Neonatal Development and Memory of Rat Pups Treated with Hypericum Perforatum During Pregnancy
Article Information
Lorena Ribeiro Silva1*, José R. Paranaíba1, Leandro Véspoli Campo1,2, Vinícius de Almeida Vieira2, Rita de Cássia da Silveira e Sá3, Martha de Oliveira Guerra1, Vera Maria Peters1
1Centro de Biologia da Reprodução – Universidade Federal de Juiz de Fora, Juiz de Fora, MG, Brasil
2Hospital Maternidade Terezinha de Jesus, Faculdade de Ciências Médicas e da Saúde de Juiz de Fora – SUPREMA, Juiz de Fora, MG, Brasil
3Departamento de Patologia e Fisiologia– CCS – Universidade Federal da Paraíba, João Pessoa, PB, Brasil
*Corresponding Author: Dr. Lorena Ribeiro Silva, Centro de Biologia da Reprodução – Universidade Federal de Juiz de Fora, Juiz de Fora, MG, Brasil
Received: 07 September 2019; Accepted: 20 September 2019; Published: 23 September 2019
Citation: Lorena Ribeiro Silva, José R. Paranaíba, Leandro Véspoli Campo, Vinícius de Almeida Vieira, Rita de Cássia da Silveira e Sá, Martha de Oliveira Guerra, Vera Maria Peters. Neonatal Development and Memory of Rat Pups Treated with Hypericum Perforatum During Pregnancy. Journal of Psychiatry and Psychiatric Disorders 3 (2019): 216-226.
Share at FacebookAbstract
Hypericum perforatum L. extract is used as treatment for depression and there are few studies on its safe usage during pregnancy.
Objective: The aim of this study was to evaluate the effects of Hypericum perforatum on the neonatal development and memory of Wistar rats’ offspring treated during this period.
Materials and Methods: Animals used throughout the present research were divided into four groups according to what they consumed: one control group (distilled water) and three treated groups (Hypericum perforatum doses of 36 mg/kg/day, 72 mg/kg/day and 144 mg/kg/day).
Results: The neonates, monitored during the neonatal period, presented the regular physical and reflexological development signs, such as body weight, day of eye opening, ear unfolding, lanugo and fur appearance, eruption of the upper and lower incisors, vaginal opening, lowering of the testicles, and the expected response in the following tests: palmar grip, postural response test, cliff avoidance, orientation test and negative geotaxis, between the second and twenty-seventh day of postnatal life, with no significant difference among groups. Memory related results from inhibitory avoidance and object recognition tasks also showed no significant change.
Conclusion: Therefore, according to the experimental model employed, Hypericum perforatum did not interfere neither in neonatal development nor in memory of pups exposed to the extract during intrauterine life.
Keywords
Hypericum perforatum; Pregnancy; Memory; Neonatal development; Rat
Hypericum perforatum articles, Pregnancy articles, Memory articles, Neonatal development articles, Rat articles
Hypericum perforatum articles Hypericum perforatum Research articles Hypericum perforatum review articles Hypericum perforatum PubMed articles Hypericum perforatum PubMed Central articles Hypericum perforatum 2023 articles Hypericum perforatum 2024 articles Hypericum perforatum Scopus articles Hypericum perforatum impact factor journals Hypericum perforatum Scopus journals Hypericum perforatum PubMed journals Hypericum perforatum medical journals Hypericum perforatum free journals Hypericum perforatum best journals Hypericum perforatum top journals Hypericum perforatum free medical journals Hypericum perforatum famous journals Hypericum perforatum Google Scholar indexed journals Pregnancy articles Pregnancy Research articles Pregnancy review articles Pregnancy PubMed articles Pregnancy PubMed Central articles Pregnancy 2023 articles Pregnancy 2024 articles Pregnancy Scopus articles Pregnancy impact factor journals Pregnancy Scopus journals Pregnancy PubMed journals Pregnancy medical journals Pregnancy free journals Pregnancy best journals Pregnancy top journals Pregnancy free medical journals Pregnancy famous journals Pregnancy Google Scholar indexed journals Memory articles Memory Research articles Memory review articles Memory PubMed articles Memory PubMed Central articles Memory 2023 articles Memory 2024 articles Memory Scopus articles Memory impact factor journals Memory Scopus journals Memory PubMed journals Memory medical journals Memory free journals Memory best journals Memory top journals Memory free medical journals Memory famous journals Memory Google Scholar indexed journals Neonatal development articles Neonatal development Research articles Neonatal development review articles Neonatal development PubMed articles Neonatal development PubMed Central articles Neonatal development 2023 articles Neonatal development 2024 articles Neonatal development Scopus articles Neonatal development impact factor journals Neonatal development Scopus journals Neonatal development PubMed journals Neonatal development medical journals Neonatal development free journals Neonatal development best journals Neonatal development top journals Neonatal development free medical journals Neonatal development famous journals Neonatal development Google Scholar indexed journals Rat articles Rat Research articles Rat review articles Rat PubMed articles Rat PubMed Central articles Rat 2023 articles Rat 2024 articles Rat Scopus articles Rat impact factor journals Rat Scopus journals Rat PubMed journals Rat medical journals Rat free journals Rat best journals Rat top journals Rat free medical journals Rat famous journals Rat Google Scholar indexed journals reflexological development signs articles reflexological development signs Research articles reflexological development signs review articles reflexological development signs PubMed articles reflexological development signs PubMed Central articles reflexological development signs 2023 articles reflexological development signs 2024 articles reflexological development signs Scopus articles reflexological development signs impact factor journals reflexological development signs Scopus journals reflexological development signs PubMed journals reflexological development signs medical journals reflexological development signs free journals reflexological development signs best journals reflexological development signs top journals reflexological development signs free medical journals reflexological development signs famous journals reflexological development signs Google Scholar indexed journals depression articles depression Research articles depression review articles depression PubMed articles depression PubMed Central articles depression 2023 articles depression 2024 articles depression Scopus articles depression impact factor journals depression Scopus journals depression PubMed journals depression medical journals depression free journals depression best journals depression top journals depression free medical journals depression famous journals depression Google Scholar indexed journals psychotropic drugs articles psychotropic drugs Research articles psychotropic drugs review articles psychotropic drugs PubMed articles psychotropic drugs PubMed Central articles psychotropic drugs 2023 articles psychotropic drugs 2024 articles psychotropic drugs Scopus articles psychotropic drugs impact factor journals psychotropic drugs Scopus journals psychotropic drugs PubMed journals psychotropic drugs medical journals psychotropic drugs free journals psychotropic drugs best journals psychotropic drugs top journals psychotropic drugs free medical journals psychotropic drugs famous journals psychotropic drugs Google Scholar indexed journals Pharmacological treatment articles Pharmacological treatment Research articles Pharmacological treatment review articles Pharmacological treatment PubMed articles Pharmacological treatment PubMed Central articles Pharmacological treatment 2023 articles Pharmacological treatment 2024 articles Pharmacological treatment Scopus articles Pharmacological treatment impact factor journals Pharmacological treatment Scopus journals Pharmacological treatment PubMed journals Pharmacological treatment medical journals Pharmacological treatment free journals Pharmacological treatment best journals Pharmacological treatment top journals Pharmacological treatment free medical journals Pharmacological treatment famous journals Pharmacological treatment Google Scholar indexed journals fetal brain development articles fetal brain development Research articles fetal brain development review articles fetal brain development PubMed articles fetal brain development PubMed Central articles fetal brain development 2023 articles fetal brain development 2024 articles fetal brain development Scopus articles fetal brain development impact factor journals fetal brain development Scopus journals fetal brain development PubMed journals fetal brain development medical journals fetal brain development free journals fetal brain development best journals fetal brain development top journals fetal brain development free medical journals fetal brain development famous journals fetal brain development Google Scholar indexed journals
Article Details
1. Introduction
Pregnancy is a period in which many physical, hormonal and social changes directly affect women’s mental health [1]. It is estimated that the prevalence of depression during pregnancy is 13% of pregnant women. But it is an underreported condition because its symptoms are often attributed to the pregnancy itself [2]. This pathology may not only affect mother’s health, but also limit maternal care as well as child’s growth and development [1]. Pharmacological treatment for depression during pregnancy should take into consideration the risk-benefit ratio, because the psychotropic drugs are able to pass the placental barrier, and may potentially influence fetal brain development [3]. The prenatal exposure with fluoxetine, a selective serotonin reuptake inhibitor, in rats has shown detrimental behavioral outcomes, which may partly be due to altered 5-HT1A receptor signaling [4]. Study conducted in Sprague-Dawley rats showed that maternal exposure to selective serotonin (5-HT) reuptake inhibitors, during pregnancy and postnatal, resulted in behavioral alterations in its progeny (spatial learning deficits, hypoactivity and induced several autism-like behavioral effects) [5]. In rats, serotonin levels are high in the immature brain as well as the expression of high affinity receptors in the perinatal period [6].
Complementary and alternative medicine is used on the treatment of psychiatric disorders, although research on benefits and risks inherent to their use is still necessary. Herbal medicine is being widely used among the population, and one of the most utilized has been Hypericum perforatum (Hp), popularly known as St. John’s wort. Hp is indicated for the treatment of mild to moderate depression, and is presented as an alternative therapy, with the use of phytotherapics [7]. Its main active substances responsible for the antidepressant action are hypericin and hyperforin. The mechanism of action is through the inhibition of neurotransmitter reuptake, such as serotonin, dopamine and noradrenaline, in addition to the activation of glutamate and gamma-aminobutyric acid (GABA) receptors and the alteration of the hypothalamic-pituitary-adrenal axis [8]. Serotonin (5-HT) appears early in fetal development and plays an important role in brain morphogenesis, being essential for its development, in addition to promoting mood and stress regulation [9]. This neurotransmitter is found in cerebral areas that are important for memory, such as the hippocampus and prefrontal cortex, and may act directly or indirectly modulating other neurotransmitters, such as glutamate, gamma-aminobutyric acid, dopamine, noradrenaline and acetylcholine [10]. Modifications in 5-HT signals may be caused by exposition to serotonin reuptake inhibitors during intrauterine life and to genetic variations of 5-HT transporter, Solute Carrier Family 6 Member 4 –(SLC6A4), and these changes in fetal signaling represent a risk for brain development and lifelong health disorders [11].
Hypericum perforatum main active substances have affinity for the central nervous system and have a long elimination half-life: hypericin t1/2=23.8h and hyperforin t1/2=19.6h [12]. The long-term use of this extract may favor its accumulation both in maternal and fetal organisms. A recent study detected the presence of Hp extract constituents in blood, retroperitoneal fat, vital organs and placenta of Wistar rats that were treated with it during pregnancy. In addition, it was also found in the liver and brain of the fetuses of those rats, indicating the passage of Hp through the placental barrier. These analyzes have been realized after the treatment, occurring during the gestational period [13].
In rodents, critical or vulnerable periods for the development of the nervous system range from the beginning of the neural plate formation to postnatal development, with full maturation of both blood-brain barrier and cholinergic and dopaminergic systems [14]. Therefore, it may be suggested that exposure to CNS-acting agents, such as Hp, would make developing fetus suffer from neural alterations as well as alter neonatal and cognitive development of offspring previously exposed during intrauterine life. Considering the previous information, this study aims to verify if the prolonged treatment of pregnant rats with Hp alters physical, neuromotor and cognitive development of offspring.
2. Objetive
To evaluate the activity of the dry extract of Hypericum perforatum on the neonatal development and the memory process in the pups of Wistar rats treated during pregnancy.
3. Materials and Methods
3.1 Animals
Forty pregnant Wistar rats and eighty pups obtained from the vivarium of the Reproduction Biology Center (CBR/UFJF). (CIAEP n. 01.0048.2013) were used. After weaning, pregnant rats and their pups were placed in polypropylene cages (49 × 34 × 16 cm), kept in acclimatized racks (ALESCO®), with airflow, and under standard laboratory conditions, with a controlled temperature of 22 ± 2°C, 40 to 60% humidity and a 12h light/dark photoperiod (the light phase starts at six o'clock in the morning). Animals were fed with rat chow pellets (Nuvilab®) and received water ad libitum. All experimental procedures were approved by UFJF’s Ethics Committee on Animal Use, certified by protocol n. 101/2012. The experimental study was carried out in the second semester of 2013.
3.2 Hypericum perforatum extract
Hypericum perforatum L. standard dried extract containing 0.3% hypericin was imported from China, registry number 20100913, and manipulated by MBPharma Ltd, lot number 10124778E.
3.3 Experimental procedures
Pregnant rats were randomly distributed into four groups, each of them composed by 10 animals: one control group, which received distilled water, and three treated groups, which received Hp aqueous extract, in doses of 36 mg/kg/day, 72 mg/kg/day and 144 mg/kg/day, throughout pregnancy (20 days) through gavage. The 36 mg/kg/day dose was calculated based on the suggested dose for humans, considering the rat’s body surface area [15]. The subsequent doses correspond to the double and quadruple of the therapeutic dose. After delivery, 10 female and 10 male newborns from each experimental group were randomly selected. They were evaluated in their postnatal period and subjected, at 90 days of age, to the memory evaluation tests. All tests occurred during the morning. The surplus newborns were used in other experiments.
3.4 Maternal toxicity evaluation
The identification of possible maternal toxic effects was made through weekly clinical evaluation, by means of the measurement of feed intake, body weight and daily observation for piloerection, alterations in ambulation, presence of stereotypy, chromodacryorrhea and diarrhea, in addition to the occurrence of pregnant individuals’ death [16].
3.5 Postnatal development evaluation
Physical development evaluation consisted of a) body weight measurement on days 4, 10, 15, 20 and 25 after delivery, b) record the day of eye opening, ear unfolding, lanugo and fur appearance, eruption of the upper and lower incisors, vaginal opening and lowering of the testicles [16, 17]. Reflexological evaluation allows the identification of possible neurobehavioral alterations from the initiation of animal life. Reflexological development evaluation consisted of the following tests: palmar grip, postural response test, cliff avoidance, orientation test and negative geotaxis. Each test was performed for 15 seconds, once a day, until the pups showed the expected response [17].
3.6 Memory evaluation
In order to evaluate the memory the following tests were performed: inhibitory avoidance and object recognition. The choice of tests encompasses memory related processes (fear-related memory and declarative-like memory). Inhibitory avoidance test, hippocampal-dependent [18], was used to access fear-related memory. The object recognition test to evaluate the declarative-like memory. Perirhinal cortex is important for the discrimination of novel and familiar individual objects [19], differential that is discriminated by object recognition test. OLMα2 cells present in the CA1 region of the hippocampus influence the formation of new object representations in an object recognition test, as well as the encoding of aversive memories in a contextual fear memory test [20]. The same rats were submitted to the two tests, with interval of one week. First test to be performed was the object recognition, because it does not use any aversive stimulus. All tests were performed during the morning, in a room protected from noise and external light. It was done by a single trained observer and were not video recorded.
3.7 Inhibitory avoidance
Inhibitory avoidance test was performed in a step down passive avoidance box (model EP104R) from INSIGHT®. This test uses an aversive stimulus to evoke memory. It consists in a training session, in which the animal is placed on a platform and, when it places all four paws on the bars floor, a 0.5 mA shock is applied for 2s. 24 h after the training, a memory retention test session is realized, in which the latency time the animal takes to exhibit the same behavior is registered and used as a learning measure. A latency timeout of 180 s was set [21].
3.8 Object recognition
The object recognition test is based on the tendency of rodents to interact more with novel objects than familiar ones, which allows the study of these animals’ memory [21]. The test was performed in an arena of Medium Density Fiberboard, with a rectangular base of 60 × 40 cm and floor covered with anti-slip material, surrounded by 30 cm high walls. The objects used were four colored plastic cylinders, three white and one black with 3 cm in diameter and 5 cm tall. The first day, the animal was placed into the arena for 5 min so as to recognize the environment. Next day (second day) the training sessions started. The animal was placed into the arena in the presence of two white cylinders for 5 min, allowing it to explore the objects. After 24 h the animal was placed into the arena for 5 min in the presence of one white cylinder and a novel object, the black cylinder. In this phase, animal’s exploration of both cylinders was timed, and recognition was treated as the act of the animal spending more time exploring the new object, that is, the black cylinder. Recognition was considered so when the animal sniffed or touched the cylinder with its snout or paws. To analyze the results, we would use the recognition rate as it follows:
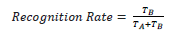
where TA is the time spent by the animal to explore the known object and TB is the time spent to explore the new object.
3.9 Statistical analysis
Data was analyzed using an ANOVA test for the parametric data, followed by the Dunnet test, or for the non-parametric data, were used for the Kruskall-Wallis test, followed by the Mann-Whitney or x2 test. It was assumed p <0.05 as the threshold level of acceptance in all statistical tests.
4. Results
4.1 Maternal evaluation
No clinical signs of maternal toxicity were observed, such as piloerection, alteration in ambulation, stereotypy, chromodacryorrhea and diarrhea in pregnant rats throughout the treatment period. There was also no body weight alteration during treatment.
4.2 Neonatal development
Hypericum perforatum doses used in this study did not result in any significant alterations on offspring physical and reflexological development signs (Tables 1 and 2) within the experimental groups.
|
Reflexologic tests |
Days of appearance of signals |
|||
|
Control |
T36mg/kg/dia Hp |
T72mg/kg/dia Hp |
T144mg/kg/dia Hp |
|
|
Grasping reflex |
2 ± 0 (10) |
2 ± 0 (10) |
2 ± 0 (10) |
2 ± 0 (10) |
|
Postural response |
2,2 ± 0,42 (10) |
2,5 ± 0,70 (10) |
2,1 ± 0,31(10) |
2,1 ± 0,31 (10) |
|
Cliff avoidance |
7,7 ± 1,70 (10) |
7,4 ± 1,07 (10) |
5,9 ± 1,19(10) |
6,1 ± 0,87 (10) |
|
Orientation test |
14 ± 1,15 (10) |
13,3 ± 1,63 (10) |
13,8 ± 0,42(10) |
13,2 ± 0,78(10) |
|
Negative geotaxis |
7,6 ± 1,07 (10) |
6,9 ± 0,73 (10) |
5,8 ± 0,63(10) |
5,9 ± 1,44 (10) |
Presented data: mean ± standard deviation (number of animals).
Table 1: Reflexologic development indicators of Wistar rat male offspring of control group (distilled water) and those treated with Hypericum perforatum (Hp) during pregnancy (p>0.05).
|
Reflexologic tests |
Days of appearance of signals |
|||
|
Control |
T36mg/kg/dia Hp |
T72 mg/kg/dia Hp |
T144mg/kg/dia Hp |
|
|
Grasping reflex |
2 ± 0 (10) |
2 ± 0 (10) |
2 ± 0 (10) |
2 ± 0 (10) |
|
Postural response |
2,4 ± 0,69 (10) |
2,5 ± 0,84 (10) |
2,7 ± 1,05 (10) |
2,6 ± 0,96 (10) |
|
Cliff avoidance |
7,4 ± 1,42 (10) |
6,8 ± 0,91 (10) |
6,5 ± 1,26 (10) |
6,2 ± 1,54 (10) |
|
Orientation test |
12,7 ± 2,11 (10) |
12,3 ± 1,63 (10) |
13,6 ± 0,96 (10) |
13,3 ± 0,82 (10) |
|
Negative geotaxis |
7,2 ± 0,78 (10) |
7,7 ± 1,49 (10) |
6,3 ± 1,56 (10) |
5,9 ± 1,44 (10) |
Presented data: mean ± standard deviation (number of animals).
Table 2: Reflexologic development indicators of Wistar rat female offspring of control group (distilled water) and those treated with Hypericum perforatum (Hp) during pregnancy (p>0.05).
However, there was a delay in the physical signal “fur appearance” in female pups in all treated groups when compared to the control group (Figure 1). However, this delay did not alter these animals’s normal development.
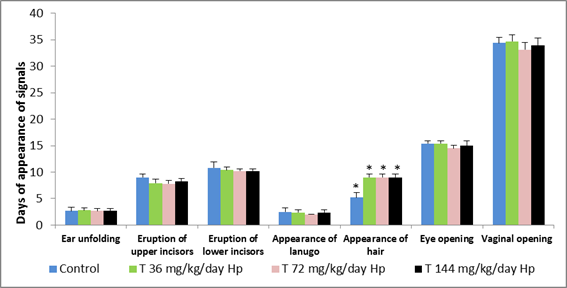
Figure 1: Physical development indicators of Wistar rat female offspring of control group (distilled water) and those treated with Hypericum perforatum during pregnancy (p>0.05). Hypericum perforatum (Hp).
4.3 Memory evaluation behavioral tests
4.3.1 Object recognition test
In general, rodents explored novel objects for a longer period of time when compared to familiar objects present in the environment. This behavior was evaluated in the object recognition test [21]. As shown in Figures 2 and 3 according to the recognition index, there was no significant difference among experimental groups with respect to the novel object exploration time.
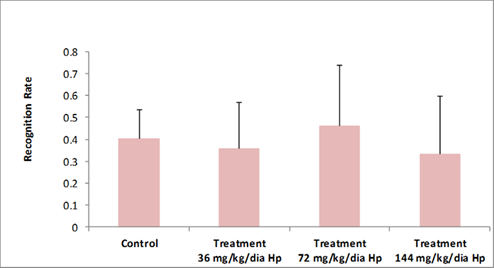
Figure 2: Total time of new object exploration, shown by the Recognition Rate, in the objects recognition test, by Wistar rat female offspring of control group (distilled water) and those treated with Hypericum perforatum. Presented data (mean ± standard deviation) (df=39, F=0.858, p= 0.472). Recognition Rate (RR), Hypericum perforatum (Hp).
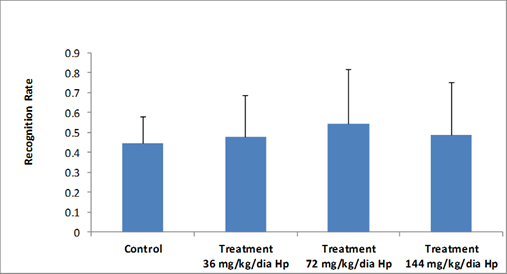
Figure 3: Total time of new object exploration, shown by the Recognition Rate, in the objects recognition test, by Wistar rat male offspring of control group (filtered water) and those treated with Hypericum perforatum. Presented data (mean ± standard deviation). (df=38, F=0.318, p= 0.812). Recognition Rate (RR), Hypericum perforatum (Hp).
4.3.2 Inhibitory avoidance test
There was no significant difference among experimental groups, as shown in Figure 4 indicating that all Hp doses had no significant effect neither on learning nor on memory.
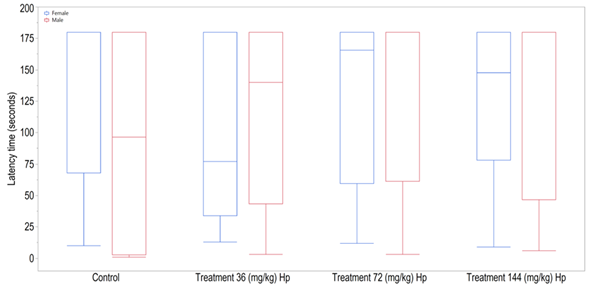
Figure 4: Latency time (seconds) observed in the inhibitory avoidance test of female and male offspring of the experimental groups (df= 79, F=0.3752, p= 0.7712).
5. Discussion
Hypericum perforatum usage on the treatment of light to moderate depression has been considered an advantageous alternative, as it causes fewer adverse effects when compared to the antidepressant drugs used in medical clinics [7]. However, there is a tendency to consider that herbal medicines are void of adverse effects, which is not true; indeed, its consumption during pregnancy and lactation period may entail some risks, with unknown consequences for the offspring.
Previous studies aiming to analyze Hp’s effects on pregnancy have showed an absence of fetal and maternal toxicity when ministered during implantation period (5 to 7 days after fecundation) [22] or organogenesis (9 to 15 days after fecundation) [23]. In the present study, the treatment underwent throughout the whole pregnancy period, with the objective of evaluating offspring postnatal development.
As observed on previous studies, mothers did not present clinical signs of toxicity, such as weight loss, alteration in ambulation, diarrhea, piloerection and death and thus, indicating absence of clinically demonstrable toxicity. Exposure to chemical agents during intrauterine development may lead to a variety of disorders that can be identified in the peri and postnatal period. Depending on the type of treatment animals undergo, somatic and neurobehavioral development may be accelerated or delayed. It is important to evaluate the physical and reflexological development signs, since they are related to behavioral teratology [19].
In this study, Hp usage during pregnancy did not alter offspring neonatal development of treated groups. The date of appearance of the physical development indicator happened between days 2 and 35 of postnatal life. Reflexological development ocurred between days 2 and 15 (Tables 1 and 2), not differing from the control group, except for those animals belonging to treated groups that suffered delay in its appearance with no consequences for the pups’ development. Study conducted aiming to establish the pattern of the physical and neuromotor development of Wistar rats (both males and females) from the breeding colony of the Reproductive Biology Center, observed that signs of physical development appeared between days 3 and 35 postnatal; and reflexological development was between days 2 and 10 [24], showcasing certain similarity to the results of the present study. However, it is well known that the signs of physical and reflexological development may vary according to the animal’s lineage and sex, in addition to the intrinsic variability of the colony and the criteria used by the researcher [22], Studies with mice that also evaluated prenatal exposure to Hp extract did not find changes in growth and physical maturation [15, 25], corroborating the data of the present study.
Hypericum perforatum acts by inhibiting neurotransmitter reuptake, such as dopamine, noradrenaline and serotonin, which play an important role in early development of the neural systems and even in the mature brain [26]. Both serotonin, noradrenaline and dopamine are important in the initial phase of long-term memory consolidation, and the release of noradrenaline into the hippocampus is essential for the formation of emotional memories [27]. Serotonin receptors are already present in the developing brain in rats, and it is known that early over-stimulation of such receptors during brain development may lead to lasting changes in brain chemistry resulting in abnormal behavior throughout life [6].
Although the Hp extract has active substances act by modulating neurotransmitter levels in the rodent brain [28], offspring exposed to Hp in this study did not present neither gain nor cognitive loss in the tests of inhibitory avoidance and object recognition. Studies evaluated descendants of mice that consumed Hp during pregnancy not find neither cognitive impact [29] nor behavioral tasks deficit [30]. It is known, however, that the effects of prenatal exposure to drugs on brain development are complex and modulated by time, dose and route of exposure to the drug. In the experimental model used in the present study, Hp extract did not interfere in postnatal development.
6. Conclusion
The experimental results did not indicate maternal toxic alterations on the physical, reflexological and cognitive development of the offspring exposed during intrauterine life.
Acknowlegments
This research was financed by the CBR– UFJF and by FAPEMIG, Rede Mineira de Farmacologia e Toxicologia e Rede Mineira de Bioterismo.
Authors’ Contributions
RCSS was the supervisor of this work. MOG guided in article writing and revision of the manuscript. LRS, LVC, VAV were responsible for the treatment of rats during all the experiment. LRS, JRP performed the behavioral test with the animals. VMP was responsible for the technical supervision of the experiment and for the animal breeding and well-being.
References
- Sherer ML, Posillico CK, Schwarz JM. The psychoneuroimmunology of pregnancy. Frontiers in Neuroendocrinology October 51 (2018): 25-35.
- Martínez-Paredes JF, Jácome-Pérez N. Review article. Depression in pregnancy. Revista Colombiana de Psiquiatría (English ed.) 48 (2019): 58-65.
- El Marroun H, White T, Verhulst FC, et al. Maternal use of antidepressant or anxiolytic medication during pregnancy and childhood neurodevelopmental outcomes: a systematic review. European Child and Adolescent Psychiatry 23 (2014): 973-992.
- Olivier JD, Vallès A, van Heesch F, et al. Fluoxetine administration to pregnant rats increases anxiety-related behavior in the offspring. Psychopharmacology 217 (2011): 419-432.
- Sprowles JLN, Hufgard JR, Gutierrez A, et al. Differential effects of perinatal exposure to antidepressants on learning and memory, acoustic startle, anxiety, and open-field activity in Sprague-Dawley rats. International Journal of Developmental Neuroscience 61 (2017): 92-111.
- Bonnin A, Peng W, Hewlett W, et al. Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience 141 (2006): 781-794.
- Chiovatto RD, Fukuda EY, Feder D, et al. Fluoxetina ou Hypericum perforatum no tratamento de pacientes portadores de transtorno depressivo maior leve a moderado? Uma revisão. Arquivos Brasileiros de Ciências da Saúde 36 (2011): 168-175.
- Russo E, Scicchitano F, Whalley BJ, et al. Hypericum perforatum: Pharmacokinetic, Mechanism of Action, Tolerability, and Clinical Drug–Drug Interactions. Phytotherapy Research, Review 28 (2014): 643-655.
- Brummelte S, Mc Glanaghy E, Bonnin A, et al. Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience 342 (2017): 212-231.
- Meneses A. 5-HT Pathways, Receptors and Transporter: Memory Functions and Dysfunctions. The Role of 5-HT Systems on Memory and Dysfunctional Memory. Emergent Targets for Memory Formation and Memory Alterations Chapter 3 (2014): 7-16.
- Oberlander TF. Fetal serotonin signaling: setting pathways for early childhood development and behavior. J Adolesc Health Review 51 (2012): S9-S16.
- Schulz HU, Schürer M, Bässler D, et al. Investigation of the Bioavailability of Hypericin, Pseudohypericin, Hyperforin and the Flavonoids Quercetin and Isorhamnetin Following Single and Multiple Oral Dosing of a Hypericum Extract Containing Tablet. Arzneimittelforschung Drug Research 55 (2005): 15-22.
- Campos LV, Vieira VA, Silva LR, et al. Rats treated with Hypericum perforatum during pregnancy generate offspring with behavioral changes in adulthood. Rev Bras Farmacognosia 27 (2017): 361-368.
- Kaufmann W. Developmental neurotoxicity. In: The laboratory rat. Krinke GJ (2000): 227-250.
- Gregoretti B, Stebel M, Candussio L, et al. Toxicity of Hypericum perforatum (St. John’s wort) administered during pregnancy and lactation in rats. Toxicol Appl Pharmacol 200 (2004): 201-205.
- Draft proposal for a new guideline 426. Guideline for testing of chemicals. Developmental neurotoxicity study Disponível em (1999).
- Bailey GP, Wise DL, Buschmann J, et al. Pre and postnatal developmental toxicity study design for pharmaceuticals..Birth Defects Res B Dev and Reprod Toxicol Review 86 (2009): 437-445.
- Zhang Y, Fukushima H, Kida S. Induction and requirement of gene expression in the anterior cingulate cortex and medial prefrontal cortex for the consolidation of inhibitory avoidance memory. Molecular Brain 4 (2011): 4.
- Balderas I, Rodriguez-Ortiz CJ, Bermudez-Rattoni F. Consolidation and reconsolidation of object recognition memory. Behavioral Brain Research 285 (2015): 213-222.
- França ASC. O papel de oscilações beta2 e de interneurônios OLMα2 da região CA1 do hipocampo de camundongos na memória de reconhecimento de objetos [tese]. Natal: Universidade Federal do Rio Grande do Norte (2016).
- Quillfeldt JA. Behavioral Methods to Study Learning and Memory in Rats (2006).
- Nepomuceno F, Las Casas L, Peters VM, et al. Desenvolvimento embrionário em ratas tratadas com Hypericum perforatum durante o período de implantação. Rev Bras Farmacognosia 15 (2005): 224-228.
- Borges LV, Carmo JC, Peters VM, et al. A toxicidade do hypericum perforatum administrado a ratas prenhes. Rev Assoc Med Bras 51 (2005): 206-208.
- Faria DE, Ribeiro LC, Reis JE, et al. Desenvolvimento físico, neuromotor e sensitivo de crias de ratas Wistar do Biotério do Centro de Biologia da Reprodução- Universidade Federal de Juiz de Fora. Bol Centro Biol Reprod 24 (2005): 55-63.
- Rayburn WF, Gonzalez CL, Christensen HD, et al. Effect of prenatally administered hypericum (St John’s wort) on growth and physical maturation of mouse offspring. Am J Obstet Gynecol 184 (2001): 191-195.
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods Experimental Neurology Review 190 (2004): 8-21.
- Izquierdo I. Memória. Porto Alegre: Artmed, 3ºEdição (2018).
- Serdarevic N, Eckert GP, Muller WE. The effects of extracts from St. John's Wort and Kava Kava on brain neurotransmitter levels in the mouse. Pharmacopsychiatry 34 (2001): 134-136.
- Rayburn WF, Gonzalez CL, Christensen HD, et al. Impact of hypericum (St.-John’s-wort) given prenatally on cognition of mice offspring. Neurotoxicol Teratol 23 (2001): 629-637.
- Rayburn WF, Christensen HD, Gonzalez CL. Effect of antenatal exposure to Saint John’s wort (Hypericum) on neurobehavior of developing mice. Am J Obstet Gynecol 183 (2000): 1225-1231.
