Nicotine-based Interventions for Adult Smokers with Diabetes: A Systematic Review
Article Information
Farhana Haseen1*, Nafis Rahman1, As-Saba Hossain2, Sohel Rana2, Asif Moinur Chowdhury2, Hasna Heena Mahmud2, Joanne Coyle1, Sophie Notley1, Gabriel Barnard1, Neil McKeganey1
1Centre for Substance Use Research (CSUR) Glasgow, United Kingdom
2Association for Community Empowerment (ACE) Dhaka, Bangladesh
*Corresponding author: Farhana Haseen, Centre for Substance Use Research (CSUR) Glasgow, United Kingdom
Received: 05 January 2024; Accepted: 08 January 2024; Published: 02 February 2024.
Citation: Farhana Haseen, Nafis Rahman, As-Saba Hossain, Sohel Rana, Asif Moinur Chowdhury, Hasna Heena Mahmud, Joanne Coyle, Sophie Notley, Gabriel Barnard, Neil McKeganey. Nicotine-based Interventions for Adult Smokers with Diabetes: A Systematic Review. Archives of Clinical and Biomedical Research. 8 (2024): 27-44.
Share at FacebookAbstract
Purpose: Starting or continuing to smoke after a diagnosis of diabetes is associated with increased health complications, decreased treatment efficacy, and worse survival outcomes. However, the extent and effectiveness of smoking cessation services among patients with diabetes are poorly understood. Nicotine replacement is one of the available interventions to aid smokers in achieving smoking cessation. This systematic review aimed to provide comprehensive evidence on the effects of nicotine-based interventions to support smoking cessation in diabetic patients.
Methods: Electronic searches were carried out on the following databases: Medline, Embase, and Cochrane Library up to November 2022. Searches were supplemented by trial registries, references from identified studies, and review articles. Studies were included if nicotine was used to deliver a smoking cessation intervention and if the impact of the interventions was reported for diabetic patients. All articles were independently screened, selected, extracted, and assessed for quality. Narrative data synthesis was conducted due to heterogeneity.
Results: Sixteen studies reporting 12 trials met the inclusion criteria, including randomized controlled trials (n=12), one cluster randomized trial, one non-randomized intervention trial, and two before-after design studies. Of the sixteen studies selected, eight studies exclusively assessed diabetic patients, and eight assessed patients with multiple comorbidities, in which diabetic patients were a subgroup of the total sample. Four studies reported interventions with nicotine that increased cessation rates significantly among the intervention group patients compared to the control group at six-month or twelve-month follow-ups. One trial reported a significant reduction in the self-reported number of cigarettes smoked/ day in the intervention group compared to the control. Additionally, there was a trend toward positive changes in levels of biomarkers of glucose control and metabolic outcomes with the use of nicotine-based interventions.
Conclusion: Nicotine, with/without behavioral support, appears to increase smoking abstinence in those diagnosed with diabetes without making a significant negative clinical impact. However, data is limited to identifying the optimal form of nicotine or effective intervention for this population. Additionally, there is no evidence of efficacy in smoking cessation interventions with next-generation tobacco harm-reduction products among people with diabetes.
Keywords
Smoking; Diabetes; Nicotine; Cessation; Intervention; Systematic review
Smoking articles; Diabetes articles; Nicotine articles; Cessation articles; Intervention articles; Systematic review articles
Article Details
1. Background
Diabetes is a major health concern that has reached alarming levels globally. The International Diabetes Federation (IDF) 10th edition Diabetes Atlas reports a continued global increase in the prevalence of diabetes and confirms diabetes as an important global challenge to the health and well-being of individuals, families, and societies. It is one of the fastest-growing global health emergencies of the 21st century. In 2021, more than half a billion people in the world live with diabetes; 537 million adults aged 20–79 years worldwide have diabetes - 1 in 10 of all adults in this age group [1]. Current evidence proves that regular smoking is a significant risk factor for micro- and macrovascular complications and mortality in patients with diabetes [2-6]. In a meta-analysis of men with and without diabetes, Yudkin et al. found that intervention for smoking was the best way to prolong life in patients with diabetes [7]. This result demonstrates the importance of encouraging patients to give up smoking as soon as possible after a diagnosis of diabetes. Several studies have shown the efficacy of methods to help patients stop smoking in the general population [8]. However, it may be more difficult for diabetic patients to quit smoking than other people [9]. Given the high burden of co-morbidities, anxiety around the disease, and the level of self-management required of people with diabetes, quitting smoking may reflect an additional challenge that they are unprepared for. Most smokers are generally reluctant to seek formal treatment for quitting smoking, with the vast majority attempting to quit without assistance [10, 11]. Evidence suggests that one reason for the low rates of successful quit attempts is that the most effective smoking cessation aids may not be used [12]. To date, the most widely used cessation aid in smoking cessation services is nicotine replacement therapy (NRT) [13, 14]. NRT is available in many different formulations, such as chewing gums, inhalers, lozenges, sprays, and transdermal patches. Their primary mechanism of action is replacing the nicotine that would otherwise be delivered by cigarette smoking with a safer mode of delivery, thus decreasing the severity of withdrawal symptoms from smoking and helping the smoker quit [13]. NRT goals are to reduce motivation to smoke and address the physiological and psychomotor withdrawal symptoms often experienced during an attempt to stop smoking; NRT-based treatment increases the likelihood of smokers remaining abstinent [15] and even doubles the chances of success in quitting smoking, regardless of the specific formulation of NRT [16-18]. The evidence that NRT helps some people to stop smoking is now widely accepted. Standard instructions for using such therapy and clinical guidelines in many countries recommend NRT as the first treatment for people seeking pharmacological help to stop smoking [8, 19-23]. Research suggests that most diabetic patients expressed an interest in smoking cessation (60%-70%) [24, 25]. Therefore, it is important to examine how nicotine replacement smoking cessation aids can be incorporated into tobacco control programs, primarily focusing on high-risk diabetic populations. One earlier review has been conducted to evaluate the effects of more intensive smoking cessation interventions compared to less intensive interventions in people with type 1 or type 2 diabetes [26]. Trials that included nicotine-based interventions were not addressed separately in this review.
Little is known about the evidence base for specific cessation services offered to smokers following diabetic diagnosis, despite the positive impact of quitting and the availability of local and national smoking cessation services. In addition, it is unknown whether adding nicotine cessation therapy to diabetic treatment programs yields higher overall abstinence from tobacco without making a significant negative clinical impact. Therefore, from a health services perspective, understanding whether a nicotine intervention is as effective in this patient group as it is with the general population is important. This review of the international evidence base was undertaken as part of a research project to support the development of a protocol to assess the overall effect of a smoking cessation intervention using oral nicotine pouches among type 2 diabetics who are current smokers. Therefore, a systematic review of studies that provided tobacco cessation therapy with nicotine among people diagnosed with diabetes was undertaken. Currently, there are too few interventions with exclusive nicotine intervention; consequently, the review considered any type of tobacco cessation intervention with a nicotine component for diabetic patients. The literature review aimed to provide comprehensive evidence on the effects of nicotine-based interventions to support smoking cessation among adult patients with diabetes as part of a research project to develop an intervention for type 2 diabetic smokers.
2. Methods
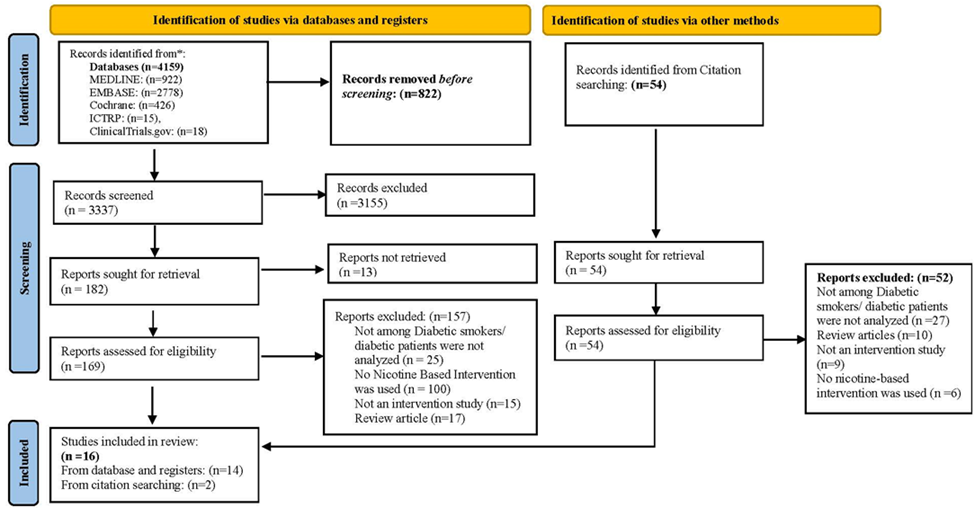
A review protocol was registered on PROSPERO, an International Prospective Register of Systematic Reviews, with Registration Number CRD42023403930 [27] and reported following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [28] (Figure 1).
2.1 Literature Search
The research team utilized a step-by-step process of systematic search strategies following Cochrane guidelines [29] applied across several databases to extract literature detailing the range and nature of nicotine-based interventions for smoking cessation among patients who are both diabetic and current smokers.
2.2 Search strategy
Medline (Dialog), Embase (Dialog), The Cochrane Library, and clinical trial registries were searched from the inception date of each database to 29th November 2022. The search strategy combined two main concepts: diabetes mellitus -AND- smoking cessation, including nicotine-based interventions. Appropriate search options were used to identify smoking cessation trial interventions where nicotine was available for type 1 or type 2 diabetes. In Medline and Embase, both thesaurus terms (e.g., Medical Subject Headings (MeSH) in Medline and EMTREE (EMB) terms in Embase) where available, and text words (e.g., words or phrases appearing in the Title, Abstract, or Author Keywords fields of references) were identified to describe each concept. For the text words, synonyms were identified for each concept (e.g., insulin-dependent diabetes and non-insulin-dependent diabetes for the ‘diabetes mellitus’ concept). In addition, clinical trials, systematic reviews, and meta-analyses were identified explicitly as part of the search strategy. A similar search strategy was applied in the Cochrane Library. The registries of clinical trials, such as clinicaltrials.gov and WHO International Clinical Trials Registry Platform (ICTRP), were searched using relevant search terms for ongoing and completed trials. The results obtained from the systematic searches were then supplemented with references from bibliographies of the identified literature and reviews. To find additional studies relevant to the research questions, a search was conducted to identify similar reviews by searching for "smoking AND diabetes AND review" in Google Scholar. Manual searches of reference lists and citation searches were also completed. No further restrictions, such as publication date and language, were imposed. No limit was placed on the recruitment setting.
2.3 Selection process
Search results from all databases were merged using the reference management software Endnote X9. Data duplication was managed by removing duplications using EndNote, which was then manually checked. A total of five reviewers were involved in selecting articles for inclusion. Two reviewers independently screened titles or abstracts for relevance. All potentially relevant articles were obtained in full text. Any articles the reviewer was unsure about for inclusion were collectively discussed, and the Principal Investigator (FH) made a final decision on inclusion.
2.4 Eligibility criteria
Reviewers assessed each full article against predefined inclusion criteria (Table 1). Inclusion criteria were set on study design, type of participants, type of intervention, and outcomes. Due to time constraints, only references available in the English language were included in the review. Any disagreements were resolved through collective discussion.
Type of study design
All types of intervention studies were included, including randomized controlled trials (RCTs), cluster-RCTs, non-randomized studies, non-controlled trials, and before and after studies. Feasibility and pilot studies were included if the effects of the intervention for diabetic patients were reported. Observational, cohort, database analysis, or cross-sectional studies were excluded.
Table 1: Inclusion and exclusion criteria
|
Inclusion criteria |
|
|
1. Type of study |
Randomized controlled trials (RCTs), cluster-RCTs, controlled non-randomized studies, non-controlled trials, and before and after studies. |
|
2. Type of intervention |
Interventions for smoking cessation include any type of nicotine. |
|
3. Type of participants |
Adults (³18 years), non-pregnant, current tobacco smokers diagnosed with diabetes. |
|
4. Type of control group |
Usual/standard care, no intervention, or another smoking cessation intervention (non-pharmacologic or pharmacologic intervention). |
|
5. Type of outcomes |
All outcomes related to cigarettes /smoking cessation, clinical, metabolic, and health outcomes, side-effects, safety and tolerance, acceptability and feasibility. |
|
Exclusion criteria |
|
|
1. Language |
Papers not in English. |
|
2. Participants |
Trials involve non-human participants. |
|
3. Outcomes |
No outcome reported for diabetic patients specifically. |
Type of participants
The target participants were adults (18 years and over) of any gender who were smokers and diagnosed with diabetes, either type 1 or type 2 at baseline. Nicotine-based interventions that targeted people with multiple chronic diseases who smoked were included, but only if diabetes represented a sub-group of the study population (e.g., participants with chronic disease and diabetes was one of them), and outcomes for diabetic patients were reported separately. Pregnant smokers with gestational diabetes were excluded. Studies on animal samples were excluded.
Type of intervention
Trials were included to evaluate any form of nicotine-based intervention, with or without pharmacological or nonpharmacological or behavioral interventions intended to support smoking cessation. Cessation treatment with nicotine was considered either as a single intervention or with other interventions. If the trial offered combined a multi-component intervention and nicotine was not the primary intervention, a component of the combination intervention for smokers was still included, such as a combination of nicotine-based products and behavior counseling or motivational interventions or cognitive behavioral therapy or non-NRT drugs (varenicline or bupropion). Trials were also included that compared different doses or forms of nicotine delivery. Studies were considered if they offered any form of nicotine, including Nicotine gum, Nicotine transdermal patch, Nicotine oral spray, Nicotine nasal spray, Nicotine inhaler, Nicotine lozenges, Nicotine sublingual tablets, Electronic cigarettes (e-cigarettes), Heated tobacco products, Snus and Oral nicotine pouches.
Comparison group
The main comparator of the nicotine-based intervention was a placebo, but other comparators eligible for inclusion were no intervention, usual/standard care, other pharmacological agents, and nonpharmacological smoking cessation intervention (e.g., counseling/motivation or optional medication)
Type of Outcomes
Trials reporting at least one of the following outcomes important for diabetic patients were included: (1) Smoking cessation, (2) Outcomes were the change in the number of cigarettes smoked, (3) Glycemic control including glycated hemoglobin (HbA1c) and blood glucose, (4) Visceral fat, (5) Lipid profile at least one of LDL, HDL, TG, Total cholesterol, (6) Body weight and/or BMI, (7) Any type of health outcomes, (8) Onset of diseases, (9) Control of diabetes, (10) Adverse event rate, (11) Safety and tolerance, (12) Acceptability/adherence and feasibility of the intervention. There were no restrictions on the duration of interventions or the length of follow-up.
2.5 Data Extraction
Two reviewers independently performed the data extraction using the structured data extraction form. Studies that did not fulfill all the criteria were excluded, and their bibliographic details were listed with the reason for exclusion (Figure 1).
2.6 Quality assessment
A methodological quality assessment of each included article was performed after data extraction and completed independently by two reviewers. Disagreements were resolved by discussion and with the Principal Investigator (FH), if necessary. The quality of included controlled trials was assessed according to the Jadad Score [30]. The scores range from 0 to 5, with trials scoring 3 or above were considered good quality trials.
2.7 Data Synthesis and Analysis
The data extraction forms of included trials were used to assess if it was viable to conduct a quantitative meta-analysis. Although most studies shared a common primary outcome (smoking cessation measured by either self-report or validated), high levels of heterogeneity make it difficult to synthesize the data from the included studies. Meta-analysis was not possible because of the heterogeneous nature of the interventions and inclusions in the studies. Heterogeneity appears in the methodological characteristics, including diversity in the key components of the interventions, duration of intervention and length of follow-up of interventions, as well as in the population and control group. In most of the included studies, nicotine was a component of multi-component interventions, and the amount of nicotine used in the interventions was also not homogenous. No sets of studies were sufficiently similar to make them suitable for inclusion in a meta-analysis; therefore, a narrative synthesis of studies was undertaken.
Studies were grouped according to interventions, outcomes, and comparison groups. The primary outcome for the review was abstinence, reported at any time, during or at the end of the intervention, or during or at the end of the follow-up.
Secondary outcomes were smoking reduction, clinical outcomes, disease outcomes, metabolic effects (physical and biological), disease control, and adverse events throughout follow-up—death, serious adverse events (death, admission to hospital, or permanent disability), discontinuation owing to side effects, and barriers, acceptability, and compliance on intervention. Any relevant outcomes reported at any time during or at the end of the intervention or during or at the end of follow-up were included in the synthesis.
3. Results
Search Results
A total of 4,159 articles were identified in electronic searches and 54 from the bibliography searches. After duplicates were removed, the titles and abstracts of 3,391 records were screened; 3,207 were removed as they did not meet the inclusion criteria, and 13 reports were not retrieved. The full text of the remaining 223 articles was assessed for eligibility, and 198 were excluded primarily.
Figure 1 contains the reasons for exclusion (e.g., participants did not have diabetes, and the intervention was not with nicotine). Most were excluded after full-text review as nicotine intervention was absent (n=106) or included patients who did not have diabetes (n=52) or were review articles (n=27). One study was not excluded, though the trial recruited smoker-patients with type 2 diabetes or prediabetes (84% had type 2 versus 16% prediabetes); hence, both groups were treated as a homogenous group within the diabetes education program [31]. After an evaluation against the inclusion criteria, 16 studies reporting 12 trials are included in this systematic review. Four papers are reported from one RCT with different follow-up durations: 1-year follow-up [32], 3-year follow-up data [33, 34] and 6.6 years of follow-up data [35]. The articles were published between March 1992 and May 2022. With more than half of the studies (n = 10) were published between 2000 and later. Table 2 shows the main characteristics of the included studies.
3.1 Description of Studies
Location
The location of the interventions varied. Geographically, 16 studies were conducted in 21 countries; the majority of trials were conducted in Europe: Sweden (n=3), where four papers reported the finding of the same trial on different time-frames; Spain (n=1), France (n=1), Italy (n=1), Germany (n=1), Switzerland (n=1); two in the USA [24, 36] and two in Canada [31, 37]. One RCT alone recruited participants in the US and 15 other countries (Argentina, Australia, Brazil, Bulgaria, Canada, Chile, Denmark, Finland, Germany, Mexico, New Zealand, Russian Federation, Slovakia, South Africa, and Spain) [38].
Study Design
Thirteen out of the 16 studies used a randomized control trial design: 12 were individual randomizations [24, 32-42] and one was cluster randomization [31] research design; another was a non-randomized intervention trial [43], and two were before-after design studies [44, 45]. Among 12 RCTs, one was a cross-over placebo control trial of a transdermal nicotine patch [40], and another was a triple-dummy, placebo- and active- (nicotine patch) controlled trial of varenicline and bupropion [38], the rest of the RCTs were two-armed, with parallel groups.
Characteristics of Participants
To provide a comprehensive review of the evidence, studies were included that exclusively recruited diabetic patients [type 1 and type 2 or type 2] and studies also that recruited patients with multiple health conditions where diabetic patients were part of the study participants. Eight studies report interventions for smokers diagnosed with diabetes [24, 31, 39-41, 43-45], and eight report interventions for multiple comorbidities: three reports from one intervention included hypertensive and diabetic patients [32-35]. The remaining four studies report interventions for patients with multiple health conditions, with diabetes being one of them [36-38, 42]. Four studies from one trial included only adult males; all other trials included both adult males and females.
Seven trials recruited adult populations relatively middle-aged: 30 to 75 years [43, 44], 50 to 72 [32-35], 40 to 70 years [42], and 25 to 54 years [45]. Three studies did not provide the age range but recruited middle-aged participants; the mean age was reported as 37 years in Sawicki et al. [41], 47 to 49 years in Lee et al. [37], and 51 years in Epifano et al. [40]. Five trials initiated recruitment from the younger population between the ages range of 18 and 21: 18 to 80 years [31], > 18 years [36], 18 to 75 years [38], 21 to 80 years [46], and 17 to 84 years [47].
Table 2: Characteristics of included studies
*Diabetic patients’ sample within the study.
COPD- Chronic obstructive pulmonary disease, CVD- Cardiovascular disease.
Type of Diabetes
Out of the eight studies that exclusively recruited diabetic patients, five studies investigated smokers with type 2 diabetes, and three included both type 1 and type 2 diabetic smokers [41, 45, 47]. None of the studies with mixed patient groups had information on the type of diabetes.
Sample Size of the Included Studies
The sample size of the included studies was widely varied. Studies included diabetic patients ranging from 12 [40] to 313 [31] participants. The sample sizes for those studies where diabetes patients were subgroups ranged from 18 [42] to 409 [38].
Baseline Smoking Behavior of Participants
All of the included trials recruited cigarette smokers; however, the definition of smoking or current smokers varied across studies. Smoking was defined as having smoked one cigarette or more per day in one trial [32-35]. Regular smoking of more than 5 cigarettes per day was defined as current smoking by Sawicki et al. [41]; adult daily smokers of 2 or more cigarettes per day for at least 3 weeks was stipulated by Lee et al. [37] and 10 or more cigarettes smoked per day and exhaled carbon monoxide reading of >10 parts per million was set as the definition of current smokers by Tønnesen et al. [38]. Radondi et al. [42] considered those who smoked at least 10 cigarettes per day and reported no periods of smoking abstinence for longer than 3 months in the past year. Hokanson et al. [24] classified current smokers as individuals who had smoked at least 100 cigarettes in their lifetime and said they smoked daily or on some days, and Canga et al. [39] defined current smokers as having smoked ≥100 cigarettes in their lifetime and smoked at least one cigarette during the last week. Persson et al. and Scemama et al. [43, 45] accepted participants as current/daily smokers who reported smoking, regardless of the number of cigarettes smoked per day. Reid et al. [31] considered smoker-patients with self-reported daily smoking of one or more cigarettes per day in the 30 days preceding recruitment. Persson et al. [44] did not provide any information on the definition of smokers. Epifano et al. [40] used a Fagerstrom score, and if the participant scored 6, they were considered a smoker.
Most interventions recruited heavy smokers (≥ 20 cigarettes/day) with a long history of smoking. Seven out of 12 interventions recruited current smokers with similar baseline smoking status. In seven trials, the mean cigarettes per day at baseline was ≥20 cigarettes/day (Range 20 to 25.8) [24, 36, 38-42]. Another two interventions recruited current smokers with baseline mean cigarettes per day between 15 and 19 [31, 37]. The number of years patients smoked varied between 17 years and 39 years across the studies reported. Four studies either required participants to have an intention to quit smoking [36, 38] or suggested that participants were likely to have had some intention or motivation to quit smoking [24, 31]; however, one study reported that the population of smokers did not appear to be very motivated to give up smoking in the trial [45].
Setting of the Trials
Most trials were conducted in a clinical setting (hospital, medical center, clinical research center, diabetes center, primary health care center) where diabetic smokers were usually treated for diabetes mellitus or other health conditions. In two trials, patients were recruited from hospitals as inpatients waiting for a surgical procedure or improvement of glycemic equilibrium and education [37, 45]. Two trials enrolled patients from another ongoing program or trial: one from a diabetes education program at a diabetes center [24] and another from the Gothenburg primary prevention trial at an out-patient hypertension clinic [32].
Intervention Provider
The trials used a variety of different providers to deliver the interventions, including research staff, therapists, nurses, educators, and physicians. Eight trials employed healthcare professionals such as physicians, primary care practice nurses, or inpatient nurses [32-37, 39, 42-45]. Two other trials employed trained diabetes educators [31] or a specially educated psychotherapist [41]. Two interventions included advice from research staff and investigators [24, 38].
Recruitment Method
Studies reported recruitment methods, including invitation emails, telephone calls, invitation letters, advertisements in newspapers, radio, and television advertising, and asking people to contact the research team to indicate a willingness to participate.
3.2 Description of the Intervention
Tobacco cessation treatment can be in the form of counseling, pharmacotherapy, or other interventions (e.g., contingency payments, education, or the provision of smoking cessation information materials, helpline). Pharmacotherapy could include NRT, offered with tapering doses under physician supervision or ad libitum, using gum, lozenge, inhaler, spray, transdermal patch, or non-NRT drugs that reduce the nicotine cravings such as varenicline or bupropion. Combined therapy could include any combination of the treatments included under counseling and pharmacotherapy. The main intervention categories observed in the included studies were counseling and NRT [32-35, 39]; motivational interviewing with counseling and NRT [24]; group session with motivational interviewing and NRT or bupropion [43]; smoking cessation consultations and NRT [45]; behavioral therapy and NRT [41]. One trial used five intervention components: counseling, smoking cessation brochures, referral to free smokers’ helpline, and transdermal NRT [37]. Another trial used counseling sessions, telephone calls with NRT, and brochures on smoking cessation [42]. One trial used a computerized journal for diabetic patients, group counseling, and NRT [44].
One cluster randomized controlled trial conducted in Ontario, Canada, used financial payments as part of smoking cessation and medication (NRT, bupropion, or varenicline as appropriate) to promote desired behavior intervention. In this trial, Reid et al. [31] offered medication discount cards given to smoker patients that provided partial reimbursement (Can $150) for smoking cessation medications and a Can $5 coffee shop gift card.
Interventions reported in three other trials included NRT only: nicotine lozenge vs. nicotine gum [36], nicotine patch [40], and NRT or varenicline or bupropion [38].
Behavioral Component
Behavioral interventions could include individual or group (or both) counseling to encourage behavioral change. Most trials focused on individual behavioral counseling; only three offered group counseling [32, 43, 44]. In all ten studies, the interventions involved motivational techniques with varying levels of intensity.
Additional behavior components described in the evidence varied in content. Interventions were low-tech, including the provision of a journal [44], quit plan booklet [31], attending the clinic or provision of leaflets and resources [39, 42], set-up negotiated cessation date [39, 41], relaxation techniques [41] or interventions including establishing a link to national smoking cessation resources including quit-lines [37, 41]. Sessions include face-to-face, telephone, and additional visits to counselors [24, 32-35, 39, 42, 43, 45].
The frequency of behavioral sessions varied across the studies, with 1-year trial consisting of six 20-minute individual counseling sessions to one telephone call at 6 months. Canga’s intervention consisted of an initial 40-minute face-to-face interview and a follow-up support program consisting of 5 contacts for 6 months [39]. In the trial by Hokanson et al. [24], the intervention group received a 20- to 30-minute face-to-face counseling session at the initial study visit and an additional 3 to 6 telephone counseling sessions over a 4- to 6-month period. Another intervention program consisted of 8 two-month group sessions and a group model for smoking cessation; the meeting lasted 45- 60 minutes [43]. In the trial by Sawicki et al. [41], a structured behavioral therapy anti-smoking program was offered consisting of 10 weekly sessions of 90 minutes. Agewall et al. [32] provided a smoking cessation program starting with 1-2 hour lessons, which were given weekly for five weeks involving patients and spouses.
Studies involved incorporating specific action planning, tailored by the participant’s level of motivation to quit smoking [39]; client-centered counseling approach using a combination of manual-based teaching [24], connected to a national guideline [41-43], motivational interviewing techniques [24], and discussions on the benefits of smoking cessation [24, 39].
Nicotine Component -Type and Dose
Sixteen studies contributed to the analysis of the efficacy of the intervention, combining one or more types of nicotine products with other types of interventions, compared to a placebo or other control group not receiving any nicotine products or another form of nicotine.
In the group of studies, there were five studies of nicotine gum [32-35, 41] and five studies of transdermal nicotine patches [37-40, 42]. One trial used NRT in the form of either a transdermal patch or gum [24], and another study used a combination of all forms of NRT (patch, gum, inhaler, lozenge, oral spray) [31]. Two studies included a direct comparison between groups of nicotine: one compared nicotine lozenge with nicotine gum [36], and another compared nicotine transdermal absorption from nicotine-containing patches with nicotine from cigarette smoking [40]. Three studies did not report which form of NRT was offered as a part of the intervention [43-45].
Out of 16 studies, 12 studies did not report the strength of nicotine used in the intervention. Tønnesen et al. [38] reported 21mg per day of nicotine patches with taper doses, and Lee et al. [37] reported a range of 21 mg to 7 mg of patches. Marsh et al. offered 4 mg nicotine lozenge or gum [36] and Epifano et al., 30 cm2 transdermal patch [40].
Two trials included a variable period of dose tapering [36-38] and two studies adjusted the initial dose with a baseline number of cigarettes smoked [37, 45]. In the trial by Lee et al. [37], a 4-week supply of 21 mg/d, a 1-week supply of 14 mg/d, and a 1-week supply of 7 mg/d patches were provided for smokers of 10 cigarettes per day or more. Smokers of <10 cigarettes per day were supplied with 4 weeks of 14 mg/d patches and 2 weeks of 7 mg/d patches. Canga et al. [39] offered transdermal NRT only to those who smoked >20 cigarettes or more. In one trial, either a transdermal patch or gum or bupropion was provided free of charge to subjects, indicating a readiness to quit among subjects in the intervention treatment group, and subjects were encouraged to use pharmacological cessation aids [24]. Scemama et al. did not report the strength of nicotine; the dose was initially adapted according to the results of the Fagerström test, and the dose was subsequently readjusted according to urinary cotinine concentrations [45]. Rodondi et al. [42] offered NRT patches tailored to individual needs. However, most studies offered participants nicotine support for 12 weeks or less.
Duration and Follow-ups of Interventions
The interventions described in the studies vary in the active phase of intervention and duration of follow-up. The duration of intervention ranged from 3 weeks to 24 weeks, and the follow-up varied between 3 months and 6.6 years. Two studies were short: Epifano et al. [40] investigated diabetic patients with a transdermal 30 cm2 patch for two days; one patch, active or placebo, was applied at midnight, and the patient was studied after 12 h, i.e., at 12.00 h on the following day. In the study by Scemama et al. [45], the patient was seen by a specialist doctor once, who discussed the various items on the form with the patient and proposed help with giving up smoking, including a prescription for NRT if necessary.
3.3 Outcome Measures
Primary Outcomes
The primary outcome of most studies was smoking cessation or abstinence (14 out of 16). Most studies addressed smoking cessation using biochemically validated measures (12 out of 16). In nine studies, smoking cessation was biochemically verified using a concentration of breath carbon monoxide (CO) [31, 37, 38], saliva cotinine [24], urinary cotinine [32-35, 39], and serum cotinine [41]. Two studies used both carbon monoxide and cotinine testing [42, 45]. Three studies did not use biochemical verification and relied on self-reported smoking cessation [36, 43, 44]. Trained researchers or nurses generally collected biochemical verification samples during study visits.
Secondary Outcomes
The self-reported mean number of cigarettes smoked per day was included as a secondary outcome in four trials [36, 39, 41, 45].
Glycemic biomarkers, an important tool to monitor glycemic control, were the most common biomarker used in eight studies [24, 32-36, 42, 45]. Measurement of HbA1c value has been widely used for routine long-term monitoring of glucose control and as a measure of risk for developing diabetes complications. Other clinical and metabolic biomarkers included blood glucose [36], lipid profile and body weight [24, 45], and hepatic glucose production, insulin secretion and action [40]. Two trials reported adverse events and adherence related to interventions for smoking cessation in participants [36, 38]. Changes in disease condition [36], diabetes control [32-36], perceived stress, depression, and diabetes distress [24] were assessed in the included trials. In the latter study, the disease status was assessed by monitoring blood glucose and HbAlc in patients with diabetes mellitus. In addition, psychosocial measures included measures of perceived stress, depression (as measured by the Center for Epidemiologic Studies Depression scale), and diabetes distress (as determined by the Problem Areas in Diabetes–2 Survey) [24].
3.4 Effect of Interventions
Smoking Cessation
The primary objective of the review was to provide evidence on the effects of nicotine-based interventions to support smoking cessation among adult patients with diabetes. Fourteen studies out of sixteen measured smoking cessation; however, nine trials reported cessation for diabetic patients (7 exclusive diabetic studies and 2 mixed patient groups). Five of the sixteen studies reported significant effects on abstinence at 6- [31, 38, 39] or 12-month follow-ups [43] among diabetic patients.
The study by Canga et al. offered the intervention consisting of a 40-min nurse visit that included counseling, education, and contracting information (a negotiated cessation date) and transdermal NRT and reported that a significant proportion of smokers achieved abstinence than control at six months; the smoking cessation incidence was 7.5 times higher in the intervention group compared with that of the control group (17% vs. 2.3% cessation incidence ratio 7.5 [95% CI 2.3–24.4]) [39]. Similar results were observed for the nicotine patch versus placebo at weeks 9-12 and at weeks 9-24 in the diabetes sub-cohort in another RCT [38]; the continued abstinence rate was 4.3 times higher (OR 4.33, 95% CI 1.82-10.27) between 9-12 and 5.0 time higher at week 9-24 (OR 5.01, 95% CI 1.77-14.17). With NRT intervention, the diabetic subgroup achieved the highest continuous abstinence rate at weeks 9-12 (27.1%) and at weeks 9-24 (20.8%) among all other smoking-related disease subgroups (COPD, Asthma, CVD, and psychiatric disorders). A cluster RCT of smoking cessation counseling with NRT or bupropion, or varenicline as appropriate, found that the CO-confirmed abstinence rate at 6 months was 11.1% in the intervention group versus 2.6% in the control group (odds ratio 3.73 [95% CI 1.20, 11.58]; P = 0.02) [31]. After 12 months, 20% in the intervention centers and 7% in the control centers reported that they had stopped smoking, and the difference was statistically significant (p<0.01) in special education with the treatment of NRT or Bupropion intervention [43].
In the rest of the trials, the likelihood of smoking cessation was higher in patients who received the intensive intervention compared with the control, although this effect was insignificant. One study [24] found the abstinence rate was marginally greater in the intervention group at a 3-month follow-up (P = 0.077), but there was no significant difference between groups at the 6-month follow-up. Another RCT [37] determined the rates of long-term smoking cessation after a perioperative smoking cessation intervention and predictors of successful cessation at 12-month follow-up and found being diabetic was not a significant predictor in achieving long-term cessation in a group of patients 12 months after surgery. A RCT with 44 diabetic patients showed only 12 reported not smoking at follow-up after 6 months, 4 in the intervention group and double (n=8) in the control group [41]. In one before-after study, only one patient had stopped smoking at the six-month visit out of 38 smokers included in the intervention [45]. In another before-after study, after 18 months of follow-up, 64% who started in the smoking cessation group had become non-smokers [44].
Except for one trial [38], there was no evidence to indicate that these smoking abstinence rates were due to the type of NRT used. Moreover, the inclusion criteria and design of the trials were not similar.
Reduction in Smoking
The smoking reduction was observed by the self-reported number of cigarettes smoked per day in three trials with diabetes patients. One trial found diabetic patients who were still smokers at the end of the trial reported the mean number of cigarettes smoked per day reduced from 20.0 at baseline to 15.5 at 6 months in the intervention group; by comparison, participants in the control group reported a reduction from 19.7 to 18.1 cigarettes daily. Changes in the mean number of cigarettes smoked between both groups showed significant differences toward a benefit in the intervention group (P<0.01) [39]. Another trial found that the self-reported mean number of cigarettes smoked had reduced from 21 cigarettes per day to 5 at the end of the intervention (6 months) [41]. In a before-after study, 3 patients reported their cigarette consumption decreased at 6 months following smoking consultation therapy with NRT, but the number of cigarettes was not quantified [45]. In the RCT to compare the safety profiles of the nicotine lozenge and nicotine gum in smokers with underlying medical conditions, Marsh et al. [36] collected data on the number of cigarettes smoked from those who smoked at the end of the intervention but did not report this outcome separately for diabetic patients.
Biochemical and Clinical Outcomes
Glycaemic Control: Studies found evidence that a combination of behavioral support and NRT was beneficial to diabetic patients in relation to blood glucose control. Among patients with diabetes mellitus, both blood glucose and HbAlc are measured as independent markers of glucose control. Nicotine-based interventions did appear to positively impact clinical outcomes as measured by HbA1c [24, 32, 33, 36, 42, 45, 48]. In one trial, researchers set a goal for the intervention to achieve HbA1c below 6.0% in the intervention patients by 12 months [32]. Four studies from that trial reported changes in HbA1c at different follow-up times: at the one-year follow-up, 15% of the intervention group reached the treatment goal of HbA1c compared to 9% in the control group [32], at 3.3- years follow-up 20% of the intervention group had reached the treatment goal compared to 4% of participants in the control group [34]; over the 3.5-year observational period, HbA1c had reduced by 17% in the intervention group (7.27% to 6.63% P <0.1) with 33% of participants had reached the treatment goal compared to 6% in the control group [33]. At further follow-up at the median of 6.6 years, the mean values and changes in serum HbA1c did not differ between the groups, although 11% of the intervention group and 5% of the control group had a mean HbA1c below 6% [35].
Two of the trials set the target to achieve a mean HBA1c level below 7% [24, 42]. The trial with diabetic patients found that HbA1c values improved significantly from baseline to 3 months for both intervention and control groups. These changes were preserved at the time of the final study follow-up visit at 6 months. More than 70% of diabetic patients in both groups achieved an HbA1c level of <7% (67% in intervention, 73% in control) after 3 months of education. These changes in HbA1c were maintained at the final study follow-up (62% in intervention, 76% in control) [24]. In another trial, a 12-month change in HbA1c was observed with no difference between the intervention and control group, and among 18 participants, none of them achieved a level of HbA1c of less than 7% [42].
A RCT to compare the safety profiles of the 4-mg nicotine lozenge and 4-mg nicotine gum in smokers indicated HbAlc values that presented blood glucose were relatively stable during the 3-month study period in both groups [36]. The proportion of patients with blood glucose levels above the normal range slightly increased during the study, with no apparent difference between the lozenge and gum groups. One before-after study showed a significant decrease (P<0.05) in glycated hemoglobin levels observed in five patients at 6 months [45].
Lipid Profile: In a trial with face-to-face motivational interviewing plus telephone counseling and NRT in the form of either a transdermal patch or gum or bupropion, diabetic participants showed improvements in plasma lipid values (low-density lipoprotein and triglycerides) from baseline to 6-month follow-up although no differences between groups were detected in this RCT [24]. Similarly, the before-after study with smoking cessation consultations and NRT observed a decrease in plasma total cholesterol and triglycerides at 3 and 6 months, but the changes were not significant [45].
Body Weight: Weight loss was observed with smoking cessation intervention for two trials with diabetic patients at 3 months. Hokanson et al. [24] reported weight loss from baseline to 3 months in both intervention groups, although it was not sustained for the cohort in the intervention group at 6 months. Similarly, Scemama et al. [45] reported weight loss in diabetic patients for the first 3 months, but no weight loss was observed at 6 months.
Adverse Events: Treatment-emergent adverse effects of the intervention were summarized for diabetic patients in one trial. The overall incidence of neuropsychiatric adverse events was 8/95 (8.5%) in those with NRT [38].
In the safety trial of nicotine lozenge compared to nicotine gum, investigators found fewer than 6% of patients with diabetes mellitus in either the lozenge or gum group considered their overall disease condition had worsened. Indeed, over 90% of patients rated their diabetic condition as having either improved or not changed at any assessment time point (Weeks 2, 4, 6, or 12) [36].
Safety Issues: The cross-over trial [40] with transdermal patches and cigarettes showed that nicotine patches and cigarette smoking did not affect endogenous insulin secretion as compared to a placebo. On the 2-day cross-over period for each treatment, plasma insulin increased, and hepatic glucose production was less suppressed post-use of the nicotine patches compared with post-use of the placebo in the first 2 hours of the study. Similarly, measures of glucose utilization were less stimulated post-use of the nicotine patch when compared with measures taken post-use of the placebo; however, these were more suppressed after cigarette smoking when measurements were taken in the last two hours of the study. Usually, nicotine impairs insulin action in the liver, adipose tissue, and muscle and may contribute to hyperglycemia in type 2 diabetes. Transdermal nicotine diminishes the action of insulin to a lesser extent than cigarette smoking. Thus, the study demonstrates that transdermal nicotine may represent a "metabolically" safe measure to help patients with type 2 diabetes to quit smoking.
Compliance and Adherence
Though most trials included NRT as an optional subcomponent of the intervention, the proportion of participants who used NRT was not reported in all the included trials. Persson et al. [43] reported that 21 (45% of the intervention group) participants used NRT, and Scemama et al. [45] reported that the NRT initiation was accepted by 45.7% of patients. Canga et al. [39] offered NRT to 105 participants (71% of the intervention group). Treatment adherence was also reported in the trial by Tønnesen et al. Mean adherence to the intervention was 77.1 days for the diabetic group, which was also similar between treatment groups overall, and, when stratified by smoking-related- disease sub-cohort (asthma, chronic pulmonary disease, cardiovascular disease), ranged between 72.3 and 75.2 days [38].
Other Effects
No group difference was observed in psychosocial measures, including measures of perceived stress, depression, and diabetes distress in one trial with type 2 diabetic adults during a 6-month follow-up [24].
3.4 Quality Assessment of the Trials
In most trials for smoking cessation with behavioral interventions, a blinded design is not feasible because of the difficulty in designing a placebo; yet most of the included studies were rated as good-quality studies (score 3). Of 16 studies, 14 RCTs were evaluated. From this assessment, three studies (18.8%) were assigned scores of 1 [43] or 2 [31, 41], and 11 studies (68.8%) were assigned scores of 3 [24, 32-37, 39, 40] or 5 [18].
4. Discussions
In this comprehensive narrative review, we have included 12 smoking cessation interventions delivered with nicotine components for diabetic patients (described in 16 studies). Most of the studies reviewed included a combination of nicotine products and behavioral support, indicating that such a combination was the most effective for diabetic, older, disadvantaged smokers, including those with substance use disorders [26, 49-51]. This review identified that the inclusion of nicotine replacement was promising in interventions with these diabetic smokers, suggesting an increased rate of smoking cessation at six-month or longer follow-ups. However, these suggestive findings were not evident in all of the trials reviewed. Previous data suggest that those adult smokers who sustain six months of abstinence with nicotine support will maintain the smoking abstinent status for the remainder of their lives [52, 53]. Other systematic reviews have suggested that any form of nicotine-based intervention, including NRT or E-cigarettes, can help people who make a quit attempt and increase their chances of successfully stopping smoking [54, 55]. NRT has been shown to have a positive long-term effect on smoking cessation in a systematic review, with the relative efficacy of a single course of NRT in sustaining smoking cessation remaining constant beyond 12 months [52]. However, the nicotine components of the interventions and the description of the commonalities and differences between the intervention products and their effects on diabetic participants are not yet understood. To our knowledge, this systematic review is the first to examine the effect of nicotine-based interventions on the diabetic population. Considering the known risk of smoking, diabetic patients who smoke should be routinely reminded that cigarette smoking adversely affects their blood glucose control, increases their insulin resistance, and increases their risk of developing disease complications. The first-line drugs that increase the likelihood of success in smoking cessation include NRT, bupropion, and varenicline [13]. Nicotinic substitutes—which have known effects on sympathetic neural stimulation and catecholamine release—can negatively impact the cardiovascular system, metabolic function, and glucose metabolism [56, 57]. Some researchers have raised concerns about using NRTs in diabetic patients with poor glycol-metabolic control, given that nicotine may increase insulin resistance [58, 59]. However, in the current review, there was no evidence of an increase in life-threatening problems in relation to nicotine use, and NRT was well tolerated, with no adverse effects reported with different doses of nicotine for diabetic patients. Data from the included studies showed that HbA1c values, as an indicator of glycemic control, improved with nicotine intervention [24, 32-35, 42, 45], and more than 95% rated their diabetic condition as having improved or not changed throughout the nicotine intervention at week 12 [36].
Additionally, findings from one included study suggested that transdermal nicotine delivery interferes with glucose metabolism much less than cigarette smoking in type 2 diabetic patients [40]; therefore, the deterioration in the action of insulin at the level of the liver, adipose tissue, and muscle from nicotine pouches was much less pronounced than after smoking cigarettes. The transient application of nicotine to patients with type 2 diabetes mellitus who smoke may represent a metabolically safe way to improve their long-term cardiovascular prognosis by assisting their efforts to quit smoking. On the basis of the review undertaken, efforts aimed at supporting diabetic smokers’ attempts to quit smoking should include consideration of nicotine provision.
Currently, nicotine-assisted reduction to stop smoking is recommended in a wide range of clinical settings, e.g., by the National Institute for Health and Clinical Excellence (NICE) [60], the US Clinical Practice Guideline [61] and the World Health Organization (WHO) [62]. The optimum dose of nicotine for diabetic patients in reduction programs is presently unknown. Moreover, most of the trials included in this review used nicotine as only part of the intervention components alongside behavioral support. As a result, it is not clear how much of the positive outcomes in terms of smoking cessation for diabetic patients are directly attributable to the provision of alternative means of consuming nicotine. Therefore, examining how nicotine-assisted smoking cessation efforts can be incorporated into tobacco control programs and clinical settings for diabetic patients is important.
Two meta-analyses investigating adverse events associated with NRTs have shown increased cardiovascular symptoms [63, 64], still not major cardiovascular events [64], and serious cardiovascular events symptoms were primarily reported in a case study for a patient who continued to smoke while using NRT [65]. Despite the increased cardiovascular risk among people with diabetes, no trial was identified that evaluated the effect of smoking cessation interventions on cardiovascular symptoms among diabetic patients. The issue surrounding the safety of such treatments was partly addressed in two trials where diabetic patients were subgroups of the total sample of patients [36, 38]. However, the follow-up period of 12 weeks or 24 weeks is likely to be too short to identify sustained effects. Trials assessing combinations of NRT, with or without behavioral interventions, may have better reflected the clinical outcomes associated with long-term follow-up. Data from robust, randomized control trials of interventions evaluating smoking outcomes, clinical and metabolic change, cardiovascular risk, and glycemic control with long-term follow-up would inform treatment strategies in the diabetic population in which smoking cessation is likely to have high absolute benefits. In recent years, many newer tobacco harm-reduction products have emerged on the market, including e-cigarettes, heated tobacco products, and oral nicotine pouches. These ‘alternative’ products are suggested for smokers as a replacement for conventional cigarettes [66, 67], thus replacing a very harmful product with a less harmful product. Although not formally regulated as a pharmaceutical product, these tobacco harm reduction products act as nicotinic substitutes. Where success has been achieved from these products in the general population [55, 68], clinicians should consider alternative strategies, including those developed on risk reduction, such as using the new emerging technological devices that release nicotine without combustion. Although little is known about the health effects associated with the long-term use of vapor, heated tobacco products, or nicotine pouches, it is widely accepted that the long-term consumption of combustible cigarettes is extremely dangerous and can lead to the development of metabolic alterations among diabetic patients. A large, worldwide, internet-based survey of 574 regular e-cigarette users with diabetes [69] found that 41.9% of diabetic patients reported improvement in diabetes control after switching from conventional cigarettes to e-cigarettes, and only 0.4% reported a worsening. Since many diabetic patients continue smoking despite the well-known health risks, these emerging nicotine delivery technologies could be a possible and much less harmful alternative. In addition to the ongoing trial of combustion-free nicotine delivery systems such as e-cigarettes and heated tobacco products among type 2 diabetic cigarette smokers [70], further RCT studies of smokers with diabetes are required to establish the benefit of nicotine replacement products among diabetic patients and inform policy decisions.
This review has a number of limitations. Firstly, a meta-analysis would provide more evidence than a narrative review. However, due to the wide range of heterogeneity in the studies, including the design, interventions, population characteristics, type of nicotine, follow-up period, treatment lengths, outcomes, etc., meta-analysis was not suitable for this review. The descriptive nature of the review, however, has meant that it was not possible to quantify the effect of individual program components in explaining smoking cessation outcomes across the various studies reviewed. Second, searches were limited to English-language publications; two relevant nicotine interventions with diabetic patients were excluded, one in German [71] and another in Russian [72]. A key strength of this review is that it addresses a knowledge gap and has collated evidence from a broad methodological base to report the interventions adopted to support smoking cessation among smokers with diabetes. The methods used for this review were complete and adhered to Cochrane review standards [29]. In addition, more than one paper from one trial was included in this review, and the authors often referenced a previous publication when describing the study methods. In these instances, the primary publication was sourced to provide a more detailed assessment of the study methodology. It is acknowledged that while a summary of characteristics and outcomes is presented, insufficient evidence is available to evaluate and summarize the relationship between individual measures statistically, and further studies are required to elucidate this. Despite this, the systematic approach to this review has identified the scope of the interventions implemented to date for a critical population of smokers diagnosed with diabetes, therefore adding to the limited body of evidence published in this area [26, 73].
Implications and Future Research
The primary goal of this review was to describe the components of nicotine interventions by examining a wide range of interventions at different developmental stages to better understand how nicotine can be used for smoking cessation in diabetic patients and produce comprehensive evidence in this area of research. This review shows that few interventions are focusing solely on nicotine intervention, and data are still limited. Future studies, sufficiently powered with rigorous methodology and biochemical measures to confirm abstinence along with clinical outcomes with appropriate follow-ups, are needed to further test nicotine as a delivery mechanism for smoking cessation interventions among diabetic patients. While smoking cessation is necessary, due to the addictive nature of nicotine, it is hard to achieve. Tobacco harm reduction approaches must be considered for smokers who repeatedly attempt to quit but have trouble sustaining abstinence. Long-term use of NRT, alternative forms of popular nicotine products, such as e-cigarettes, heated tobacco products, oral nicotine pouches, and smoking reduction strategies, deserve increased consideration for vulnerable groups.
This review has identified areas that need further examination to improve the quality of research in this area. Future areas for research include identifying the most effective means to reach vulnerable populations; ascertaining what specific combination treatments have the most significant effects on smoking outcomes; what impact the mode of delivery or frequency of treatment has on abstinence in this population; and understanding the components of the intervention in order to improve its efficacy and feasibility.
The current review was undertaken to support the development of a clinical trial to assess the long-term (12-month) effect of a nicotine-based intervention (Oral Nicotine Pouches) among type 2 diabetes in Bangladesh, one of the top ten countries with diabetic adults [1]. None of the studies included in this review were conducted in Low- and Middle-Income Countries (LMICs) where diabetes is highly prevalent. In 2021, almost 80% of diabetes patients lived in LMICs, and it is estimated that 94% of those with diabetes will stay in LMICs in 2045 [1]. Like prevalence, more than 80% of diabetes deaths occur in LMICs, which makes it the ninth leading cause of mortality in LMICs [74]. Due to inadequate infrastructure for diabetes care and limited resources, many developing countries struggle to cope with both smoking and the diabetes epidemic [75]. Given that smokers with diabetes are susceptible to the detrimental effects of cigarette smoke, identifying and delivering effective smoking cessation interventions in LMICs may be an important area for future investigation. This review offers a solid ground to design studies to identify nicotine-based interventions that promote longer-term abstinence up to the 12-month follow-up period, using a measure of abstinence, critical clinical outcomes, compliance, and sustainability to inform policy decisions in LMICs.
Conclusions
In conclusion, this narrative review has focused on smoking cessation interventions using nicotine among adult patients with diabetes. This review suggests that smoking cessation interventions utilizing NRT combined with behavioral support appear to increase smoking abstinence rates in patients with diabetes and positively affect other clinical or disease outcomes. However, the studies included in this review were heterogeneous in design, intervention, sample size, and inclusion criteria. Exclusive nicotine-based interventions are required to strengthen the evidence base as standard guidelines in the US support NRT to stop smoking for every adult regardless of the individual's physical condition, even patients with a history of cardiovascular disease [61]. Similarly, current National Health Services in the UK offers NRT or e-cigarettes to all smokers aged 12 and over, including pregnant women [60, 76]. Further work is needed to explore the role of nicotine in diabetic clinical care.
Declaration
Funding
This literature review was funded with a grant from the Foundation for a Smoke-Free World (FSFW) (Reference: FSFW W3-053), a US non-profit 501(c)(3) private foundation.
Disclaimer
The contents, selection, and presentation of facts, as well as any opinions expressed in the review, are the sole responsibility of the authors and under no circumstances shall be regarded as reflecting the positions of the FSFW.
Author’s contributions
Dr. Haseen conceptualized the review. Dr. Haseen conducted the search of the literature, and Dr. Rahman, Dr. Hossain, Mr. Rana, Ms. Mahmud, and Mr. Chowdhury identified articles and reviewed them for eligibility. Dr. Coyle and Ms. Notley conducted the quality checking procedures. Dr. Haseen reviewed all eligible studies and analyzed them. Dr. Haseen wrote the first draft of the report, and all authors contributed to and approved the final version.
Acknowledgments
The authors would like to thank Beata Coffey, Information Specialist at the Royal Society of Medicine (RSM) Library, for help in developing the Medline and Embase search strategies.
References:
- International Diabetes Federation, IDF Diabetes Atlas. 2021: Brussels, Belgium.
- Pan A, Yeli Wang, Mohammad Talaei, et al. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 3 (2015): 958-967.
- Al-Delaimy WK, JoAnn E Manson, Caren G Solomon, et al. Smoking and risk of coronary heart disease among women with type 2 diabetes mellitus. Arch Intern Med 162 (2002): 273-279.
- Campagna D, A Alamo, A Di Pino, et al. Smoking and diabetes: dangerous liaisons and confusing relationships. Diabetol Metab Syndr 11 (2019): 85.
- Qin R, Tao C, Qingqing L, et al. Excess risk of mortality and cardiovascular events associated with smoking among patients with diabetes: meta-analysis of observational prospective studies. Int J Cardiol 167 (2013): 342-350.
- Pan A, Yeli W, Mohammad T, et al. Relation of Smoking With Total Mortality and Cardiovascular Events Among Patients With Diabetes Mellitus: A Meta-Analysis and Systematic Review. Circulation 132 (2015): 1795-1804.
- Yudkin JS. How can we best prolong life? Benefits of coronary risk factor reduction in non-diabetic and diabetic subjects. Bmj 306 (1993): 1313-1318.
- Fiore MC, WC Bailey, and SJ Cohen. Treating Tobacco Use and Dependence: 2008 Update. 2008, U.S. Department of Health and Human Services. Public Health Service, Rockville (2008).
- Haire-Joshu D. Smoking, cessation, and the diabetes health care team. Diabetes Educ 17 (1991): 54-64.
- Zhu S, T Melcer, J Sun, et al. Smoking cessation with and without assistance: a population-based analysis. Am J Prev Med 18 (2000): 305-311.
- West R, and X Zhou. Is nicotine replacement therapy for smoking cessation effective in the "real world"? Findings from a prospective multinational cohort study. Thorax 62 (2007): 998-1002.
- West R, and J Brown. Smoking and Smoking Cessation in England London (2012).
- Tobacco TC, A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med 35 (2008): 158-176.
- Gross J, and ML Stitzer. Nicotine replacement: ten-week effects on tobacco withdrawal symptoms. Psychopharmacology (Berl) 98 (1989): 334-341.
- West R, and S Shiffman. Effect of oral nicotine dosing forms on cigarette withdrawal symptoms and craving: a systematic review. Psychopharmacology (Berl) 155 (2001): 115-122.
- Stead LF, Rafael P, Chris B, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 11 (2012): Cd000146.
- Hajek P, R West, J Foulds, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med 159 (1999): 2033-2038.
- Tønnesen P, and KL Mikkelsen. Smoking cessation with four nicotine replacement regimes in a lung clinic. Eur Respir J 16 (2000): 717-722.
- West R, A McNeill, and M Raw. Smoking cessation guidelines for health professionals: an update. Thorax 55 (2000): 987-999.
- Ministry of Health, New Zealand Smoking Cessation Guidelines. 2007: New Zealand: Wellington (2007).
- Woolacott NF, L Jones, C A Forbes, et al. The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation. Health Technol Assess 6 (2002): 1-245.
- Zwar N, Robyn R, Ron B, et al. Smoking cessation guidelines for Australian general practice. Aust Fam Physician 34 (2005): 461-466.
- Le Foll B, P Melihan-Cheinin, G Rostoker, et al. Smoking cessation guidelines: evidence-based recommendations of the French Health Products Safety Agency. Eur Psychiatry 20 (2005): 431-441.
- Hokanson JM, Robyn L A, Deborah J H, et al. Integrated tobacco cessation counseling in a diabetes self-management training program: a randomized trial of diabetes and reduction of tobacco. The Diabetes educator 32 (2006): 562-570.
- Schwartz JL. Methods of smoking cessation. Med Clin North Am 76 (1992): 451-476.
- Nagrebetsky A, Rachel B, Nia R, et al. Smoking cessation in adults with diabetes: a systematic review and meta-analysis of data from randomised controlled trials. BMJ Open 4 (2014): e004107.
- Haseen F, et al. A literature review to assess the efficacy of nicotine-based interventions for adult smokers with diabetes, PROSPERO CRD42023403930 (2023).
- Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372 (2021): n71.
- Higgins JPT, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane (2022).
- Jadad AR, R A Moore, D Carroll, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17 (1996): 1-12.
- Reid RD, Janine M, Evyanne W, et al. Prospective, Cluster-Randomized Trial to Implement the Ottawa Model for Smoking Cessation in Diabetes Education Programs in Ontario, Canada. Diabetes care 41 (2018): 406-412.
- Agewall S, et al. Multiple cardiovascular risk factor intervention in treated hypertensive men: what can be achieved? Nutr Metab Cardiovasc Dis 3 (1993): 128-135.
- Suurküla M, S Agewall, B Fagerberg, et al. Multiple risk intervention in high-risk hypertensive patients: A 3-year ultrasound study of intima-media thickness and plaques in the carotid artery. Arteriosclerosis, Thrombosis, and Vascular Biology 16 (1996): 462-470.
- Agewall S, J Wikstrand, O Samuelsson, et al. The efficacy of multiple risk factor intervention in treated hypertensive men during long-term follow up. Journal of Internal Medicine 236 (1994): 651-659.
- Fagerberg B, J Wikstrand, G Berglund, et al. Mortality rates in treated hypertensive men with additional risk factors are high but can be reduced: a randomized intervention study. American journal of hypertension 11 (1998): 14-22.
- Marsh HS, Carolyn MD, Jae HC, et al. Safety profile of a nicotine lozenge compared with that of nicotine gum in adult smokers with underlying medical conditions: a 12-week, randomized, open-label study. Clinical therapeutics 27 (2005): 1571-1587.
- Lee SM, Jennifer L, Philip MJ, et al. Long-term quit rates after a perioperative smoking cessation randomized controlled trial. Anesthesia and Analgesia 120 (2015): 582-587.
- Tønnesen P, D Lawrence, and S Tonstad. Medication-assisted quit rates in participants with smoking-related diseases in EAGLES: Post hoc analyses of a double-blind, randomized, placebo-controlled clinical trial. Tobacco induced diseases 20 (2022): 46.
- Canga N, J De Irala, E Vara, et al. Intervention study for smoking cessation in diabetic patients: A randomized controlled trial in both clinical and primary care settings. Diabetes Care 23 (2000): 1455-1460.
- Epifano L, A Di Vincenzo, C Fanelli, et al. Effect of cigarette smoking and of a transdermal nicotine delivery system on glucoregulation in type 2 diabetes mellitus. European journal of clinical pharmacology 43 (1992): 257-263.
- Sawicki PT, U Didjurgeit, I Mühlhauser, et al. Behaviour therapy versus doctor's anti-smoking advice in diabetic patients. Journal of internal medicine 234 (1993): 407-409.
- Rodondi N, Tinh-Hai C, David N,et al. Impact of carotid plaque screening on smoking cessation and other cardiovascular risk factors: a randomized controlled trial. Archives of internal medicine 172 (2012): 344-352.
- Persson L-G, and A Hjalmarson. Smoking cessation in patients with diabetes mellitus: results from a controlled study of an intervention programme in primary healthcare in Sweden. Scandinavian journal of primary health care 24 (2006): 75-80.
- Persson LG, K Lindström, and H Lingfors. Quality improvement in primary health care using computerised journal, exemplified by a smoking cessation programme for diabetic patients. Scandinavian journal of primary health care 18 (2000): 252-253.
- Scemama O, E Hamo-Tchatchouang, A L Le Faou, et al. Difficulties of smoking cessation in diabetic inpatients benefiting from a systematic consultation to help them to give up smoking. Diabetes Metab 32 (2006): 435-441.
- Hokanson JM, Robyn LA, Deborah JH, et al. Integrated tobacco cessation counseling in a diabetes self-management training program: a randomized trial of diabetes and reduction of tobacco. Diabetes educator 32 (2006): 562?570.
- Canga N, J De Irala, E Vara, et al. Intervention study for smoking cessation in diabetic patients: a randomized controlled trial in both clinical and primary care settings. Diabetes care 23 (2000): 1455-1460.
- Eisenberg MJ, Lisa MB, Kristian BF, et al. The efficacy of smoking cessation therapies in cardiac patients: a meta-analysis of randomized controlled trials. Can J Cardiol 26 (2010): 73-79.
- Bauld L, Kirsten B, Lucy McC, et al. The effectiveness of NHS smoking cessation services: a systematic review. J Public Health (Oxf) 32 (2010): 71-82.
- Thurgood SL, Ann McN, David Clark-Carter, et al. A Systematic Review of Smoking Cessation Interventions for Adults in Substance Abuse Treatment or Recovery. Nicotine Tob Res 18 (2016): 993-1001.
- Wilson A, Ashleigh G, Johnson G, et al. A systematic narrative review of the effectiveness of behavioural smoking cessation interventions in selected disadvantaged groups (2010-2017). Expert Rev Respir Med 11 (2017): 617-630.
- Etter JF, and JA Stapleton. Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control 15 (2006): 280-285.
- Kralikova E, et al. The clinical benefits of NRT-supported smoking reduction. . Nicotine Tob Res 4 (2002): 243.
- Hartmann-Boyce J, Samantha CC, Weiyu Ye, et al. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev 5 (2018): Cd000146.
- Hartmann-Boyce J, Hayden McR, Nicola L, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 4 (2021): Cd010216.
- Driva S, Aliki K, Serena T, et al. The Effect of Smoking Cessation on Body Weight and Other Metabolic Parameters with Focus on People with Type 2 Diabetes Mellitus. Int J Environ Res Public Health 19(2022): 13222.
- Benowitz NL, and SG Gourlay. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol 29 (1997): 1422-1431.
- Eliasson B. Cigarette smoking and diabetes. Prog Cardiovasc Dis 45 (2003): 405-413.
- Eliasson B, MR Taskinen, and U Smith. Long-term use of nicotine gum is associated with hyperinsulinemia and insulin resistance. Circulation 94 (1996): 878-881.
- NICE, NICE guideline [NG209]. Tobacco: preventing uptake, promoting quitting and treating dependence. 2021 National Institute for Health and Clinical Excellence (2021).
- Agency for Healthcare Research and Quality, Clinical Guidelines for Prescribing Pharmacotherapy for Smoking Cessation. Agency for Healthcare Research and Quality: Rockville, MD (2012).
- WHO, WHO report on the global tobacco epidemic 2021: addressing new and emerging products., W.H. Organization, Editor. World Health Organization: Geneva (2021).
- Mills EJ, Ping W, Ian L, et al. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis 8 (2010): 8.
- Mills EJ, Kristian T, Shawn E, et al. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation 129 (2014): 28-41.
- Dacosta A, J M Guy, B Tardy, et al. Myocardial infarction and nicotine patch: a contributing or causative factor? Eur Heart J 14 (1993): 1709-1711.
- McNeill A, Leonie S Brose, Robert Calder, et al. Evidence review of e-cigarettes and heated tobacco products 2018. A Report Commissioned by Public Health England. 2018, Public Health England: London, UK (2018): 1-243.
- Royal College of Physicians, Nicotine without Smoke: Tobacco Harm Reduction. 2016, Royal College of Physicians: London, UK (2016).
- McCaffrey S, Lewis J , Becker E , et al. Six-Week Actual Use Study to Evaluate the Impact of Oral Tobacco-Derived Nicotine Pouches on Cigarette Smoking and Smokeless Tobacco Product Use Behaviors (2021).
- Farsalinos KE, Giorgio R, Dimitris T, et al. Characteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumers. Int J Environ Res Public Health 11 (2014): 4356-4373.
- Krysinski A, Cristina R, Sarah J, et al. International randomised controlled trial evaluating metabolic syndrome in type 2 diabetic cigarette smokers following switching to combustion-free nicotine delivery systems: the DIASMOKE protocol. BMJ Open 11 (2021): e045396.
- Brath H, D Lasar, I Buchhäusl, et al. [Intensified smoking cessation for diabetic patients--preliminary results]. Acta Med Austriaca 26 (1999): 163-167.
- Ostrovskaia TP. [Results of clinical investigation of anti-nicotine drug patches]. Med Tekh (194): 42-43.
- Moore D, Paul A, Martin C, et al. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. Bmj 338 (2009): b1024.
- Khan MAB, Muhammad J H, Jeffrey K K, et al. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health 10 (2020): 107-111.
- Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34 (2011): 1249-1257.
- NICE, NICE public health guidance 10. Smoking cessation services in primary care, pharmacies, local authorities and workplaces, particularly for manual working groups, pregnant women and hard to reach communities. . National Institute for Health and Clinical Excellence (2008).