Mobile de Microbiologie Clinique, Groupe Hospitalier Paris Saint Joseph - Paris (France)
3Service informatique, Groupe Hospitalier Paris Saint-Joseph - Paris (France)
4Obstetric department, Groupe Hospitalier Paris Saint-Joseph - Paris (France)
5Université de Paris Cité, CRESS, Obstetrical Perinatal and Pediatric Epidemiology Research Team, EPOPé, INSERM, INRA, Paris, France
*Corresponding author: Jean-Claude Nguyen Van. Groupe Hospitalier Paris Saint-Joseph, Service de Microbiologie Clinique, 185 rue Raymond Losserand, 75014 Paris.
Received: 7 April 2023; Accepted: 14 April 2023; Published: 09 May 2023
Citation: Jean-Claude Nguyen Van, Benoit Pilmis, Amir Khaterchi, Olivier Billuart, Gauthier Péan De Ponfilly, Alban Le Monnier, Elie Azria, Assaf Mizrahi. Organizational Impact of an ID NOW™ COVID-19 Point-Of-Care Testing for SARS-Cov2 Detection in A Maternity Ward. Archives of Clinical and Biomedical Research. 7 (2023): 306-311.
Share at FacebookAbstract
Background: SARS-CoV-2 has been responsible for more than 676 million cases of COVID-19 worldwide. RT-PCR is considered the “gold standard” for the diagnosis of patients suspected of having COVID-19. During the heightened waves of the pandemic, more rapid tests have been required. Point-of-care tests (POCT) for COVID-19 include antigen tests, serological tests, and other molecular-based platforms. The ID NOW™ COVID-19 assay (Abbott) performs an isothermal gene amplification of a target encoding the RNA-dependent RNA polymerase of SARSCoV-2. The main objective of this study was to evaluate the organizational impact following the implementation of a POC testing platform ID NOW™ in a maternity ward.
Materials and Methods: This retrospective study included pregnant women admitted for Groupe Hospitalier Paris Saint-Joseph Paris. The study was conducted over 2 periods lasting 6 months each. The first period (P1) corresponded to the 2nd wave in France (July to December 2020) whereas the second (P2) period focused on the 3rd wave (February to July 2021). During P1, viral detection was performed by RT-PCR at the hospital’s laboratory. During P2, it was performed with the ID NOW™ COVID-19 test directly in the delivery room by nursing staff after training and certification. Our primary endpoint was the length of time in the birth room from admission to discharge in the postpartum period.
Results: 2447 pregnant women were included, 1053 during P1 and 1394 during P2. The median age, percentage of singleton pregnancies, mean gestational age, percentage of nulliparous individuals, percentage of vaginal deliveries, and COVID19 positivity rate were comparable between the two periods. During P2, the length of stay in the delivery room was significantly shorter than during P1 (17.9 vs 14.7 hours, p<0.001).
Conclusion: Analysis of the data from this study following the implementation of the ID NOW™ POCT in the maternity ward indicates a significant decrease in the length of stay in the birth room. This outcome needs to be confirmed in a multicenter cohort, in particular to precise the specific impact of COVID-19 care on delays.
Keywords
Organizational impact; Point-of-care testing POCT; ID NOW COVID-19; SARS-CoV-2; Maternity ward
Article Details
1. Introduction
SARS-CoV-2 has been responsible for more than 676 million cases of COVID-19 worldwide [1]. The onset of the pandemic revealed our limited supply of molecular tests. Increasing testing for COVID-19 was identified as an essential component of the pandemic control strategy. Thus, rapid and accurate tests were urgently needed. Molecular testing in a point-of-care (POC) strategy in the maternity ward became apparent. Indeed, POC testing (POCT) is associated with large reductions in time to results [2] and could lead to improvements in work flow in the delivery room.
The implementation of POCT for influenza for several years and coronavirus in the emergency department of our hospital has allowed us to benefit from this significant experience [3,4]. The ID NOW™ COVID-19 test is a rapid molecular in vitro diagnostic test based on isothermal nucleic acid amplification using nicking enzyme amplification reaction (NEAR) technology [5,6]. Pregnant women, particularly those with associated comorbidities, seem to be at higher risk of severe complications of SARS-CoV-2 infection than non-pregnant women [7,8]. In addition, a delay in the turnaround for a COVID-19 PCR test result performed in the central laboratory may result in a delay in transfer to the postnatal suite.
RT-PCR is considered the “gold standard” for the diagnosis of COVID-19. During the heightened waves of the pandemic, more rapid tests were required. POCT for COVID-19 includes antigen tests, serological tests, and other molecular-based platforms. The development of rapid molecular diagnostic tests with sensitivity and specificity comparable to the current gold-standard RT-PCR techniques can significantly help expand testing [9,10]. The ID NOW™ COVID-19 assay (Abbott) involves rapid isothermal gene amplification of a target segment encoding the RNA-dependent RNA polymerase gene of SARS-CoV-2. In a previous study, we showed that this ID NOW™ assay had comparable performance to RT-PCR [5]. The main objective of this current study was to evaluate the organizational impact of the implementation of the ID NOW™ POCT platform in our maternity ward.
2. Materials and Methods
2.1 Study design and setting
This was a pre–post study in a single center. All parturients were recruited from the maternity ward of the tertiary hospital Groupe Hospitalier Paris Saint Joseph, France.
This retrospective study included pregnant women admitted for childbirth in our hospital in Paris. Inclusion criteria were spontaneous labor after 37 weeks of amenorrhea in women with monofetal pregnancies. The study was conducted over 2 periods, each lasting 6 months. The first period (P1) corresponded to the second wave of COVID-19 (July to December 2020) and the second period (P2) the third wave (February to July 2021). During P1, the virus was detected by RT-PCR in the hospital’s laboratory. During P2, it was detected with the ID NOW™ COVID-19 assay directly in the delivery room by trained and certified nursing staff. The assay was performed directly from the dry swab obtained from pregnant women as soon as they went into labor. Once they gave birth, after a legal monitoring period of 2 hours, they were transferred to the postnatal suite.
The primary endpoint was the time in the delivery room from admission to discharge in the postpartum period. The secondary endpoint was the percentage of COVID-19 tests obtained after the time from onset of labor through the required 2 hours of legal delay before moving to the postnatal suite (2 hours postpartum).
The third endpoint was the level of satisfaction of the practitioners with the platform assessed in the form of a paper questionnaire.
2.2 Testing methods
The ID NOW™ COVID-19 assay (Abbott, Chicago, IL, USA) is an isothermal nucleic acid amplification-based test. It is a rapid molecular diagnostic test that uses NEAR technology for detecting SARS-CoV-2 RNA, targeting the RdRp gene 6. Individual samples can only be tested one at a time. Testing was completed in accordance with the manufacturer’s instructions. Nasopharyngeal swabs were collected with flexible nasopharyngeal flocked swabs, and during P2, swabs were directly tested by the ID NOW™ COVID-19 assay at the POC by emergency-department trained nurses or trained and certified midwives. Following an initial 3-min warm-up of the test system, a dry swab is added to elution buffer in the sample receiver and mixed for 10 s. Using the sample transfer device, 200 μL sample is transferred into the test cartridge, the lid is closed, and the instrument automatically initializes the assay, which runs for 10 min. The ID NOW™ assay provides a qualitative result to the user. The instrument software interprets amplification data, and final results are reported as positive, negative, or invalid. Samples that yield an initial invalid result are repeated. If an invalid result is generated twice, the final result is reported as invalid. The connectivity between POCT instruments and the electronic medical record is provided by the middleware AegisPOC™ Point of Care Management Solutions.
2.3 Statistical analysis
Percentages were calculated based on documented data (missing data were excluded from percentages). Inter-group comparisons involved the Mann-Whitney test for quantitative data and Fisher exact test for categorical data. Statistical analysis involved using StatView 5.0. All tests were two-tailed and p < 0.05 (calculated by c test, Student’s t test, or Mann- Whitney test) was considered significant.
2.4 Practitioners’ perceptions regarding COVID-19 POC implementation in the maternity ward
At the end of the study, user satisfaction with the ID NOW™ COVID-19 test was assessed by a qualitative/quantitative paper questionnaire that was completed by participating nurses and midwives.
2.5 Ethical statement
This study followed the guidelines of the Standards for Reporting of Diagnostic Accuracy studies (STARD) and was previously approved by the institutional research board, which issued a favorable ethical opinion. The study was registered as the single protocol DELOCOVIDMATER at ClinicalTrials.gov (NCT05175716; https://clinicaltrials.gov/ct2/show/NCT05175716). Non-objection consent for participation was obtained from each participant, in accordance with French law.
3. Results
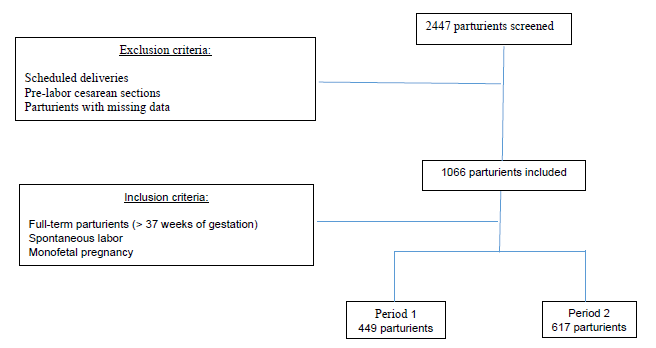
We screened 2447 pregnant women, 1053 during P1 and 1394 during P2. 1066 total parturients were included in the study, 449 included during P1 and 617 during P2 (Figure 1).
The median age and gestational age; mean labor hours; percentage of singleton pregnancies, vaginal deliveries and postpartum hemorrhage; and COVID-19 positivity rate were comparable between the two periods (Table 1). The median time in the delivery room was significantly shorter during P2 than P1 (10.4 vs 14.59 hr, p<0.001) and for nulliparous and multiparous women, the median time in the delivery room was significantly shorter in P2 than P1 (16.08 vs 19.02 hr, p<0.001, and 10.36 vs 11.54 hr, p<0.05, respectively). The proportion of COVID-19 tests obtained after the time from onset of labor to delivery plus 2 hr of medical postpartum was 73/448 (19.4%) and 4/596 (0.6%) in P1 and P2 (p <0.05).
|
Period 1 |
Period 2 |
p value |
|
|
n = 449 |
n = 617 |
||
|
Age (years), median (IQR) |
33 (30-36) |
33 (30-36) |
NS |
|
Gestational age (years), median (IQR) |
37.9 (37-38.6) |
38 (37-38.6) |
NS |
|
Vaginal delivery, n (%) |
428 (95.3) |
590 (95.6) |
NS |
|
Labor (hours), mean (IQR) |
7h40 (4h-11h) |
7h05 (3.5h-9.5h) |
NS |
|
COVID-19 positivity rate, n (%) |
10 (2.2) |
17 (2.7) |
NS |
|
Postpartum hemorrhage, n (%) |
12 (2.7) |
8 (1.3) |
NS |
|
Nulliparity, n (%) |
232 (51.7) |
265 (42.9) |
p = 0.005 |
|
Time in the delivery room (hours), median (IQR) |
14h59 (10h40-22h) |
10h40 (17h59-22h) |
p< 0.001 |
|
Time in the delivery room (hours) for nulliparous women, median (IQR) |
19h02 (13h47-25h22) |
16h08 (12h12-20h32) |
p< 0.001 |
|
Time in the delivery room (hours) for multiparous women, median (IQR) |
11h54 (8h31-16h12) |
10h36 (7h21-14h48) |
p <0.05 |
|
COVID-19 tests obtained after legal delay, n (%)* |
73/448 (19.4) |
4/596 (0.6) |
p <0.05 |
Table 1: Comparison of parturients during period 1 and period 2
IQR, interquartile range; NS, not significant
* COVID-19 tests obtained after the time from onset of labor to delivery plus 2 hr of medical postpartum
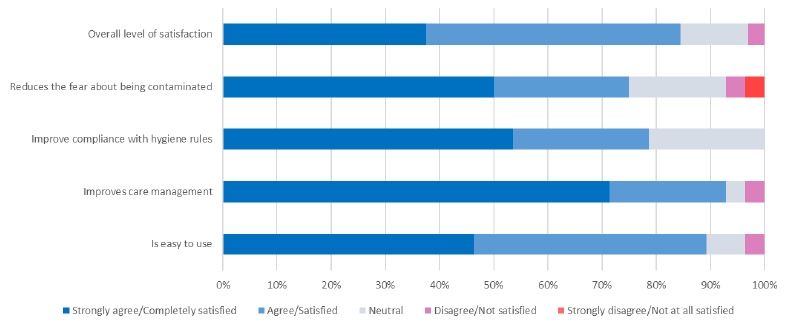
At the end of the study, we collected users’ opinions on the ID NOW™ COVID-19 assay. Survey responses are shown in Table 2. A total of 63 practitioners participated in the survey, including 44 midwives (70%), 18 nurses (28%), and 1 physician. Most practitioners reported that the implementation of ID NOW™ COVID-19 assay was straightforward. In addition, ease of use and duration of care scored high (90.6% and 93.0%, respectively). Improved hygiene compliance in addition to reduced fear of being contaminated scored well (75%). The overall level of satisfaction with use of the POCT was 85%. POCT improved compliance with health regulations in the delivery room and allowed for systematic screening of parturients upon entry to the delivery room.
|
Question |
Response |
|
Was the test easy to use? scale of 1 (unsatisfied) to 5 (very satisfied) |
1 = Not at all satisfied: 0% 2 = Not satisfied: 3.6% 3 = Neutral: 7.1% 4 = Satisfied: 42.9% 5 = Completely satisfied: 46.4% |
|
Do you think that test reduces the time required for the medical management of parturients compared to the reference RT-PCR technique in the laboratory? |
Strongly disagree: 0% Disagree: 3.6% Neutral: 3.6% Agree: 21.4% Strongly agree: 71.4% |
|
Do you believe that the test improves compliance with health regulations in the delivery room? |
Strongly disagree: 0% Disagree: : 0% Neutral: 21.4% Agree: 25% Strongly agree: 53.6% |
|
Do you think that test reduces the fear of healthcare workers of being infected by a patient with SARS-CoV-2? |
Strongly disagree: 3.6% Disagree: 3.6% Neutral: 17.8% Agree: 25% Strongly agree: 50% |
|
What is your overall level of satisfaction with the use of the test in the delivery room on a scale of 1 to 5? |
1 = Not at all satisfied: 2 = Not satisfied: 3.1% 3 = Neutral: 12.5% 4 = Satisfied: 46.9% 5 = Very satisfied: 37.5% |
4. Discussion
Rapid and accurate detection of SARS-CoV-2 is essential to ensure early and appropriate parturient management. New diagnostic technologies with rapid turnaround and good performance are an opportunity to improve the organization in the birthing room. Here we investigated the efficacy of POCT for COVID-19 in parturient women in our hospital. During P1, the second COVID-19 wave in France, the virus was detected in women by RT-PCR in the hospital’s central laboratory. During P2, the third wave, it was detected with the ID NOW™ platform directly in the delivery room by trained and certified nursing staff. We found median age and gestational age; mean labor hours; percentage of singleton pregnancies, vaginal deliveries and postpartum hemorrhage; and COVID-19 positivity rate comparable between the two periods but the median time in the delivery room was significantly shorter during P2 than P1.
During the first wave of COVID-19, there was pressure on the Groupe Hospitalier Paris Saint-Joseph laboratory to deliver COVID-19 test results quickly, particularly by maternity care teams. This pressure was even greater at night because COVID-19 diagnostic testing was performed only in the central laboratory during the day. This led to disorganization in the laboratory and tension between the laboratory and the maternity ward. Therefore, we decided to implement ID NOW™ COVID-19 POCT for SARS-CoV2 detection in the maternity ward, using our POCT experience gained in the emergency department [3] [4].
The implementation of an automated system in POC requires strict adherence and attention to the approved testing procedure. In our hospital, testers were required to be trained and certified by the central laboratory staff. A project manager was identified to monitor on a daily basis and communicate on positivity rates.
The organizational impact after the implementation of the ID NOW™ POCT platform for parturients could be evaluated by comparing the median time in the delivery room with and without the ID NOW™ platform. To obtain a homogeneous population, the inclusion criteria were full-term patient >37 weeks of gestation, spontaneous labor and mono-fetal pregnancy. Implementation of ID NOW™ led to a significantly lower time to results than with RT-PCR testing in the central laboratory.
We observed a significant reduction in the mean time in the delivery room, from 14.59 to 10.4 hr. This reduction in time spent in the delivery room could be explained by the reduction in time to results. In our study, pregnant women had COVID-19 POCT as soon as they went into labor. Once they had given birth, after a legal monitoring period of 2 hr, they were transferred to the postnatal suite.
During P1, 73/448 (19.4%) parturients could not be transferred from the delivery room to the postnatal suite because they did not have their PCR results. Implementation of ID NOW™ allowed for significantly decreasing this number to 4/596 (0.6%) parturients during P2.
Nurses and midwives who used the ID NOW™ COVID-19 assay received training from two experts and were required to undertake a proficiency test prior to participation. As reported by Oliver et al. [11] the crucial areas for POCT accreditation are method performance verification, internal and external quality assurance, staff training, competency, and continuous improvement. To this end, we have organized the supervision and support of the maternity ward staff with the help of a referral microbiologist, a quality assurance engineer and two referral technicians. This organization allowed us to ensure staff training, equipment maintenance, connectivity to the laboratory information system, quality control and external quality assurance procedures, all required for accreditation according to the ISO 22870 standard [2] [12].
For connectivity, we used the AegisPOC™ Point of Care Management Solutions, which is a web-based, open platform connecting POC devices. It allowed the laboratory to manage training and certification of individuals performing POCT as well as data-sharing from the POC device on one middleware.
POCT is usually more expensive than testing performed in the central laboratory and requires a significant amount of support from the laboratory to ensure quality testing and meeting accreditation requirements [12]. This point is important and requires careful consideration before embarking on a process that requires time, human resources (reference technicians, quality specialists and microbiologists) and material resources (connected prescription, middleware). The cost-effectiveness could be evaluated in a future study as well as the impact of implementation of the COVID-19 POCT on direct and indirect hospital costs during the pandemic.
Limitations
To our knowledge, this is the first study to evaluate the organizational impact of the POCT in the delivery room, but this work has several limitations. First, it was a retrospective monocentric study. Results may be specific to the population of our hospital. Use of rapid diagnostic test at triage in other care settings would depend on the organization of each maternity ward and, importantly, the availability of compliant and trained staff. Second, the optimal assessment of the organizational impact of COVID-19 POCT implementation should be evaluated in an unbiased randomized controlled trial. Nevertheless, in the tense situation and according to the expectations of the clinicians (gynecologists/obstetricians), it was almost impossible to carry out a randomized study.
We observed excellent compliance with the POCT by nurses and midwives throughout the study, and the low number of invalid tests (< 4%) attests to good compliance with the procedure.
This is a collaborative and exemplary study. When we interviewed the medical and paramedical staff in the delivery room, they responded that “no one would want to go back” to the previous situation. Additional studies are needed to confirm these results.
5. Conclusion
Analysis of the data from this study after implementation of the ID NOW™ COVID-19 POCT in the maternity ward indicates a significant decrease in time in the birthing room and high user satisfaction. This outcome needs to be confirmed in a multicenter cohort, in particular to define the specific impact of COVID-19 care on delays in transfer to the postnatal suite.
Conflicts of interest
This work was not funded. None of the authors declare any personal or financial conflict of interest in relation to this manuscript.
Acknowledgements
The author thank the entire maternity ward team, especially Alice Auvray, Anaïs Cohen and Sonia Tahiri for the coordination within the ward.
We are also grateful to the members of clinical research: Dr Emmanuelle Sacco, Dr Nesrine Ben Nasr, Dr Hélène Beaussier and Prof. Gilles Chatellier.
Thanks to Soifiya Madi Bounou and Lalia Cisse for technical support and Laura Smales and Erika Wells for professional proofreading.
Special thanks to Dr Henri Bendelac for hepful discussions.
Author Contributions
BP contributed to conceptualization, data curation, formal analysis, methodology, validation
AK contributed to data curation, formal analysis
OB data curation, formal analysis
GPP contributed to writing the original draft
ALM contributed to supervision, project administration
EA contributed to conceptualization, project administration, supervision, validation
AM contributed to data curation, formal analysis, methodology, validation, writing the original draft
JCNV contributed to conceptualization, data curation, formal analysis, methodology, project administration, supervision, validation, visualization, writing the original draft
JCNV drafted the manuscript, to which all authors provided critical comments and a final consent for the publishing
References
- John Hopskins University. Coronavirus Ressource Center. 3 (2022).
- Florkowski C, Don-Wauchope A, Gimenez N, et al. Point-of-care testing (POCT) and evidence-based laboratory medicine (EBLM) – does it leverage any advantage in clinical decision making? Crit Rev Clin Lab Sci 54(7-8) (2017): 471-494.
- Trabattoni E, Le V, Pilmis B, et al. Implementation of Alere i Influenza A & B point of care test for the diagnosis of influenza in an emergency department. Am J Emerg Med. Published online 18 (2017).
- Gerlier C, Pilmis B, Ganansia O, et al. Clinical and operational impact of rapid point-of-care SARS-CoV-2 detection in an emergency department. Am J Emerg Med 50 (2021): 713-718.
- Nguyen Van JC, Gerlier C, Pilmis B, et al. Prospective Evaluation of ID NOW COVID-19 Assay Used as Point-of-Care Test in an Emergency Department. Emergency Medicine 9(2021).
- Abbott Laboratories. ID Now COVID-19 package insert. Abbott Laboratories, Chicago 10 (2022).
- DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol 224(5) (2021): 510.
- Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol 226(2) (2022):177-186.
- Valera E, Jankelow A, Lim J, et al. COVID-19 Point-of-Care Diagnostics: Present and Future. ACS Nano 15(5) (2021):7899-7906.
- Vandenberg O, Martiny D, Rochas O, et al. Considerations for diagnostic COVID-19 tests. Nat Rev Microbiol 19(3) (2021): 171-183.
- Oliver P, Fernandez-Calle P, Buno A. POCT Accreditation ISO 15189 and ISO 22870: Making the Point. EJIFCC 32(2) (2021):131-139.
- Shaw JLV. Practical challenges related to point of care testing. Pract Lab Med 4(2016): 22-29.