Myelofibrosis with Transient Pancytopenia and CD-68 Marker Positivity: A Case to Review
Article Information
Muhammad Sohaib Asghar*, Saad Aslam, Durre Naman, Maryam Zafar, Narmin Khan, Haris Alvi, Uzma Rasheed, Abubakar Tauseef
Resident Physician, Dow University Hospital, Karachi, Pakistan
*Corresponding author: Muhammad Sohaib Asghar, Resident Physician, Dow University Hospital, Karachi, Pakistan
Received: 25 September 2019; Accepted: 03 October 2019; Published: 26 October 2019
Citation:
Muhammad Sohaib Asghar, Saad Aslam, Durre Naman, Maryam Zafar, Narmin Khan, Haris Alvi, Uzma Rasheed, Abubakar Tauseef. Myelofibrosis with Transient Pancytopenia and CD-68 Marker Positivity: a Case to Review. Archives of Internal Medicine Research 2 (2019): 056-060.
Share at FacebookAbstract
Myelofibrosis is characterized by a group of clonal neoplastic proliferation under the influence of cytokines such as fibroblast growth factor (particularly by megakaryocytes) which leads to the replacement of normal hematopoietic bone marrow with connective tissue via collagen fibrosis. It most commonly affects patients in the fifth and sixth decade of their life. Constitutional symptoms such as fatigue along with massive splenomegaly, easy fatigability, and easy bruising are reported most commonly in such patients. Here we present a case of a 76-year-old male who was diagnosed with myelofibrosis having transient pancytopenia and CD-68 marker positive.
Keywords
Myelofibrosis, Splenomegaly, Bone marrow
Myelofibrosis articles Myelofibrosis Research articles Myelofibrosis review articles Myelofibrosis PubMed articles Myelofibrosis PubMed Central articles Myelofibrosis 2023 articles Myelofibrosis 2024 articles Myelofibrosis Scopus articles Myelofibrosis impact factor journals Myelofibrosis Scopus journals Myelofibrosis PubMed journals Myelofibrosis medical journals Myelofibrosis free journals Myelofibrosis best journals Myelofibrosis top journals Myelofibrosis free medical journals Myelofibrosis famous journals Myelofibrosis Google Scholar indexed journals Splenomegaly articles Splenomegaly Research articles Splenomegaly review articles Splenomegaly PubMed articles Splenomegaly PubMed Central articles Splenomegaly 2023 articles Splenomegaly 2024 articles Splenomegaly Scopus articles Splenomegaly impact factor journals Splenomegaly Scopus journals Splenomegaly PubMed journals Splenomegaly medical journals Splenomegaly free journals Splenomegaly best journals Splenomegaly top journals Splenomegaly free medical journals Splenomegaly famous journals Splenomegaly Google Scholar indexed journals Bone marrow articles Bone marrow Research articles Bone marrow review articles Bone marrow PubMed articles Bone marrow PubMed Central articles Bone marrow 2023 articles Bone marrow 2024 articles Bone marrow Scopus articles Bone marrow impact factor journals Bone marrow Scopus journals Bone marrow PubMed journals Bone marrow medical journals Bone marrow free journals Bone marrow best journals Bone marrow top journals Bone marrow free medical journals Bone marrow famous journals Bone marrow Google Scholar indexed journals myeloproliferative disorder articles myeloproliferative disorder Research articles myeloproliferative disorder review articles myeloproliferative disorder PubMed articles myeloproliferative disorder PubMed Central articles myeloproliferative disorder 2023 articles myeloproliferative disorder 2024 articles myeloproliferative disorder Scopus articles myeloproliferative disorder impact factor journals myeloproliferative disorder Scopus journals myeloproliferative disorder PubMed journals myeloproliferative disorder medical journals myeloproliferative disorder free journals myeloproliferative disorder best journals myeloproliferative disorder top journals myeloproliferative disorder free medical journals myeloproliferative disorder famous journals myeloproliferative disorder Google Scholar indexed journals leuko-erythroblastosis articles leuko-erythroblastosis Research articles leuko-erythroblastosis review articles leuko-erythroblastosis PubMed articles leuko-erythroblastosis PubMed Central articles leuko-erythroblastosis 2023 articles leuko-erythroblastosis 2024 articles leuko-erythroblastosis Scopus articles leuko-erythroblastosis impact factor journals leuko-erythroblastosis Scopus journals leuko-erythroblastosis PubMed journals leuko-erythroblastosis medical journals leuko-erythroblastosis free journals leuko-erythroblastosis best journals leuko-erythroblastosis top journals leuko-erythroblastosis free medical journals leuko-erythroblastosis famous journals leuko-erythroblastosis Google Scholar indexed journals splenomegaly articles splenomegaly Research articles splenomegaly review articles splenomegaly PubMed articles splenomegaly PubMed Central articles splenomegaly 2023 articles splenomegaly 2024 articles splenomegaly Scopus articles splenomegaly impact factor journals splenomegaly Scopus journals splenomegaly PubMed journals splenomegaly medical journals splenomegaly free journals splenomegaly best journals splenomegaly top journals splenomegaly free medical journals splenomegaly famous journals splenomegaly Google Scholar indexed journals appetite articles appetite Research articles appetite review articles appetite PubMed articles appetite PubMed Central articles appetite 2023 articles appetite 2024 articles appetite Scopus articles appetite impact factor journals appetite Scopus journals appetite PubMed journals appetite medical journals appetite free journals appetite best journals appetite top journals appetite free medical journals appetite famous journals appetite Google Scholar indexed journals jaundice articles jaundice Research articles jaundice review articles jaundice PubMed articles jaundice PubMed Central articles jaundice 2023 articles jaundice 2024 articles jaundice Scopus articles jaundice impact factor journals jaundice Scopus journals jaundice PubMed journals jaundice medical journals jaundice free journals jaundice best journals jaundice top journals jaundice free medical journals jaundice famous journals jaundice Google Scholar indexed journals nausea articles nausea Research articles nausea review articles nausea PubMed articles nausea PubMed Central articles nausea 2023 articles nausea 2024 articles nausea Scopus articles nausea impact factor journals nausea Scopus journals nausea PubMed journals nausea medical journals nausea free journals nausea best journals nausea top journals nausea free medical journals nausea famous journals nausea Google Scholar indexed journals vitamin B12 articles vitamin B12 Research articles vitamin B12 review articles vitamin B12 PubMed articles vitamin B12 PubMed Central articles vitamin B12 2023 articles vitamin B12 2024 articles vitamin B12 Scopus articles vitamin B12 impact factor journals vitamin B12 Scopus journals vitamin B12 PubMed journals vitamin B12 medical journals vitamin B12 free journals vitamin B12 best journals vitamin B12 top journals vitamin B12 free medical journals vitamin B12 famous journals vitamin B12 Google Scholar indexed journals
Article Details
1. Introduction
Myelofibrosis was first described by Gustav Heuck [1]. It was characterized as a myeloproliferative condition by William Dameshek [2]. Primary myelofibrosis (PMF) is a chronic progressive myeloproliferative disorder with a median survival of about 5.5 years (ranging from less than 1 year to greater than 30 years. The median age at which PMF gets diagnosed is 65 years. The hallmarks of PMF are leuko-erythroblastosis, splenomegaly and constitutional symptoms like a low-grade fever, weight loss, night sweats, fatigue, pruritis, and cachexia. Diagnostic investigations include typical complete blood picture, peripheral film, and bone marrow biopsy. Allogenic bone marrow transplantation is the definitive treatment of the patient with Myelofibrosis.
2. Case Presentation
A 75-year-old male of Asian descent with no significant past medical history presented to us with complaints of high-grade fever, generalized weakness, weight loss and decrease appetite. He is also complaining of left diffuse hypochondriac fullness, associated with easy fatigability. He denied jaundice, rigors, chills, racing of heart, chest pain, shortness of breath, nausea, vomiting, burning micturition, abdominal pain, darkening of the urine and diffuse bone pains. The patient was started on Intravenous Ceftriaxone, vitamin B12, folate, and iron supplements for his fever, generalized weakness, and easy fatigability. Physical examination was unremarkable except for mild hepatomegaly and massive splenomegaly. The laboratory findings were as follows:- Hemoglobin: 6.5 g/dl , white cell count: 1.5 microliter of blood, platelets: 50,000 microliter of blood, erythrocyte sedimentation rate (ESR) of 34 mm/hour, peripheral film showed anisocytosis, bizarre shaped cells, nucleated precursor red blood cells and classic tear-drop shaped cells (leukoerythroblastic reaction), serum total bilirubin of 0.98 umol/L with direct bilirubin of 0.33 umol/L, reticulocyte count of 0.2%, while rest of the labs were normal. Computed Tomography (C.T) scan was done which showed massive splenomegaly with splenic infarcts (Figure 1).
A trephine bone marrow biopsy was planned from posterior iliac crest which showed hypocellular imprint. Hematoxylin and Eosin staining revealed marked loss of architecture with histiocytosis forming multinucleated giant cells and epithelioid cells were observed with granuloma formation as well as erythroid and myeloid precursors. Also, grade 3 fibrosis was noted using a reticulin stain (Figure 2). The differential considerations included chronic malaria, tropical splenomegaly, Chronic myeloid leukemia, and myelofibrosis. Our history, clinical examination, investigations, and radiological imaging were suggestive of myelofibrosis presented with signs of transient Pancytopenia. Immunohistochemistry was performed which showed cells stained positive for CD-markers LCA, CD3 and C68 but TdT, CD20, C30, PAX5, EMA and cytokeratin all came out to be negative. Our patient was also CD-34 negative, the absence of CD-34 marker proved the reason behind a hypocellular imprint on bone marrow biopsy. JAK/STAT gene testing was sent which was positive. Based on WHO Guidelines, our patient had positive A1, A2 and four B criteria confirming the diagnosis of Myelofibrosis with transient Pancytopenia. The patient was treated initially with folic acid supplements, blood transfusions, dexamethasone followed by hydroxyurea, to which the patient didn’t respond so we scheduled the patient for allogeneic bone marrow transplant.
3. Discussion
Myelofibrosis was first described as a disease entity in 1879 by Gustav Heuck [1]. It was characterized as a myeloproliferative condition in 1951 by William Dameshek [2]. Primary myelofibrosis (PMF) is a chronic progressive myeloproliferative disorder with a median survival of about 5.5 years (ranging from less than 1 year to greater than 30 years), which is much shorter than that of other myeloproliferative disorders [3]. The median age at which PMF get diagnosed is 65 years. The hallmarks of PMF are leuko-erythroblastosis and splenomegaly, as were present in our patient. Risk factors predisposing the patient to the development of myelofibrosis include old age and exposure to radiation or industrial solvents [3]. The diagnosis of myelofibrosis is supported clinically by the presence of constitutional symptoms like a low-grade fever, weight loss, night sweats, fatigue, pruritis, and cachexia. All these features depict the hypercatabolic state [3]. Furthermore, on clinical examination moderate to the massively enlarged spleen was always noted in the patient giving the sensation of fullness and pain in the left upper quadrant, referring to the left shoulder [3]. The diagnosis of PMF, as defined by the World Health Organization [4], is based on a combination of clinical, morphological, cytogenetic and molecular features [5]. To diagnose myelofibrosis, the criteria should fulfill A1 + A2 and any two of the B criteria.
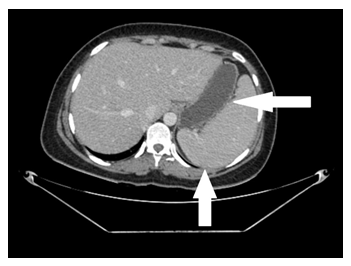
Figure 1: Computed Tomography of Abdomen showing Splenomegaly with infarcts.
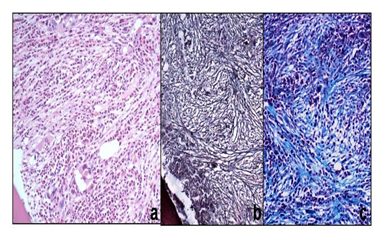
Figure 2: histology showed hypocellular imprint with grade 2 fibrosis using a reticulin stain.
|
A1 |
Bone marrow fibrosis ≥3 (on 0–4 scale) |
|
A2 |
Pathogenetic mutation (e.g. in JAK2 or MPL), or absence of both BCR?ABL1 and reactive causes of bone marrow fibrosis |
|
B1 |
Palpable splenomegaly |
|
B2 |
Unexplained anemia |
|
B3 |
Leuko?erythroblastosis |
|
B4 |
Tear?drop red cells |
|
B5 |
Constitutional symptoms |
|
B6 |
Histological evidence of extramedullary hematopoiesis |
Table 1: Criteria.
Laboratory investigations supporting the diagnosis of myelofibrosis include Complete blood count showing the presence of anemia and the peripheral film would show the classic tear-drop cells, bizarre RBCs, enlarged platelets and Leuko?erythroblastosis [4]. With the typical complete blood picture and peripheral film, bone marrow biopsy is usually planned which showed hypercellular marrow along with marrow fibrosis unless the patient also had an absence of CD 34 marker [6]. A bone marrow biopsy alone is not the confirmatory test for diagnosing PMF. so, immunohistochemical tests are usually planned to further evaluate the patient. It showed the positivity of CD-3, CD-68, and LCA markers but the disease is considered most severe if there is an absence of CD 34 marker [6]. Most patients with PMF carry a driver mutation in JAK/STAT, MPL or CALR genes [5, 8]. Our patient presented with fever, weight loss, easy fatigability and left hypochondriac fullness, showing massive splenomegaly on abdominal examination. His laboratory investigations showed signs of Pancytopenia and classic teardrop cells and Leuko?erythroblastosis. So, biopsy and immune-histochemistry were planned which showed grade 3 fibrosis and positive CD 3, CD 68, and LCA, with JAK/STAT gene positivity and absence of CD 34 markers. CD68 positivity in association with Primary myelofibrosis is sparsely reported [9, 10, 11]. The patient is initially managed with empiric or symptomatic treatment, which didn’t produce beneficial results [7]. Allogenic bone marrow transplantation is the definitive treatment in the patient with Myelofibrosis [8]. The current criteria of patient selection for the procedure include age ≥50 years with ≥ 2 adverse features including anemia (hemoglobin <10g/dL, cytogenic abnormalities, circulating blasts (>1%) or ≥ 2 gene mutations should be considered for non-ablative stem cell transplantation following diagnosis [8].
4. Conclusion
Keeping in mind the above diagnostic methods, management and treatment options that extensive research work has provided us the knowledge to better treat and manage Myelofibrosis. Although allogenic-Bone Marrow Transplantation remains the only curative option, it is accompanied by a higher number of morbidity and mortality as well.
References
- Heuck G. Zwei Falle von Leukamie mit eigenthumlichem Blut- resp. Knochenmarksbefund. [Two cases of leukemia with peculiar blood and bone marrow findings, respectively]. Arch Pathol Anat Physiol Virchows 78 (1879): 475-496.
- Dameshek W. Some speculations on the myeloproliferative syndromes. Blood 6 (1951): 372–375.
- Reilly JT1, McMullin MF, Beer PA, Butt N, Conneally E, Duncombe A, et al. British Committee for Standards in Haematology. Guideline for the diagnosis and management of myelofibrosis. Br J Haematol 158 (2012): 453-471.
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114 (2009): 937-951.
- Kreipe H, Büsche G, Bock O, Hussein K. Myelofibrosis: molecular and cell biological aspects. Fibrogenesis Tissue Repair 5 (2012): S21.
- Ni H, Barosi G, Rondelli D, Hoffman R. Studies of the site and Distribution of CD34+ cells in idiopathic Myelofibrosis. Am J Clin Pathol 123 (2005): 833-839.
- Katsuto Takenaka, Kazuya Shimoda, Koichi Akashi. Recent advances in the diagnosis and management of primary myelofibrosis. Korean J Intern Med 33 (2018): 679–690.
- Paula de Melo Campos; Primary Myelofibrosis: current therapeutic options. Rev Bras Hematol Hemoter 38 (2016): 257–263.
- Otawa M, Kawanishi Y, Ando K, Iwama H, Shohji N, Nishimaki J, et al. Rapidly progressive fibrosis and increased CD68-positive cells in the bone marrow at the terminal stage of adult T-cell leukemia accompanied by polycythemia vera. Rinsho Ketsueki 41 (2000): 1254-1259.
- Renee V Gardner, Paulina E Rojas. Myelofibrosis As Initial Presentation of Disseminated Langerhans Cell Histiocytosis. Pediatric Research 45 (1999): 146.
- Misung Kim, Jooryung Huh. Primary myelofibrosis and extramedullary blastic transformation with hemophagocytosis. Korean J Hematol 47 (2012): 244.
