Novel Protection by Omega-3-FAs against Strychnine-Induced Tonic-Convulsion in Mice: Synergy with Carbamazepine
Article Information
M. El-Mowafy1*, M. A. Abdel-Dayem2
1Department of Biochemistry, Medical Lab-Consultant and Professor of Biochemistry, Egypt
2Department of Pharmacology and Toxicology, Faculty of Pharmacy, Mansoura University and Department of Pharmacology/ Biochemistry, Horus university, New-Damiette, Egypt
*Corresponding Author: A. M. El-mowafy PhD., Medical Lab-Consultant and Professor of Biochemistry, Egypt
Received: 14 August 2021; Accepted: 23 August 2021; Published: 31 August 2021
Citation: Abd M Elmowafy, Abdul Dayem. Novel protection by omega-3-FAs against strychnine-induced tonic-convulsion in mice: synergy with carbamazepine. Journal of Food Science and Nutrition Research 4 (2021): 227-239.
Share at FacebookAbstract
Background/Aim: The utility of ω-3-FAs (DHA and EPA) against epilepsy was evident in clonic-convulsion animal-models, in their chronic- than acute-modes. However, their efficacy against tonic-convulsion-models remained unclear. Besides, while some-antiepileptics (AEDs), like carbamazepine (CBZ), adversely impacted the bioavailability of dietary ω-3-FAs, it remains unclear whether co-ω-3-FAs may change efficacy/blood-levels of CBZ. This work investigated the capacity of both acute- versus chronic-regimens of ω-3-FAs to: 1) alleviate the-tonic, strychnine-induced convulsions in mice, and 2) synergize with CBZ-evoked anti-tonic-convulsions, and then further-probe whether this has altered plasma-CBZ levels (clearance).
Methods: Both acute (1.0 hr)- and chronic (14 day)-regimens of the ω-3-FAs, DHA and EPA (120-1000mg/kg p.o.), were administered in a mouse strychnine convulsion-model (2mg/kg i.p.), and seizure frequency, latency and animal-survival were determined versus the positive-control CBZ (12mg/kg p.o). Further, synergy between submaximal-doses of DHA(EPA) and CBZ was verified. Lastly, pharmacokinetic interaction was verified in rats by determining plasma CBZ-levels in the presence- and-absence of ω-3-FAs.
Results: Both DHA and EPA dose-dependently enhanced seizure latency (2-folds) and protected mice against strychnine-induced convulsion (up to 75%). Besides, interestingly, similar responses and animal-survival rates obtained in acute and chronic models. Moreover, either DHA or EPA synergized with CBZ effects beyond their individual responses (3.6-4.3 folds, respectively). Such concurrent DHA/CBZ fully protected the mice, while the joint-EPA/CBZ spared only 88% of the animals. Lastly, pharmacokinetic studies revealed that CBZ levels were unchanged with co-administration of ω-3-FAs.
Conclusions: The study reveal
Keywords
Omega-3-Fas, Strychnine, Tonic-convulsions, Seizure, Carbamazepine- pharmacokinetics- anticonvulsants
Omega-3-Fas articles; Strychnine articles; Tonic-convulsions articles; Seizure articles; Carbamazepine articles, pharmacokinetics articles, anticonvulsants articles
Omega-3-Fas articles Omega-3-Fas Research articles Omega-3-Fas review articles Omega-3-Fas PubMed articles Omega-3-Fas PubMed Central articles Omega-3-Fas 2023 articles Omega-3-Fas 2024 articles Omega-3-Fas Scopus articles Omega-3-Fas impact factor journals Omega-3-Fas Scopus journals Omega-3-Fas PubMed journals Omega-3-Fas medical journals Omega-3-Fas free journals Omega-3-Fas best journals Omega-3-Fas top journals Omega-3-Fas free medical journals Omega-3-Fas famous journals Omega-3-Fas Google Scholar indexed journals Strychnine articles Strychnine Research articles Strychnine review articles Strychnine PubMed articles Strychnine PubMed Central articles Strychnine 2023 articles Strychnine 2024 articles Strychnine Scopus articles Strychnine impact factor journals Strychnine Scopus journals Strychnine PubMed journals Strychnine medical journals Strychnine free journals Strychnine best journals Strychnine top journals Strychnine free medical journals Strychnine famous journals Strychnine Google Scholar indexed journals Tonic-convulsions articles Tonic-convulsions Research articles Tonic-convulsions review articles Tonic-convulsions PubMed articles Tonic-convulsions PubMed Central articles Tonic-convulsions 2023 articles Tonic-convulsions 2024 articles Tonic-convulsions Scopus articles Tonic-convulsions impact factor journals Tonic-convulsions Scopus journals Tonic-convulsions PubMed journals Tonic-convulsions medical journals Tonic-convulsions free journals Tonic-convulsions best journals Tonic-convulsions top journals Tonic-convulsions free medical journals Tonic-convulsions famous journals Tonic-convulsions Google Scholar indexed journals Seizure articles Seizure Research articles Seizure review articles Seizure PubMed articles Seizure PubMed Central articles Seizure 2023 articles Seizure 2024 articles Seizure Scopus articles Seizure impact factor journals Seizure Scopus journals Seizure PubMed journals Seizure medical journals Seizure free journals Seizure best journals Seizure top journals Seizure free medical journals Seizure famous journals Seizure Google Scholar indexed journals Carbamazepine- pharmacokinetics- anticonvulsants articles Carbamazepine- pharmacokinetics- anticonvulsants Research articles Carbamazepine- pharmacokinetics- anticonvulsants review articles Carbamazepine- pharmacokinetics- anticonvulsants PubMed articles Carbamazepine- pharmacokinetics- anticonvulsants PubMed Central articles Carbamazepine- pharmacokinetics- anticonvulsants 2023 articles Carbamazepine- pharmacokinetics- anticonvulsants 2024 articles Carbamazepine- pharmacokinetics- anticonvulsants Scopus articles Carbamazepine- pharmacokinetics- anticonvulsants impact factor journals Carbamazepine- pharmacokinetics- anticonvulsants Scopus journals Carbamazepine- pharmacokinetics- anticonvulsants PubMed journals Carbamazepine- pharmacokinetics- anticonvulsants medical journals Carbamazepine- pharmacokinetics- anticonvulsants free journals Carbamazepine- pharmacokinetics- anticonvulsants best journals Carbamazepine- pharmacokinetics- anticonvulsants top journals Carbamazepine- pharmacokinetics- anticonvulsants free medical journals Carbamazepine- pharmacokinetics- anticonvulsants famous journals Carbamazepine- pharmacokinetics- anticonvulsants Google Scholar indexed journals corn oil articles corn oil Research articles corn oil review articles corn oil PubMed articles corn oil PubMed Central articles corn oil 2023 articles corn oil 2024 articles corn oil Scopus articles corn oil impact factor journals corn oil Scopus journals corn oil PubMed journals corn oil medical journals corn oil free journals corn oil best journals corn oil top journals corn oil free medical journals corn oil famous journals corn oil Google Scholar indexed journals
Article Details
1. Introduction
Epilepsy is an abnormal neurological excitation that manifests as spontaneous, frequent hyperexcitability (seizures). It associates with a surge in inflammatory mediators and disruption of ion-channel gating, thereby emanating into a range of coherent morbidities and mortalities [1-3]. Thus far, the use of CNS-depressant anticonvulsant medications, antiepileptics, (AEDs), to mitigate seizures and their consequences has only achieved partial success. Thus, about 30% of patients remain refractory to AEDs [4,5], Additionally, patients using AEDs frequently experience a range of annoying or organ-disruptive adverse-drug reactions (ADRs), including fatigue, sedation, nausea, liver-toxicity and blood-dyscrasias [5,6]. Thus, research was inspired and expedited towards the discovery of more efficacious and less-noxious therapies, thereby overcoming such glitches and bridging deficits in availing satisfactory-therapy for patients afflicted with epilepsy. The n-3 polyunsaturated fatty acids, commonly designated as omega-3-FAs (?-3-FAs), are essential FAs that are not synthesized in human cells. Their isolation and biological evaluations availed numerous health benefits against a plethora of health disorders, including epilepsy [5-7]. Thus, they are intended to be received in diets from specific, predefined seafoods and plants, from which they were also isolated and offered as pharmaceutical supplements. ?-3-FAs were recognized as vital components in normal brain development, transmission and function [8-10]. Therefore, first, they were appreciated as a complementary medicine of support for patients with epilepsy [11,12]. Furthermore, subsequent molecular and signaling studies have revealed their functional roles in modifying cellular effectors, signaling, division, ion-channels, and gene activity, findings which spurred characterization of their therapeutic utility against epilepsy [13-16]. Additional meticulous and functional-studies revealed multifaceted clues that ?-3-FA may well protect against convulsions by raising conduction thresholds, delaying the refractory period, and dampening excitation of neurons [18-21]. In vivo studies in a variety of animal seizure-models that target “brain” transmission, such as fluorothyl, pentylenetetrazole (PTZ), and kainate, lent further support for the utility of ?-3-FA, alone or in combination with established anticonvulsants, to combat seizures [8]. Unlike pentylenetetrazole (PTZ) that elicits the typical brain-based “generalized clonic seizure (GCS)” animal-model, strychnine produces a characteristic spine-based “tonic generalized extension (TGE)”. Noteworthy, AEDs and experimental anticonvulsants may well display variable sensitivities towards the two-models [2]. However, the joint administration of the AED, carbamazepine (CBZ) with dietary, or supplemental ?-3-FAs, adversely impacted the levels of ?-3-FAs, thereby possibly posing resistance to their efficacy, aggravating their ADRs, and endangering patient health status subsequent to CBZ [22]. However, conversely, our understanding of the exact dynamics and therapeutic-impact of ?-3-FAs on the levels and utility of AEDs have been unclear. To date, some controversy has revolved around whether chronic administration of ?-3-FAs (weeks-months), rather than their acute use, and thus the invoked alterations of neuronal composition, is inevitable for their anticonvulsant actions [23-25]. Furthermore, the efficacy of ?-3-FAs against “spinal-cord”-based convulsions, as with the present strychnine-induced tonic-convulsions, has not been identified. Thence, this study was undertaken to 1) examine the capacity of ?-3-FAs, EPA and DHA, to ameliorate strychnine-induced tonic seizures and incurred animal mortality in either of their acute (hours) and chronic (weeks) administrations. Besides, 2) probe the potential of ?-3-FAs to synergize with the standard-AED, CBZ, and if evident, whether this has involved any alterations of CBZ’s plasma levels (clearance), that is, a pharmacokinetic drug-interaction.
2. Materials and Methods
2.1 Drugs and chemicals:
2.1.1 Standard Antiepileptic drug: Carbamazepine, a white pure powder, is a gift from Novartis, and was prepared as a homogeneous suspension in 0.5% carboxymethylcellulose (CMC) in 0.9% NaCl solution (saline).
Omega-3-FAs source and doses:
- Docosahexaenoic Acid (DHA) was purchased as soft gelatin capsules from America’s nutrition, U.S.A. Each capsule provides 100mg DHA.
- Eicosapentaenoic acid (EPA) was purchased as soft gelatin capsules from America’s nutrition, U.S.A. Each capsule provides 500mg EPA.
DHA and EPA were diluted in corn oil. The doses used for EPA and DHA in this study were in the range of those used in other studies applied for the same animals. These were determined after appropriate preliminary experiments. The time course range of the pharmacokinetic experiments in this study was similar to those used in other studies of the same animal-model. This was determined via appropriate preliminary experiments.
- Strychnine Sulfate, a white, water-soluble powder, was purchased from Sigma-Aldrich, U.S.A.
- Carbamazepine ELISA-assay kit: was obtained from Dade Behring, Atterbury, Milton Keynes, United Kingdom.
2.2. Animals
2.2.1A Mice: Male albino mice weighing (20-25) gm were used in the epilepsy-model experiments.
2.2.2B Rats: Adult male Sprague-Dawley rats weighing 200-250g were used in pharmacokinetic experiments, to allow for enough collections of serum samples and comparison with relevant literature reports that were also conducted with rats. All animals were purchased from a local source and maintained under standard conditions of temperature about 30°C with regular 12h light/12h dark-cycle, and allowed free-access to standard laboratory food and water.
2.2.3C Research ethics approval: The protocol of the study was conducted with the formal approval of the “animal research ethics committee” and the “postgraduate research committee” at this university.
2.3 Methods
2.3A. Strychnine epilepsy models in mice:
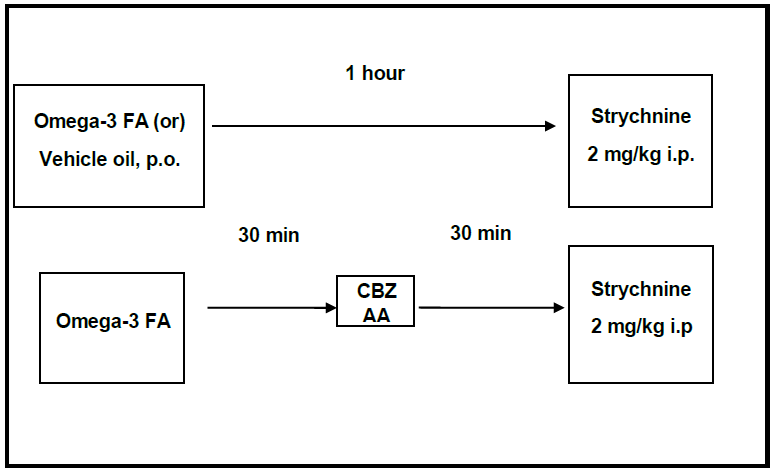
2.3A.1. Strychnine acute antiepileptic study: 14-mouse-groups, 8 mice each, were randomly constituted. 10 such groups had received different doses of the ?-3-FAs orally, 1hr before strychnine (2mg/kg i.p.) was injected [26]. The positive control group received the ED50 dose of CBZ (12mg/kg, p.o). Strychnine was injected after 30min from CBZ administration [27]. The combination groups received the ?-3-FAs then CBZ, respectively; at 30min intervals, before strychnine was injected.
|
Group (1) |
Received equivalent amount of vehicle p.o. 1 hr before strychnine (2mg/kg i.p) was injected |
|
Group (2) |
Received ED50 of CBZ (12mg/kg p.o) 30 min before strychnine (2mg/kg i.p.) was injected. |
|
Group (3) |
Received 120 mg/kg DHA 1hr before strychnine (2mg/kg i.p) was injected. |
|
Group (4) |
Received 250 mg/kg DHA 1hr before strychnine (2mg/kg i.p) was injected. |
|
Group (5) |
Received 500mg/kg DHA 1hr before strychnine (2mg/kg i.p) was injected. |
|
Group (6) |
Received 750mg/kg DHA 1hr before strychnine (2mg/kg i.p) was injected. |
|
Group (7) |
Received 1000mg/kg DHA 1hr before strychnine (2mg/kg i.p) was injected. |
|
Group (8) |
Received 75mg/kg 1hr before strychnine (2mg/kg i.p) was injected. |
|
Group (9) |
Received 200mg/kg EPA 1hr before strychnine (2mg/kg i.p) was injected. |
|
Group (10) |
Received 300 mg/kg EPA 1hr before strychnine (2mg/kg i.p) was injected. |
|
Group (11) |
Received 500mg/kg EPA 1hr before strychnine (2mg/kg i.p) was injected. |
|
Group (12) |
Received 1000mg/kg EPA 1hr before strychnine (2mg/kg i.p) was injected. |
|
Group (13) |
Received 750mg/kg DHA, then after 30min, received CBZ (12mg/kg p.o.), then after another 30min strychnine (2mg/kg i.p) was injected. |
|
Group (14) |
Received 500mg/kg EPA, then after 30min, received CBZ (12mg/kg p.o.), then after another 30min, strychnine (2mg/kg i.p) was injected. |
Table 1: The following details mouse-grouping and their treatments
2.3A.2 Strychnine chronic-antiepileptic study: Two groups, 8 mice each, received ?-3-FAs as a single submaximal daily dose, every day for 14 days. In the last day, strychnine (2mg/kg i.p.) was injected 1hr after the last dose.
|
Group (1) |
Received DHA 250mg/kg daily for 14 days before strychnine (2mg/kg i.p.) was injected. |
|
Group (2) |
Received EPA 300mg/kg daily for 14 days before strychnine (2mg/kg i.p.) was injected. |
2.3.1B CBZ-pharmacokinetic studies in rats: CBZ was determined by ELISA, using a kit obtained from Dade Behring, Atterbury, Milton Keynes, United Kingdom, as will be detailed next. For this purpose, 6 rat-groups, 6-animal each, received the antiepileptic drug (CBZ) alone or in combination with ?-3-FAs (DHA or EPA). The FAs were administered 1hr. before CBZ. Blood samples were collected after giving CBZ at the intervals of; 30min, 1hr, 3hr, and 6hr. Samples were centrifuged and the separated serum was used for determination of CBZ concentration.
Animal grouping and treatments for Pharmacokinetic studies:
|
Group (1) |
Received effective dose of CBZ (25mg/kg p.o.) [28]. |
|
Group (2) |
Received DHA (250mg/kg p.o.) [29], and after 1hr, received CBZ (25mg/kg p.o.). |
2.4 Carbamazepine ELISA assay
Principle: CBZ assay is a homogeneous enzyme-immunoassay technique for quantitative analysis of CBZ (free and protein-bound) in serum or plasma. CBZ in the sample and labeled-G6PDH-CBZ compete for limited antibody binding sites. Therefore, bound-enzyme (and its activity) decreases as CBZ increases in the sample. The enzyme activity is measured through conversion of oxidized NAD to NADH; resulting in an absorbance change that can be measured spectrophotometrically. The assay is a fully-automated one that runs through a programmed protocol utilizing a Dad-Behring instrument. Thus, results are calculated automatically by the analyzer based on a standard curve that is constructed jointly with the assay of samples. No additional data manipulations are required.
2.5 Statistical analyses
Statistical analysis was achieved by using the GraphPad Prism program-version-5 (GraphPad Software Inc.). Statistical significance between two groups was evaluated by Student’s t-test for unpaired data. Comparisons among means of the multiple data groups were conducted using the one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. Statistical significance was predefined at P < 0.05.
3. Results
3A. Strychnine epilepsy models
3A.1. Strychnine acute antiepileptic study:
The tonic onset of convulsion was measured in different mouse groups and the results were expressed as means ± SEM, as shown in figures (1A-B). CBZ, at its ED50 value (12 mg/kg), significantly (2 folds)-delayed the onset of tonic convulsion from that of the strychnine-only, positive control group (P<0.001). On the other hand, while the lower DHA dose (120mg/kg) had no effect on the tonic onset of convulsion, higher DHA doses (250-1000mg/kg) significantly and dose-responsively delayed the tonic convulsions (1.5-2.1 folds) (P<0.001). (Figure 1A). Furthermore, combining CBZ with DHA raised the tonic onset of convulsion far beyond their individual effects (up to 4.3 folds) (P<0.001), thereby implying a synergic response. EPA, at doses of 75 or 200mg/kg, didn’t significantly affect the tonic convulsion onset. However, higher EPA doses (300, 1000mg/kg) delayed the tonic convulsion from by (1.3-1.9 folds) from the control group response (P<0.001), (Figure 1B). The joint administration of EPA (1000mg/kg) with CBZ also elicited remarkable synergy that mounted to (3.6 folds) of the control response (Figure 1B). Overall, however, EPA scored an inferior anticonvulsant efficacy relative to that achieved by the DHA.
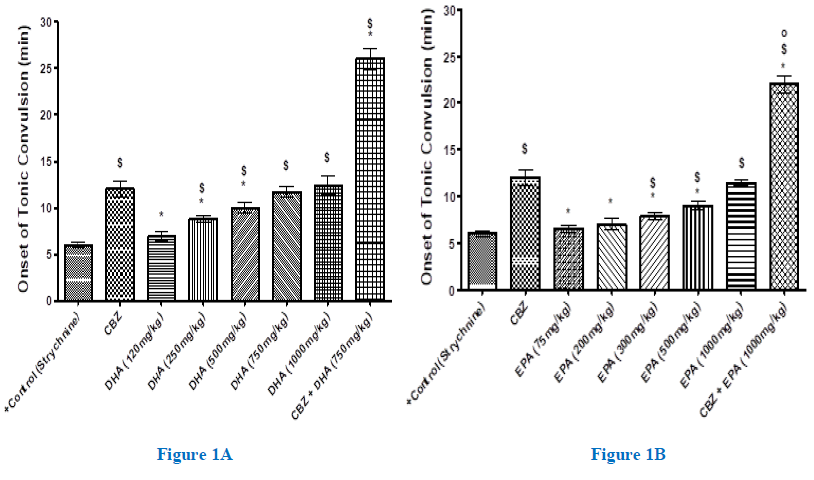
Figure 1(A-B): Onset of strychnine (2 mg/kg, i.p.)-induced tonic convulsion (min) in mice pretreated with and without increasing doses of DHA (A) or EPA (B) given 1.0 hr. prior to strychnine, against the standard-antiepileptic carbamazepine (CBZ). Each group comprised 8-mice. Animals that survived the convulsion after 30 mins, were considered recovered. Data are expressed as means ± SEM, and significance was determined with ANOVA and was considered at P<0.05. $, significant from positive-control; *, significant from CBZ; o, significant from the corresponding DHA value.
The number of animals that survived convulsion rose by increasing the DHA dose, as shown in figure (2A). Thus, while DHA (120mg/kg) preserved only 25% of mice, DHA (750, 1000mg/kg) elicited the most protective effects and spared 75% of animals. Besides, increasing EPA concentration also enhanced the animal survival, though to a lesser extent than DHA. Accordingly, EPA (1000 mg/kg) showed 63% of animals (Figure 2B). Unlike animals of the positive control group, all synergy-animals had survived in response to treatment with DHA, while only 88% did survive in case of (EPA). The survived mice either didn’t pass through a tonic convulsion or gently passed through it, before completely recovered thereafter. Linear regression suggested a significant positive correlation between the onset of tonic-convulsion and the number of survived animals, R2= 0.76 (data not displayed).
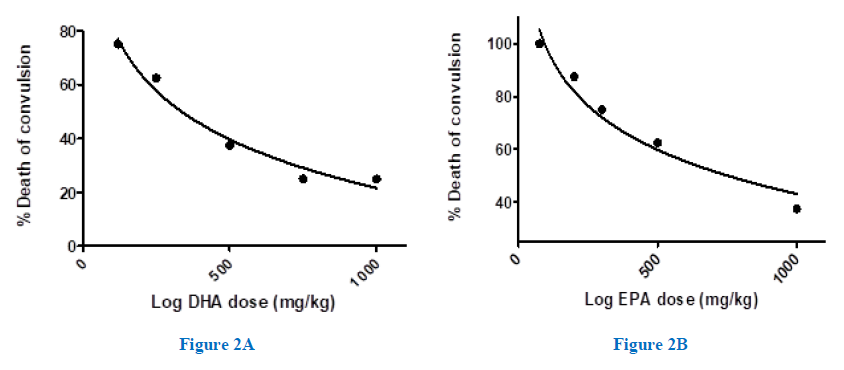
Figure 2A,2B: Dose-response relationship for protection by the omega-3-FAs against strychnine-induced convulsion and death in mice. The %death of animals taking different doses of DHA-(A) or EPA-(B), 1.0 hr. before the injection of strychnine (2mg/kg, i.p.), was determined in each group (n=8).
A.2. Strychnine, chronic-antiepileptic study:
Some controversy has revolved around the utility of acute ?-3-FAs regimen versus that of chronic regimen in clonic-models of epilepsy [23-25]. Therefore, we currently examined whether the chronic cumulative buildup of ?-3-FAs may elicit different response in abating the current strychnine, spinal-cord-based tonic model of convulsion. Treatment of the animals with DHA (250 mg/kg) or EPA (300mg/kg) daily dose for two weeks before strychnine was injected, produced statistically-similar seizure latency responses, and animal-survival outcomes, to their corresponding (acute) single-doses (Figure 3).
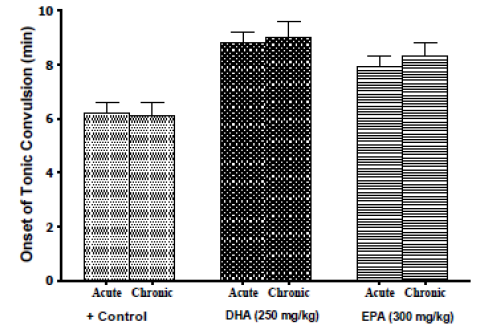
Figure 3: Protection by chronic (2 week)-treatment of DHA (250mg/kg) or EPA (300mg/kg), versus that of similar single-dose acute-treatment, against strychnine-induced convulsion in mice. Each group comprised 8-mice. Animals that survived convulsion after 30 mins were considered recovered. Data are expressed as means ± SEM, and significance was determined with ANOVA, with significance to be considered at P<0.05.
- Pharmacokinetic Carbamazepine assay in rats:
Serum carbamazepine (CBZ) concentration was measured at different time intervals, in rats pre-treated with and without DHA (250 mg/kg, p.o) (Figure 4). Results were expressed as means ± SEM, n=6. Statistical analysis was performed with ANOVA.
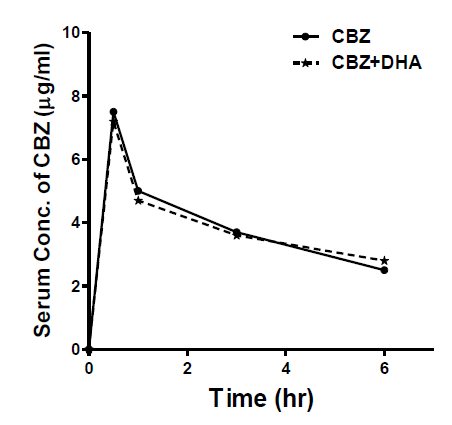
Figure 4: Time course of rat-serum CBZ levels, in the presence and absence of DHA (250 mg/kg, p.o.), given 1hr prior to CBZ (25 mg/kg, p.o.). CBZ concentrations were determined with ELISA, in rat sera (n=6), at the given time-points. Results were calculated as (Means ± SEM). Statistical analysis was determined with ANOVA, with significance to be considered at P<0.05.
Pre-treatment with DHA didn’t significantly alter the serum concentration of CBZ at different time intervals, as compared to the animals that received CBZ alone. Table 1 displays the computed pharmacokinetic parameters in the presence and absence of DHA.
|
Group |
AUC (mg.h/l) |
Cmax (mg/l) |
Tmax (h) |
t ½ (h) |
Vd/F (l/kg) |
Cl/F (l/h/kg) |
|
CBZ |
38.8±3.1 |
7.48±0.67 |
0.5 |
4.42±0.41 |
4.253±0.58 |
0.667±0.05 |
|
CBZ+DHA |
42.1±5.8 |
7.35±0.69 |
0.5 |
5.79±0.95 |
4.702±0.36 |
0.56±0.1 |
Table 1: Computed pharmacokinetic parameters following oral administration of Carbamazepine (25 mg/kg p.o.) alone or in combination with DHA (250 mg/kg p.o.) in rats.
Cmax= maximum plasma concentration.
Tmax=time needed to attain Cmax
Cl= clearance.
Vd= apparent volume of distribution.
T1/2 = elimination half-life.
F: oral availability.
AUC= area under serum concentration-time curve.
4. Discussion
Outcomes of the present study indicate that either DHA or EPA, in a potency-rank order, raised seizure latency in a spinal cord-based, strychnine-tonic convulsion model, and protected the mice from death, dose-dependently. Moreover, responsiveness to these ?-3-FAs was evident equally in the acute and chronic routes of administration. Besides, at their submaximal doses, these ?-3-FAs synergized with CBZ-evoked suppression of seizure, thereby conferring responses that surpassed either of their individual responses. Subsequently, independent rat-pharmacokinetic determinations of plasma-CBZ in presence and absence of the ?-3-FAs, DHA, ruled out pharmacokinetic interaction. Almost one-third of epileptic patients are refractory to known seizure-curbing drugs, and sudden-death of epilepsy and/or its cardiovascular sequelae, formally collectively termed “sudden unexplained death in epilepsy (SUDEP)”, have been a paramount hurdle against recovery from this disease [31-33]. Thus, in this vein, the emerging therapeutic-profiles identified for ?-3-FAs against diverse inflammatory, cytokine- and stress-mediated disorders including cardiovascular-diseases, cancer, diabetes and obesity, opened new prospects of interventions against epilepsy. This perception for ?-3-FAs spurred progressive unfolding of multifaceted-protective benefits in epilepsy via clinical and laboratory studies [33,34]. Indeed, because of the wide safety-margin of ?-3-FAs, doses up to 2880 mg/day were prescribed to epileptic patients, which indeed evoked positive outcomes with regards to seizure severity as well as cardiovascular dynamics, including triglyceride- and HDL-levels and myocardial efficiency [33-35]. Overall, collectively, such outcomes in humans through clinical studies, proposed that low to moderate doses of ?-3 fatty acids can elevate seizure-thresholds and dampen associated inflammatory and stressful responses that are now believed to culminate into fatality to epilepsy, “SUPED” [34]. A recent such clinical trial involved refractory epileptic-patients, who were administered 600 mg mixed ?-3-FAs daily dose for 16 weeks (versus placebo). Intriguingly, fruitful findings with seizure delay were obtained that associated with cogent molecular suppression of cytokine mediators, modulation of ion channel activity and alteration of gene functions [35,36]. Therefore, safety, versatility, numerous health benefits, reduced therapy-cost, and ease of use via diverse routes, presented ?-3-fatty acids as fascinating alternative or joint-therapy tools in managing drug-resistant epilepsy [37,38]. These envisions are further consolidated with our present finding that the spectrum of anticonvulsant effects now encompasses the spinal transmission, as evidenced by trying neurodepressive-ability of ?-3-FAs against strychnine-induced convulsion. Thus, a 2-fold delay in seizure onset and 75% reduction in animal mortality were presently obtained. Strychnine is an alkaloid obtained from the seeds of Strychnos nuxvomica. It was used for long as a rodenticide and pesticide but is also employed in the illicit manufacture of some narcotics, predominantly cocaine. It induces cogent excitatory effect on the central nervous system by blocking glycine uptake at inhibitory synapses in the ventral horns of the spinal cord, thereby inducing its characteristic, persistent tonic-extension [39,40]. Early studies with ?-3-FAs in epilepsy-models suggested that their acute responses are unlikely and that chronic treatments, for weeks-months, are required to elicit anticonvulsant effects on their own [23]. These long-term effects for ?-3-FAs were ascribed to their incorporation into brain-membranes and alteration of channel-gating and signaling [18]. This dogma prevailed for some time, until a few acute studies were also proven efficient in dulling seizures [25]. However, such reports have dealt with merely brain-based clonic-type of excitations. This study was the first to target palliative effects of ?-3-FAs in a spinal cord-based tonic-convulsion model. Thus, it was crucial to probe the efficacy for both of the short-term (acute) and long-term (chronic) modes of treatment. The present findings of equipotent responses for the used acute- and chronic-regimens of ?-3-FAs, therefore, attest for their versatility and wide-spectrum against seizures, initial observations that AED, like CBZ, may unilaterally interfere with bioavailability of ?-3-FAs, coupled with the recommendations from clinical studies with ?-3-FAs on their value as supplements to boost efficacy and mitigate resistance to marketed AEDs, spurred us to examine the joint drug-effects of CBZ and ?-3-FAs in the present strychnine model of convulsion [22,41]. Indeed, synergic responses were obtained that exceeded their individual actions on both seizure latency (4+ folds) and animal survival (100%). Whilst this synergy concept offers avenues towards implementing fruitful therapy regimens, it further permits possible deploying of lower CBZ doses, and thus, drug safety. These envisions are substantiated with earlier studies that delineated hepatoprotection against valproate toxicity [15,42], as well as overall cardiovascular protective outcomes [34]. This present sole and synergy profiles of ?-3-FAs in mitigating seizures and their sequelae are essential characters of successful drug candidates in the management of complex diseases like, diabetes, cancer and epilepsy. Whereas the present work rules out any kinetic interaction (clearance) for DHA with CBZ, it has not precisely delineated the molecular mechanism/s whereby it confers its anticonvulsant effects. Indeed, a myriad of mechanisms were described for such ?-3-FAs protective actions in the clonic models, leading from cell-membrane to genome modifications, through tens of signaling effectors [8,34], which would mandate detailed subsequent independent investigations. Collectively, this study pioneers reports on the efficacy of ?-3-FAs against tonic-clonic epilepsy model both in acute or chronic modes, a profile that remains functional and synergic with conventional AEDs, like CBZ, thereby revealing broad-spectrum utility of ?-3-FAs in the armamentarium against epilepsy.
Acknowledgements
- A. A. was supported with a Master’s fellowship from Mansoura University, Egypt.
- M. E. Served as the thesis advisor and mentor of the student.
Declaration of interest statement
The authors report no conflict of interest.
References
- Sinha S, Siddiqui KA. Definition of intractable epilepsy. Neurosciences 16 (2011): 3-9.
- Burnham WM. Anti-seizure drugs. In H Kalant, DM Grant, J Mitchell (eds) Principles of Medical Pharmacology, 7th Elsevier Canada, Toronto, (2006) pp 223.
- Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 58 (2017): 512-521.
- Shorvon SD. The epidemiology and treatment of chronic and refractory epilepsy. Epilepsia 37 (1996): S1-S3.
- Vining EPG. Clinical efficacy of the ketogenic diet. Epilepsy Res 37 (1999): 181-190.
- El-Mesery M, Al-Gayyar M, Salem H, et al A. Chemopreventive and renal protective effects for docosahexaenoic acid (DHA): implications of CRP and lipid peroxides. Cell Div 11 (2009): 4-6.
- Cunnane SC, McAdoo KR, Horrobin DF. n-3 Essential fatty acids decrease weight gain in genetically obese mice. Br J Nutr 56 (1986): 87-95.
- Christopher M DeGiorgio, Ameer Y Taha. Omega-3 fatty acids (?-3 fatty acids) in epilepsy: animal models and human clinical trials. Expert Review of Neurotherapeutics 16 (2016): 10.
- Clandinin MT, Chapell JE, Leong S, et al. Extrauterine fatty acid accretion in infant brain: implications for fatty acid requirements. Early Hum Dev 4 (1980): 131-138.
- Crawford MA, Golfetto I, Ghebremeskel K, et al. The potential role for arachidonic and docosahexaenoic acids in protection against some central nervous system injuries in preterm infants. Lipids 38 (2003): 303-315.
- Yuen AW, Sander JW, Fluegel D, et al. Omega-3 fatty acid supplementation in patients with chronic epilepsy: a randomized trial. Epilepsy Behav 7 (2005): 253-258.
- Schlanger S, Shinitzky M, Yam D. Diet enriched in omega-3 fatty acids alleviates convulsion symptoms in epilepsy patients. Epilepsia 43 (2002): 103-104.
- Bromfield E, Dworetzky B, Hurwitz S, Eluri Z, Lane L, Replansky S, Mostofsky D. A randomized trial of polyunsaturated fatty acids for refractory epilepsy. Epilepsy Behav 12 (2008): 187-190.
- Rapoport SI. In vivo approaches to quantifying and imaging brain arachidonic and docosahexaenoic acid metabolism. J Pediatr 143 (2003): S26-S34.
- El-Mowafy AM, AbdelDayem MA, Abdel-Aziz A, et al. Eicosapentaenoic acid ablates valproate-induced liver oxidative stress and cellular derangement without altering its clearance rate: dynamic synergy and therapeutic utility. Biochim Biophys Acta 11 (2011): 460-467.
- Kitajka K, Sinclair AJ, Weisinger RS, et al. Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression. Proc Natl Acad Sci U S A 101 (2004): 10931-10936.
- Lauritzen I, Blondeau N, Heurteaux C, et al. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J 19 (2000): 1784-1793.
- Vreugdenhil M, Bruehl C, Voskuyl RA, et al. Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc Natl Acad Sci USA 93 (1996): 12559-12563.
- Xiao Y, Li X. Polyunsaturated fatty acids modify mouse hippocampal neuronal excitability during excitotoxic or convulsant stimulation. Brain Res 846 (1999): 112-121.
- Yehuda S, Carasso RL, Mostofsky DI. Essential fatty acid preparation (SR-3) raises seizure threshold in rats. Eur J Pharmacol 254 (1994): 193-198.
- Young C, Gean PW, Chiou LC, et al. Docosahexanoic acid inhibits synaptic transmission and epileptiform activity in the rat hippocampus. Synapse 37 (2003): 90-94.
- Yuen AW, Sander JW, Flugel D, et al. Erythrocyte and plasma fatty acid profiles in patients with epilepsy: does carbamazepine affect omega-3 fatty acid concentrations? Epilepsy Behav 12 (2008): 317-323.
- Willis S, Samala R, Rosenberger TA, et al. Eicosapentaenoic and docosahexaenoic acids are not anticonvulsant or neuroprotective in acute mouse seizure models. Epilepsia 50 (2009): 138-142.
- Trépanier MO, Taha AY, Mantha RL, et al. Increases in seizure latencies induced by subcutaneous docosahexaenoic acid are lost at higher doses. Epilepsy Res 99 (2012): 225-232.
- Trépanier MO, Kwong KM, Domenichiello AF, Chen CT, Bazinet RP, Burnham WM. Intravenous infusion of docosahexaenoic acid increases serum concentrations in a dose-dependent manner and increases seizure latency in the maximal PTZ model. Epilepsy Behav 50 (2015): 71-76.
- Kaputlu I and Uzbay T. L-NAME inhibits pentylenetetrazole and strychnine-induced seizures in mice. Brain Res 753 (1997): 98-101.
- Rostock A, Tober C, Rundfeldt C et al. A new anticonvulsant with a very good margin of safety. Epilepsy Research 28 (1997): 17-28.
- Mizuno K, Okada M, Murakami T, et al. Effects of carbamazepine on acetylcholine release and metabolism. Epilepsy Res l40 (2000): 187-195.
- Nguyen KA, Carbone JM, Silva VM, et al. The PPR activator docosahexaenoic acid prevents acetaminophen hepatotoxicity in male CD-1 mice. J Toxicol Environ Health A 58 (1999): 171-186.
- Mori Y, Nobukata H, Harada T, et al. Long-Term Administration of highly purified eicosapentaenoic acid ethyl ester improves blood coagulation abnormalities and dysfunction of vascular endothelial cells in rats. Endocr J 5 (2003): 603-611.
- Alan W C, Yuen, Josemir W Sander. Is omega-3 Fatty Acid Deficiency a Factor Contributing to Refractory Seizures and SUDEP? A Hypothesis. Seizure 13 (2004): 104-107.
- Scorza FA, Scorza CA, Cavalheiro EA. Omega-3 fatty acids and SUDEP prevention. Lancet Neurol 15 (2016): 1303.
- Devinsky O, Hesdorffer DC, Thurman DJ, et al. Omega-3 fatty acids and SUDEP prevention - Authors' reply. Lancet Neurol 15 (2016): 1303-1304.
- Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 3 (2020): 10-19.
- DeGiorgio CM, Miller P, Meymandi S, et al. n-3 fatty acids (fish oil) for epilepsy, cardiac risk factors, and risk of SUDEP: clues from a pilot, double-blind, exploratory study. Epilepsy Behav 13 (2008): 681-684.
- Omrani S, Taheri M, Omrani MD et al. The effect of omega-3 fatty acids on clinical and paraclinical features of intractable epileptic patients: a triple blind randomized clinical trial. Clin Trans Med 8 (2019): 3.
- Abdel-Dayem MA, Elmarakby AA, Abdel-Aziz AA, et al. Valproate-induced liver injury: modulation by the omega-3 fatty acid DHA proposes a novel anticonvulsant regimen. Drugs RD 14 (2014): 85-94.
- Sarmento Vasconcelos V, Macedo CR, de Souza Pedrosa A, et al Polyunsaturated fatty acid supplementation for drug-resistant epilepsy. Cochrane Database Syst Rev 8 (2016): CD011014.
- Perper JA. Fatal strychnine poisoning: a case report and review of the literature. J Forensic Sci 30 (1985): 1248-1255.
- Kaputlu I, Uzbay T. L-NAME inhibits pentylenetetrazole and strychnine-induced seizures in mice. Brain Res 753 (1997): 98-101.
- DeGiorgio CM, Taha AY. Omega-3 fatty acids (?-3 fatty acids) in epilepsy: animal models and human clinical trials. Expert Rev Neurother 16 (2016): 11-21.
- Reimers A, Ljung H. The emerging role of omega-3 fatty acids as a therapeutic option in neuropsychiatric disorders. Ther Adv Psychopharmacol 9 (2019): 204-210.
