Molecular Analysis of CYP2C19 Alleles and Clopidogrel Resistance Among Bangladeshi Patients with Acute Coronary Syndrome
Article Information
Mohsin Ahmed1*, Sayeedur Rahman Khan2, Aminur Razzaque3, Mohammad Arifur Rahman4, Shariful Islam5, Mainul Islam5, Kudrat-E-Khuda CM3, Monwarul Islam AKM1, Mohammad Ullah Firoze6, Md Matiur Rahman7, Tanveer Ahmad7, Shiblee SA8
1Associate Professor, Department of Cardiology, National Institute of Cardiovascular Diseases (NICVD), Dhaka, Bangladesh
2Medical Officer, Department of Cardiology, National Institute of Cardiovascular Diseases (NICVD), Dhaka, Bangladesh
3Assistant Professor, Department of Cardiology, National Institute of Cardiovascular Diseases (NICVD), Dhaka, Bangladesh
4Senior Consultant, Department of Cardiology, Sarkari Karmachari Hospital, Dhaka, Bangladesh
5Assistant Registrar, Department of Cardiology, National Institute of Cardiovascular Diseases (NICVD), Dhaka, Bangladesh
6Associate Professor, Department of Cardiology, Dhaka Medical College & Hospital, Dhaka, Bangladesh
7Junior Consultant, Department of Cardiology, United Hospital Limited, Dhaka, Bangladesh
8Senior Research Executive, IPDI Foundation, Dhaka, Bangladesh
*Corresponding author: Dr.Mohsin Ahmed, Associate Professor, Department of Cardiology, National Institute of Cardiovascular Diseases (NICVD), Dhaka, Bangladesh.
Received: 11 May 2023; Accepted: 29 May 2023; Published: 19 June 2023
Citation: Mohsin Ahmed, Sayeedur Rahman Khan, Aminur Razzaque, Mohammad Arifur Rahman, Shariful Islam, Mainul Islam, Monwarul Islam AKM, Mohammad Ullah Firoze, Md Matiur Rahman, Tanveer Ahmad, Kudrat-E-Khuda CM, Shiblee SA. Molecular Analysis of CYP2C19 Alleles and Clopidogrel Resistance Among Bangladeshi Patients with Acute Coronary Syndrome. Cardiology and Cardiovascular Medicine. 7 (2023): 151-157.
Share at FacebookAbstract
Background: Antiplatelet therapy is a cornerstone in the management of the Acute Coronary syndrome and the prevention of stent thrombosis after PCI. After aspirin clopidogrel is the widely used antiplatelet drug in our country. Many studies showed that clopidogrel resistance is the emerging problem for managing ACS patients and after coronary intervention. But exact data about clopidogrel resistance of Bangladeshi population is still unknown.
Objectives: The purpose of the present study was to determine the prevalence of clopidogrel resistance among the Bangladeshi patients with Acute Coronary Syndrome.
Methods: In this observational study a total 200 patients with Acute Coronary Syndrome were enrolled to see the prevalence of Clopidogrel resistance.
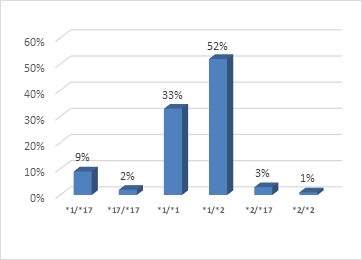
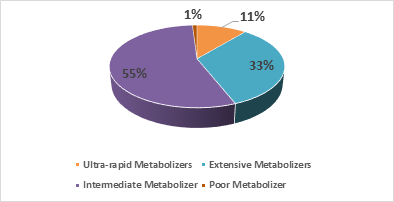
Results: The mean age of the study population was 52.32±9.86 years and the study showed male predominance (84 % was male and 16 % was female). Female patients and smoker patients had higher incidence of clopidogrel resistance. Dominant CYP2C19 genotype was CYP2C19 (*1/*2) which was 52%. 33% of study population had CYP2C19 (*1/*1) genotype, 9% had CYP2C19 (*1/*17) genotype, 3 % had CYP2C19 (*2/*17) genotype, 2% had CYP2C19 (*17/*17) genotype and only 1% had CYP2C19 (*2/*2) genotype. In this study we found 44 % study population was clopidogrel sensitive (Ultrarapid metabolizer: 11%, Extensive metabolizer: 33%). 56% study population had clopidogrel resistance (Intermediate metabolizer: 55%, Poor metabolizer: 1%).
Conclusion: 56% of our study population was clopidogrel resistance among them 55% was intermediate metabolizer and only 1% was poor metabolizer.
Keywords
Clopidogrel resistance; CYP2C19; Allele; Genotype
Clopidogrel resistance articles; CYP2C19 articles; Allele articles; Genotype articles
Article Details
1. Introduction
Platelet activation and aggregation play an important role in the pathogenesis of Acute Coronary Syndromes (ACS) and also in thrombotic complications during and after Percutaneous Coronary Interventions (PCI). Clopidogrel resistance is a widely used term that remains to be clearly defined. There is currently no clear and consensual definition of what clopidogrel resistance means. It has been used to reflect failure of clopidogrel to achieve its antiaggregatory effect [1]. Decreased response or resistance to antiplatelet drugs is associated with subsequent major adverse clinical events (MACE) [2].
The interpatient variability in clopidogrel response is multifactorial. It can be due to extrinsic or intrinsic mechanisms. A. Extrinsic mechanisms include: 1. Patient non-compliance, 2. Under-dosing or inappropriate dosing of clopidogrel, 3. Drug-drug interactions involving CYP3A4 and B. Intrinsic mechanisms include: 1. Genetic polymorphisms of the P2Y12 receptor and of the CYP3As, 2. Increase release of ADP, 3. Alternative pathways platelet activation [1].
There is growing interest in the use of laboratory tests to monitor patients treated with clopidogrel. There are a number of laboratory tests capable of detecting clopidogrel induced platelet inhibition. The currently available laboratory testing are: 1. Platelet function tests: a. Light transmission aggregometry (LTA), b. PFA-100, c. VerifyNow P2Y12, d. Plateletworks, e. VASP phosphorylation; 2. Genotyping for CYP2C19 variants [3].
Pharmacokinetic and antiplatelet tests of the active metabolite of clopidogrel show that the drug levels and antiplatelet effects differ depending on the genotype of the CYP2C19 enzyme. Mega et al showed that carriers of at least one CYP2C19 reduced function allele had a 32.4% reduction in circulating active clopidogrel metabolite compared with noncarriers [4].
Based on CYP2C19 genotype, individuals are categorized in different phenotypes:
- Ultra-rapid metabolizers (*1/*17 or *17/*17) : An individual carrying two increased activity alleles (*17) or one functional allele (*1) plus one increased-activity allele (*17)
- Extensive metabolizer (*1/*1) : An individual carrying two functional (*1) alleles
- Intermediate metabolizers (*1/*2, *1/*3, *2/*17 or *3/*17) : An individual carrying one functional allele (*1) plus one loss-of function allele (*2–*8) or one loss-of-function allele (*2–*8) plus one increased-activity allele (*17)
- Poor metabolizers (*2/*2, *2/*3 or *3/*3) : An individual carrying two loss-of-function alleles (*2–*8) [5]
There is a strong physiologic basis for the association between poor metabolizer CYP2C19 alleles and increased risk of cardiovascular events. Various studies showed that the existence of CYP2C19 polymorphisms may be responsible for increased cardiovascular events and all-cause mortality [6,7].
The concept of Clopidogrel resistance is an emerging clinical entity and increasingly evoked now a days, but the prevalence has not yet been clearly described in our country. Exact data on Bangladeshi population regarding the Clopidogrel resistance is not available. Few previous studies about Clopidogrel resistance in our country showed about fifty percent of Bangladeshi population are Clopidogrel resistant. A study by Santanu Guha et al, has shown about 19% patients were Clopidogrel resistant in India [8]. According to Haq et al. [9] about 46.7% and Munwar et al. [10] about 56.6% Bangladeshi population are Clopidogrel resistant, which is higher when compared with other studies. The aim of this study was to identify the prevalence of Clopidogrel resistance in Bangladeshi population by CYP2C19 assay.
So, this study was conducted to determine the prevalence of Clopidogrel resistance (by CYP2C19 assay) among the patients who got admitted with ACS. This information will help for the better management of CAD and PCI.
2. Materials and Methods
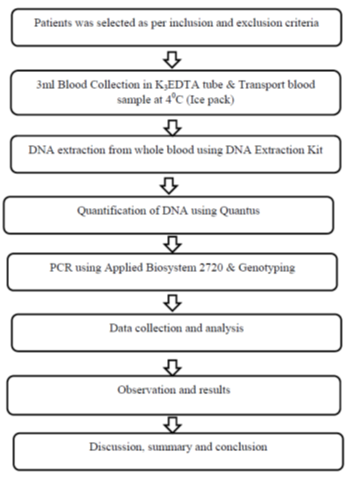
This observational study was carried out in the Department of Cardiology, National Institute of Cardiovascular Diseases from July, 2019 to June, 2020. This study included patients who got admitted with the clinical diagnosis of Acute Coronary Syndrome from July, 2019 to June, 2020. By non-randomized purposive sampling of all consecutive patients who got admitted with Acute Coronary Syndrome and considering the inclusion and exclusion criteria’s total 200 patients were included in this study. Aim of the present study was to determine the prevalence of Clopidogrel resistance (by CYP2C19 assay) among the Bangladeshi patients with Acute Coronary Syndrome.
2.1 Enrollment of subjects
2.1.1 Inclusion Criteria:
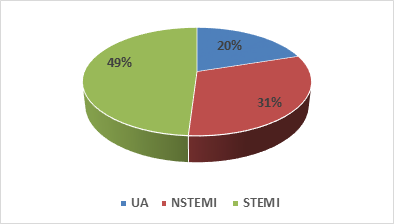
Both male and female patients who got admitted for ACS (UA, NSTEMI and STEMI).
2.1.2 Exclusion Criteria:
- Family or personal history of bleeding disorders.
- Patient who was getting anticoagulation therapy.
- Platelet count <100×109/L
- Concomitant co-morbid conditions (severe liver and kidney diseases)
- Patient who did not give consent.
2.1.3 Methodology
- All patients who got admitted in the Department of Cardiology, NICVD, Dhaka fulfilling the inclusion criteria was considered as a study population.
- Meticulous history was taken regarding symptoms and detailed clinical examination was performed in each patient.
- Patient’s baseline 12 lead ECG was performed and blood sample was taken for Troponin I.
- Patient was diagnosed as UA/NSTEMI/STEMI as per clinical presentation, ECG changes and cardiac marker.
- Demographic data such as age, sex, height, weight, BMI and risk factors of all patients were recorded.
- Blood sample was collected and sent for biochemical investigations and Clopidogrel pharmacogenetic (CYP2C19 assay).
- 3ml blood was collected in K3EDTA tube and blood sample was transported at 4°C (Ice pack).
- DNA extraction from whole blood was done using DNA Extraction Kit (Eppendrof Centrifuge 5415R).
- Quantification of DNA was done using Quantus Fluorometer.
- PCR (Polymerase Chain Reaction) was done using Applied Biosystem 2720.
- Final analysis (Genotyping).
2.1.4 Data collection
Data were collected in a predesigned data collection form.
2.1.5 Stastistical Analysis
- After processing all available data, statistical analysis of their significance were done.
- Obtained data were expressed in frequency, percentage, mean and standard deviation as applicable.
- Comparison between groups were done by Student’s T-test for continuous variables.
- Categorical data were analyzed by chi-square test.
- The whole analysis was done with the help of computer based SPSS (Statistical programme for social science) programme version 23.0.
- P-value of <0.05 was considered as significant.
2.1.6 Study flow chart (Figure 1)
3. Observation and Results
This observational study was conducted in the Department of Cardiology, National Institute of cardiovascular Diseases and Hospital, Dhaka, from July, 2019 to June, 2020. The purpose of the present study was to find out the prevalence of clopidogrel resistance in patients presenting with Acute Coronary Syndrome. Considering inclusion and exclusion criteria total number of 200 patients were included. Observations and results are presented in different Tables 1, 2 and Figure 2-5.
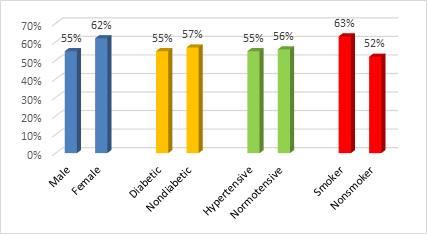
The baseline characteristics of the study population are presented in Table 1. Figure 2 and 3 demonstrating distribution of CYP2C19 genotype and phenotype variants among the ACS patients. Clopidogrel resistance in patients with different risk factors is shown in Figure 4.
The mean age was 52.32 ± 9.86 years. This study shows male predominance, of all patients 84 % (168) was male and 16 % (32) was female. Dyslipidemia was 43%, diabetes mellitus was 40%, smoking was 35%, hypertension was 30%, obesity was 12% and family history of CAD was 8%.
Table 1: Baseline characteristics of study population (n=200).
|
Patients characteristics |
n (%) |
|
Age (Years) |
52.32 ± 9.86 |
|
Sex: Male |
168 (84) |
|
Diabetes |
80 (40) |
|
Hypertension |
60 (30) |
|
Dyslipidemia |
86 (43) |
|
Smoking |
70 (35) |
|
Obesity |
24 (12) |
|
Family History |
16 (8) |
Table 2: Frequency and comparison of clopidogrel resistance (Phenotypes) in Unstable angina, NSTEMI and STEMI patients.
|
Diagnosis |
Ultra-rapid metabolizer |
Extensive metabolizer |
Intermediate metabolizer |
Poor metabolizer |
P value* |
|
UA |
2 |
10 |
28 |
0 |
.0315S |
|
NSTEMI |
12 |
16 |
34 |
0 |
|
|
STEMI |
8 |
40 |
48 |
2 |
|
|
Total |
22 |
66 |
110 |
2 |
|
|
NS= Not significant |
|||||
Total 110 patients were intermediate metabolizer. Among them 48 patients were diagnosed as STEMI, 34 were NSTEMI and 38 were Unstable angina (Table 3).
Table 3: Frequency and comparison of different CYP2C19 alleles in Unstable angina, NSTEMI and STEMI patients.
|
Diagnosis |
*1/*17 |
*17/*17 |
*1/*1 |
*1/*2 |
*2/*17 |
*2/*2 |
P value* |
|
UA |
2 |
0 |
10 |
24 |
4 |
0 |
.001S |
|
NSTEMI |
12 |
0 |
16 |
32 |
2 |
0 |
|
|
STEMI |
4 |
4 |
40 |
48 |
0 |
2 |
|
|
Total |
18 |
4 |
66 |
104 |
6 |
2 |
|
|
NS= Not significant |
|||||||
Dominant CYP2C19 allele was (*1/*2), 104 patients had this allele, followed by (*1/*1), (*1/*17), (*17/*17) & (*2/*2).
4. Discussion
Clopidogrel is a prodrug which selectively and irreversibly inhibits P2Y12 receptor of platelet. So clopidogrel needs hepatic biotransformation to form its active metabolites. Only 15% of clopidogrel is available for this biotransformation and other 85% prodrug is hydrolyzed. CYP2C19 hepatic enzyme is responsible for biotransformation of prodrug but CYP2C19 gene is highly polymorphic. The wild-type CYP2C19*1 allele is associated with functional CYP2C19-mediated metabolism [5]. CYP2C19*2 is the most common among the various reduced function genes studied and is the prime indicator of Clopidogrel low responsiveness [11]. The frequency of other CYP2C19 variant alleles (*3 - *8) with reduced or absent enzymatic activity typically below 1% [5]. Another allelic variant of CYP2C19 i.e. CYP2C19*17 which is associated with increased enzyme activity is shown to have higher platelet inhibition as compared to the wild type variant [12].
In this observational study we used Real Time PCR for CYP2C19 assy. The mean age of the study population was 52.32 ± 9.86 years. This study shows male predominance, of all patients 84% was male and 16% was female. Among the study population 55% male patients was clopidogrel resistance where as 62% female was clopidogel resistance. Sadath A. pareed et al, also found more clopidogrel resistance in female patients in their study [13].
In our study we found clopidogrel resistance is higher in smoker patients than nonsmoker (63% vs 52%) but not statistically significant. Santanu Guha et al, showed clopodogrel resistance is higher in both smoker and diabetic Indian population [8].
In this study we observed dominant CYP2C19 genotype was CYP2C19 (*1/*2) which is 52% of all study population. We also found 33% of study population had CYP2C19 (*1/*1) genotype, 9% had CYP2C19 (*1/*17) genotype, 3 % had CYP2C19 (*2/*17) genotype, 2% had CYP2C19 (*17/*17) genotype and only 1% had CYP2C19 (*2/*2) genotype. In another Bangladeshi study Munwar et al revealed that prevelance of CYP2C19 (*2/*2) genotype was 8.4% and CYP2C19 (*1/*2) genotype was 48.2% [10]. Sadath A. Pareed mentioned that about 30 to 55% persons harbor a loss of the function of the CYP2C19 allele mostly CYP2C19*2 [13]. CYP2C19 loss of function allele is very common in Indian population as compared to other worldwide population [14]. Hasan Khalaf et al, in a study on Arabic population found that the percentage of non-functioning allele *2 was about 33% [15]. In another Indian study Raju et al. [14] reported that CYP2C19*17 frequency was 10.2% in the western region population of India.
In this study we found 44 % study population was clopidogrel sensitive and 56% study population had clopidogrel resistance. Among the clopidogrel sensitive patient 11% was ultra-rapid metabolizer and 33% were extensive metabolizer. On the other hand 55% was intermediate metabolizer and only 1% was poor metabolizer in clopidogrel resistance patients. We also found 70% of UA patient, 54.8% of NSTEMI and 51% of STEMI patient had clopidogrel resistance.
A study by Santanu Guha et al. [8] has shown 26.4% patients were responders, 54.2% semi responders and about 19% patients were clopidogrel resistant in Indian population. In this study they used optical platelet aggregometry to diagnose the clopidogrel resistance rather than CYP2C19 assy. They also showed that after doubling the dose clopidogrel resistant patients showed adequate response but 4 out of 12 resistant patients showed inadequate response. May be these 4 patients who did not response to double dose of clopidogrel were genetically poor metabolizer like our study. Sadath A. Pareed et al [13] in a prospective study showed that prevalence of clopidogrel resistance was 32% in Indian population. They have used “VerifyNow P2Y12 assay” and only explained about clopidogrel sensitive and resistance group. In a Bangladeshi study Haq et al. [9] found about 46.7% patients were clopidogrel resistant and this resistance was defined as <20% inhibition of P2Y12 by VerifyNow measured 2-24 hours after 600 mg of clopidogrel loading dose. But there is wide variation of defining clopidogrel resistance in this method. Some investigator used <40% inhibition is cut off on the other hand some used <15% as a cut off value. Accordingly to Munwar et al. [10] about 56.6% Bangladeshi population are Clopidogrel resistant and they used CYP2C19 assay to determine the clopidogrel resistance. They revealed that among the resistant case, 8.4% patients are Homozygous Positive or poor metabolizer with probable genotype CYP2C19*2 (*2/*2) and 48.2% patients were Heterozygous positive or intermediate metabolizer with probable genotype CYP2C19 (*1/*2). The impact of different genotypes on Clopidogrel Active Metabolites (CAM) and P2Y12 Percent Inhibition (PRI) were also determined by Bin Sayeed et al, in another Bangladeshi study. That study also informed that CAM concentration as well as PRI by clopidogrel varied significantly based on genotypic variation of CYP2C19*2 and CYP2C19*17 individually [16].
Clopidogrel is widely prescribed drug in Bangladesh for the secondary prevention of Acute Coronary syndrome and prevention of stent thrombosis after PCI. The treatment expense of ACS and PCI is still too high for our people. For the developing country like us it is a social and financial burden. Using newer antiplatelet drugs add more expense to the treatment cost, resulting premature discontinuation of DAPT. Ultimately end up with more adverse cardiovascular events and stent thrombosis. Since we found that 55% of our population is intermediate metabolizer, so we can overcome this resistance by increasing the dose of the clopidogrel from 75mg to 150 mg [17]. But in case of poor metabolizer which is only 1% of our population, we have to choose other antiplatelet drugs to overcome this resistance.
5. Conclusion
From this study it may be concluded that 56% of our study population is clopidogrel resistance among them 55% is intermediate metabolizer and only 1% is poor metabolizer. Current guidelines do not advice routine platelet function testing to identify the clopidogrel resistance. But it is good to do a clopidogrel genotyping of a patient with ACS specially high risk patients and who will undergo PCI. We also suggest using double dose of clopidogrel when clopidogrel resistance is not possible to evaluate. Though we have alternative antiplatelet drugs like prasugrel and ticagrelor but increased bleeding risk, increased treatment expense as well as two times daily dosing of ticagrelor make these antiplatelet less convenient for many patients.
Limitations
- Single center study.
- Study population was small.
- Only genotyping was done but others platelet function tests were not used.
Recommendation
Further multi-center nationwide study with large sample size is recommended for strong evidences in favour of this study.
References
- Nguyen TA, Diodati JG, Pharand C. Resistance to clopidogrel: A review of the evidence, Journal of the American College of Cardiology 45 (2005): 1157-1164..
- Michelson AD, Frelinger AL, Furman MI. Resistance to antiplatelet drugs. European Heart Journal Supplements 8 (2006): G53-G58.
- Smock KJ, Saunders PJ, Rodgers GM, et al. Laboratory evaluation of clopidogrel responsiveness by platelet function and genetic methods. Am J Hematol 86 (2011): 1032-1034.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 360 (2009): 354-362.
- Scott SA, Sangkuhl K, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther 94 (2013): 317-323.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 373 (2009): 309-317.
- Sibbing D, Stegherr J, Latz W, et al. Cytochrome P450 2C19 loss of function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J 30 (2009): 916-922.
- Guha S, Sardar P, Guha P, et al. Dual antiplatelet drug resistance in patients with acute coronary syndrome. Indian Heart J 61 (2009): 68-73.
- Haq MM, Ahsan CH, Amin MN, et al. Comparison of P2Y12 receptor inhibition by clopidogrel and prasugrel in patients undergoing percutaneous coronary intervention. Bangladesh Med Res Counc Bull. 39 (2013): 139-145.
- Munwar S, Islam AW, Bhuiyan AH, et al. Clopidogrel Resistance in Patient Undergoing Percutaneous Coronary Intervention (PCI): A Single Center Experience in Apollo Hospitals Dhaka. J Am Coll Cardiol 73 (2019): S52-S53.
- Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the anti-platelet effect and clinical efficacy of Clopidogrel therapy. JAMA 302 (2009): 849-857.
- Price MJ, Tantry US, Gurbel PA. The influence of CYP2C19 polymorphisms on the pharmacokinetics, pharmacodynamics, and clinical effectiveness of P2Y12 inhibitors. Rev Cardiovasc Med 12 (2011): 1-12.
- Sadath AP, Vijayaraghavan G, Kartha CC, et al. Antiplatelet drug resistance in Indians. Ann Clin Cardiol 2 (2020): 36-41.
- Raju S. Genetic Aspect of Clopidogrel Resistance: An Indian Scenario. Indian J Cardio Biol Clin Sci 3 (2016): 107.
- Khalaf H, Al Meman AA, Rasool S. Impact of Cytochrome P450 2C19*2 and *3 on Clopidogrel Loading Dose in Saudi Patients with Acute Coronary Syndrome. Drug Metab Lett 10 (2016): 65-70.
- Bin Sayeed MS, Hasan Apu MN, Munir MT, et al. Prevalence of CYP2C19 alleles, pharmacokinetic and pharmacodynamic variation of clopidogrel and prasugrel in Bangladeshi population. Clin Exp Pharmacol Physiol 42 (2015): 451-457.
- Louca JI, Bassem GS, Habib W, et al. The effect of doubling the dose of clopidogrel on platelet aggregation in patients with clopidogrel resistance, The Egyptian Heart Journal 66 (2014): 259-262.