Microsatellite Instability with BRAF V600E Associated with Delayed Presentation but Poor Survival in Stage III Colorectal Cancer
Article Information
Sophiya Karki*, 1, Weijing Sun2, Rashna Madan3, Kamal Lamsal4, Sarah Schmitt3, Andrew Godwin3,5, Anup Kasi*, 2
1University of Kansas School of Medicine, University of Kansas Medical Center, Kansas City, KS, USA
2Division of Medical Oncology, University of Kansas University Cancer Center, Kansas City, KS, USA
3Department of Pathology Laboratory Medicine, University of Kansas Medical Center, Kansas City, KS, USA
4KU School of Business, University of Kansas, Kansas City, KS, USA
5Kansas Institute for Precision Medicine, University of Kansas Medical Center, Kansas City, KS, USA
*Corresponding author: Anup Kasi and Sophiya Karki. University of Kansas Medical Center Kansas City, KS, USA.
Received: 22 March 2023; Accepted: 31 March 2023; Published: 19 April 2023
Citation:
Sophiya Karki, Weijing Sun, Rashna Madan, Kamal Lamsal, Sarah Schmitt, Andrew Godwin, Anup Kasi. Microsatellite Instability with BRAF V600E Associated with Delayed Presentation but poor Survival in stage III Colorectal Cancer. Fortune Journal of Health Sciences. 6 (2023): 167-173.
Share at FacebookAbstract
Colorectal cancer (CRC) has tremendous molecular and genetic heterogeneity, making it a difficult cancer to treat. Two of the key prognostic indicators of CRC include microsatellite instability (MSI) and BRAF V600E mutation. Here, we performed a retrospective survival analysis on 145 stage II and III CRC patients treated at the University of Kansas Cancer Center between 2009 and 2020. Of the 145 patients, BRAF V600E was observed in 15% patients and MSI in 28% patients. Median survival was not reached for stage II. For stage III, patients with BRAF V600E showed poor overall survival, which worsened with concurrent presence of MSI [x2=6.4, p=0.01]. Eighty-five percent of this group was found to have right-sided CRC. For stage III, overall survival (OS) was 27 months, 37 months, 87 months and not reached for MSI-H/BRAF V600E, MSS/BRAF V600E, MSS/BRAF WT and MSI-H/BRAF WT, respectively. Although associated with poor prognosis, presence of MSI in BRAF V600E patients was associated with delayed disease presentation (mean age 77) compared to those with stable microsatellite (mean age 63) [p=0.01]. Although median survival between the groups could not be assessed for stage II due to very few deaths and/or inadequate length of study, comparison of survival trend suggests that BRAF V600E, rather than MSI, is what drives prognosis in stage II CRC. Our findings suggest that prognostic value of MSI is more relevant for stage III than stage II CRC. Patients with MSI-H and BRAF V600E have advantage of late presentation, although at the cost of poor overall prognosis.
Keywords
Colorectal Cancer, Microsatellite instability, BRAF mutation, Mismatch Repair
Colorectal Cancer articles, Microsatellite instability articles, BRAF mutation articles, Mismatch Repair articles
Article Details
1. Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-associated deaths in the United States [1], with 25 % of CRC already metastasized at diagnosis and the remaining 50% developing metastatic disease later on [2]. Thus, identification of molecular and genetic signatures that aid with early diagnosis, prognosis and therapy is essential. Two of the several molecular features associated with metastatic CRC (mCRC) include BRAF V600E mutation and microsatellite instability (MSI) that result from mutation or loss of mismatch-repair (MMR) genes including, MLH1, PMS2, MSH2 and MSH6 [3]. Around 65% of CRCs are sporadic, with less than 5% CRCs with a genetic component [4]. MSI instability falls under sporadic cases and constitute ~ 15-20% CRC, with ~20% seen in Stage II, 12% in stage I and ~4% of stage IV [5]. Loss of MMR genes increase with age, making MSI-H CRC more common in older patients [6]. Some of the distinctive features of this subset include proximal localization, immune-infiltration and poor differentiation [7]. MSI-H CRC has been shown to have relatively good prognosis when diagnosed at an early stage, although is associated with refractoriness to standard chemotherapy at later stages [8, 9]. More recently, immunotherapy has emerged as a potential treatment option for this subtype due to its immunogenicity. However, MSI often occurs with mutation in other cellular components, needing a more sophisticated strategy to target both the immune and the cellular aspect in conjunction.
One of the components of cellular machinery impacted in CRC include mutation in BRAF, which is a protein kinase involved in MAP Kinase signaling cascade that supports cell growth, proliferation and survival, which by itself is associated with poor prognosis when mutated [10]. V600E mutation renders BRAF constitutively active and constitutes 90% of all BRAF mutations in CRC [11]. Nearly 10% CRC have been reported to have BRAF mutation [12, 13]. Interestingly, 30-50% CRC patients with sporadic MSI-H also have BRAF mutation [14]. Although poor prognosis associated with co-occurrence of MSI and BRAF mutation has been well documented for metastatic CRC [14], the prognostic value of these mutations in combination in earlier (stage II/III) CRC is not well studied [2, 3].
2. Materials and Methods
2.1 Patient Cohort
We performed a retrospective study on 145 stage II/III CRC patients treated at the University of Kansas Cancer Center between September 2009 and July 2020 from the University of Kansas Health System’s Epic electronic medical records system. The patients were stratified based on their BRAF mutation status and MSI status (stable: MSS vs high instability: MSI-H). MSI-H/BRAF mutant (n=17), MSS/BRAF mutant (n=5), MSI-H/BRAF WT (n=24) and MSS/BRAF WT (n=99) were categorized based on PCR and/or IHC staining (Figure 1A, B). All patients had undergone resection followed by adjuvant chemotherapy that included combination of FOLFOX (Oxaliplatin /Fluorouracil /leucovorin). Pair-wise comparison of overall survival between MSI-H/BRAF mutant, MSS/BRAF mutant, MSI-H/BRAF WT and MSS/BRAF WT was then performed.
2.2 Molecular data
MSI and BRAF mutation status was determined by KU Cancer Center pathology laboratory on FFPE samples using either PCR or IHC methods, or extracted from external medical records.s.
2.3 Data analysis
The baseline characteristic and clinical outcomes were compared between subtypes. Kaplan-Meier survival analysis was done using R package with death as the “event” and time to follow-up as “censored”. Survival was measured in months as time reached from date of diagnosis to last follow up date or date of death. Log-rank test was used to compare trends between the curves.
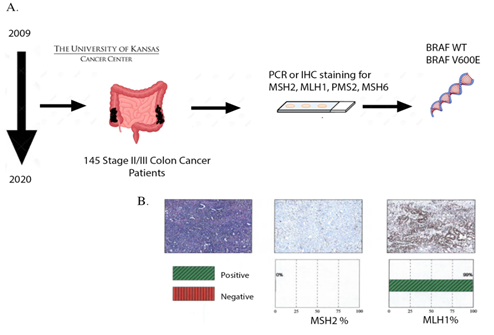
Figure 1: Schematic of the study. A, Stage II or III CRC patients stratified based on their MSI status and BRAF mutation status (MSI-H/BRAF mutant (n=17), MSS/BRAF mutant (n=5), MSI-H/BRAF WT (n=24) and MSS/BRAF WT (n=99) based on PCR and IHC staining. B, Representative figure showing MSI by loss of MSH2 by IHC.
3. Results
Table 1 shows baseline characteristics of patients in each group. At baseline, male and female patients were almost equally represented in our study. In ~73% patients, cancer was in colon, with the rest either in rectum or unknown. Of the total, 12% had MSI-H/BRAF V600E mutation, 16.6% had MSI-H/BRAF WT and only 3.5% had MSS/BRAF V600E. ~68% were mutation-naïve with MSS and BRAF WT. Of total, 67% were Stage III CRC. White patients represented majority of patient (82%) compared to Black (6%), Hispanic (6%) and Others (6%) (Figure 2A). In our study, ~85 % of patients with MSI-H/BRAF V600E had right-sided tumor (Figure 2B).
3.1 At baseline BRAF V600E, presence of MSI does not affect survival in Stage III CRC
Survival curve of stage III patients with BRAF V600E was not significantly different in presence or absence of MSI (Figure 3A) with median survival of 27 months for MSI-BRAF V600E and 37 months for MSS/BRAF V600E. However, there was difference in mean age at diagnosis between the two groups. Patients with MSI-BRAF V600E presented late at mean age of 77, whereas those without MSI presented at mean age of 63 (Figure 4A).
3.2 At baseline MSI-H, presence of BRAF V600E worsens survival in Stage III CRC
Survival curve of stage III patients with MSI-H was significantly different in presence of BRAF V600E compared to BRAF WT (Figure 3D) as shown by Wilcoxon test with median survival of 27 months for MSI-H BRAF V600E and median survival not reached for MSI-H BRAF WT. Interestingly, MSI alone without BRAF mutation tended to present earlier (mean age 61) compared to MSI with BRAF V600E (mean age 77) (Figure 4A).
3.3 Combined presence of MSI-H and BRAF V600E has worse survival compared to mutation naïve MSS/BRAF WT patients
Survival curve of stage III patients with MSI-H and BRAF V600E was significantly different in presence of MSI than absence (Figure 3D) as shown by both Log-rank and Wilcoxon test with median survival of 27 months for MSI-H BRAF V600E and median survival of 87 months for MSS/BRAF WT. Unlike patients with MSI-H BRAF V600E who presented late, patients without either of these mutations presented at varying ages (Figure 4A).
3.4 Prognostic value of MSI and BRAF V600E unclear at Stage II CRC
Survival analysis between the groups in Stage II CRC was limited due to fewer events early in the disease. However, survival trends showed that BRAF V600E mutation and not MSI, was associated with worsening survival (Figure 4C-D). Furthermore, patients diagnosed with early-stage II disease had no correlation with age at diagnosis (Figure 4B).
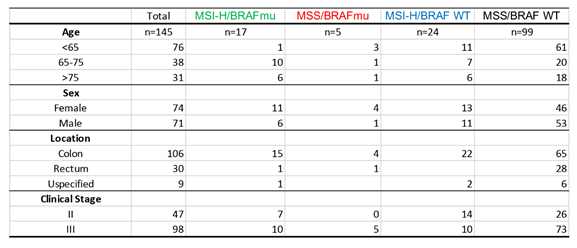
Table 1: Baseline characteristics of included participants according to MSI/ BRAF status
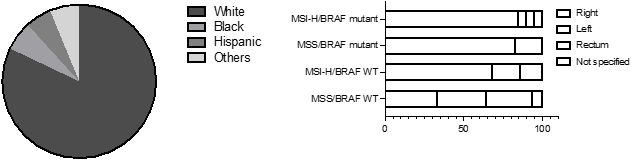
Figure 2: A, Distribution of patients by ethnicity. B, left vs right sided localization of CRC: Left, splenic flexure, descending column, sigmoid colon. Right, cecum, ascending column, hepatic flexure.
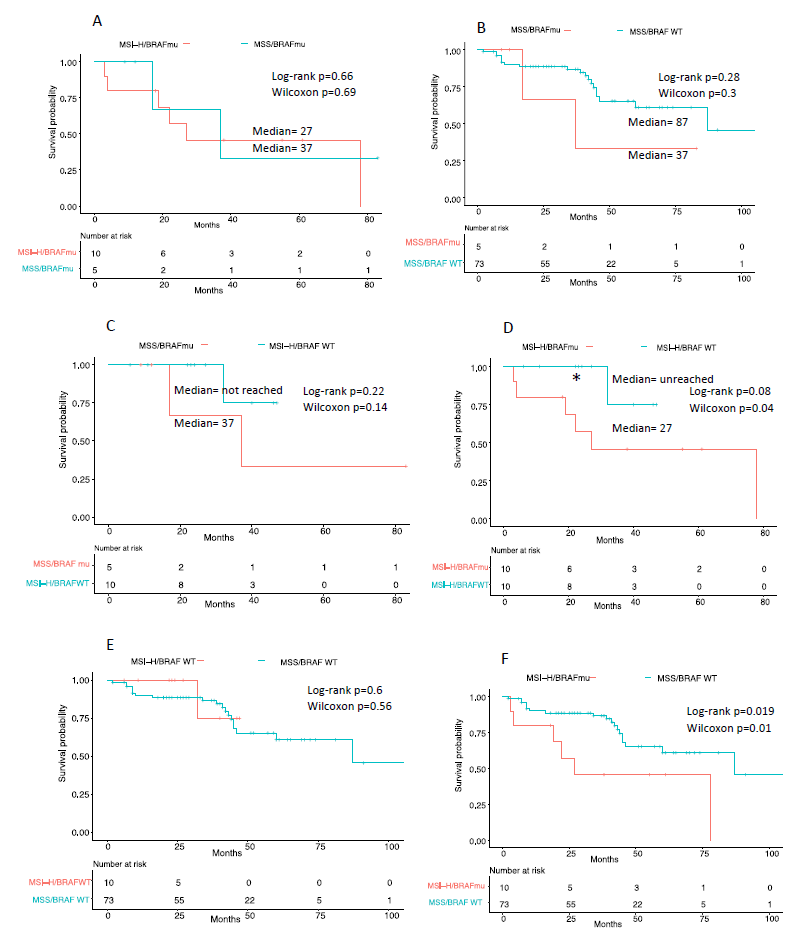
Figure 3: Kaplan-Meier survival curve comparison between groups of Stage III CRC. A, Comparison between MSI-H/BRAF V600E and MSS/BRAF V600E; B, MSS/BRAF V600E and MSS/BRAF WT; C, MSS/BRAF V600E and MSI-H/BRAF WT; D, MSI-H/BRAF V600E and MSI-H/BRAF WT; E, MSI-H/BRAF WT and MSS/BRAF WT; F, MSI-H/BRAF V600E and MSS/BRAF WT. Time (in months) indicate time from date of diagnosis to last follow up date or date of death. Log-rank test and Wilcoxon test with significance at *=p<0.05, **p<=0.01).
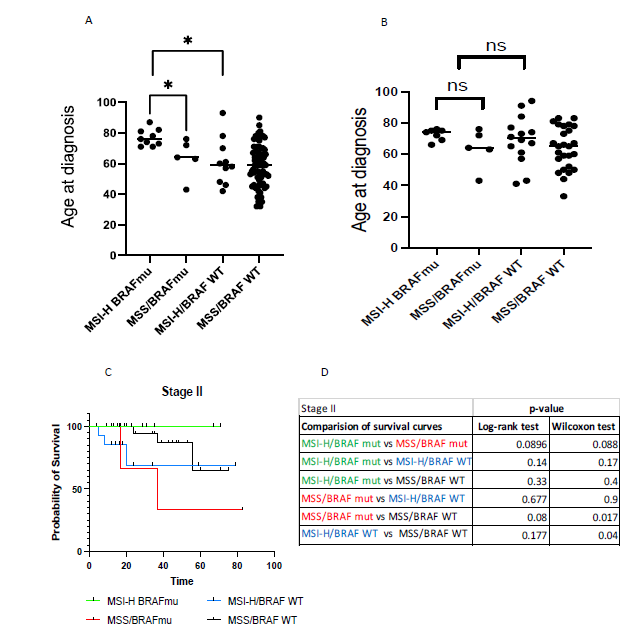
Figure 4: A, Age at diagnosis for each group of Stage III. B, Age at diagnosis for each group of Stage II. Unpaired student t-test comparing mean age at diagnosis. Significance at *=p<0.05, **p<=0.01. C, Kaplan-Meier survival curve comparison between groups of Stage II CRC. Time (in months) indicate time from date of diagnosis to last follow up date or date of death. D, Log-rank test and Wilcoxon test with significance at *=p<0.05, **p<=0.01). Each group is color-coded as shown.
4. Discussion
Our study was able to validate some important prior findings such as poor survival associated with BRAF V600E alone. There have been contradictory findings in the literature regarding left vs right-sided CRC prognosis [15, 16]. Our findings showed poor survival associated with right-sided CRC. The percentage of patients represented in our study for each group is comparable to what has been noted in the literature with majority being MSS/BRAF WT (68%) and most minority being MSS/BRAF V600E group (3.5%). Most of MSI is sporadic and tends to increase in frequency with age. We saw similar outcome in our study, although the findings were more significant when MSI presented with BRAF V600E. Males and females were equally represented in our study, which points to CRC being the third most diagnosed cancer in both men and women.
There was one distinct discrepancy between our study and the published literature. There is notably more representation of white patients in our study, although the incidence of CRC has been noted to be disproportionately higher in Blacks compared to white (by ~20%) [17]. This points to likely disparities between the two ethnicities in terms of access to care, cancer screening and other socio-economic factors. We provide stage-specific prognostic value of MSI and BRAF V600E, with proposition that BRAF V600E drives prognosis in early-stage CRC with MSI adding unfavorably to the prognosis in stage III and beyond. Our study suggests that the impact of MSI is favorable in stage II but unfavorable in stage III onwards, especially in conjunction with BRAF V600E mutation. Our study also highlights that MSI in stage II disease does not affect survival drastically. On the other hand, BRAF mutation negatively impacts survival even at earlier stages of the disease, highlighting the need for BRAF-targeted therapy for CRC at any stage. There are some discrepancies in sample size in our study. Since 40-60% of CRC with MSI also have BRAF mutation, MSS/BRAF group had comparatively smaller sample size compared to other groups. A larger study would likely mitigate this shortcoming. There are limited number of studies looking at stage-specific outcomes of MSI and BRAF. Therefore, our study provides a basis for further investigation into the stage-specific outcomes of MSI and BRAF mutations in the future.
5. Conclusion
Our findings show that stage III patients with MSI-H and BRAFV600E have the advantage of relatively late onset disease (median age 71-77 yrs) compared to MSS-stable CRC (56-63), however, at a cost of poor prognosis. In stage III patients with BRAF V600E at baseline, presence of MSI does not affect their survival, however, those with MSI at baseline, presence of BRAF V600E worsens survival. Overall, based on our study MSI-H/BRAF V600E patients have the worst outcome overall. Median survival is 27 months for MSI-H/BRAF V600E compared to 37 months for MSS/BRAF V600E and 87 months for MSS/BRAF WT. Unlike stage III patients, prognosis with MSI and BRAF mutation based on survival analysis was not feasible for stage II patients due to few events throughout the length of the study. Survival trends point to dominating effect of BRAF V600E vs MSI in Stage II disease. Interestingly, unlike with stage III, patients diagnosed with stage II disease show no association with age at diagnosis.
Acknowledgement
I would like to acknowledge Dr. Kasi for supervising me throughout the process and assisting me with manuscript edits. A.K.G. is the Chancellors Distinguished Chair in Biomedical Sciences Endowed Professor
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- American Cancer Society. Cancer Statistics Center.
- Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: Metastases to a single organ. World journal of gastroenterology 21 (2015): 11767-11776.
- Karki S, Umar S, Kasi A. Treating Colorectal Cancer with Immunotherapy: Implications for Single versus Combination Therapy. Curr Colorectal Cancer Rep 16 (2020): 107-117.
- Burt R. Inheritance of Colorectal Cancer. Drug Discov Today Dis Mech 4 (2007): 293-300.
- Koopman M, Kortman GAM, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. British journal of cancer 100 (2009): 266-273.
- Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 138 (2010): 2073-2087.e3.
- Pages F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391 (2018): 2128-2139.
- Jo W-S, Carethers JM. Chemotherapeutic implications in microsatellite unstable colorectal cancer. Cancer biomarkers: section A of Disease markers 2 (2006): 51-60.
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 23 (2005): 609-18.
- Safaee Ardekani G, Jafarnejad SM, Tan L, et al. The prognostic value of BRAF mutation in colorectal cancer and melanoma: a systematic review and meta-analysis 7 (2012): e47054-e47054.
- Sanz-Garcia E, Argiles G, Elez E, et al. BRAF mutant colorectal cancer: prognosis, treatment, and new perspectives. Ann Oncol 28 (2017): 2648-2657.
- Molina-Cerrillo J, San Román M, Pozas J, et al. BRAF Mutated Colorectal Cancer: New Treatment Approaches. Cancers 12 (2020): 1571.
- De' Angelis GL, Bottarelli L, Azzoni C, et al. Microsatellite instability in colorectal cancer. Acta bio-medica: Atenei Parmensis 89 (2018): 97-101.
- Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 117 (2011): 4623-32.
- Mangone L, Pinto C, Mancuso P, et al. Colon cancer survival differs from right side to left side and lymph node harvest number matter. BMC Public Health 21 (2021): 906.
- Warschkow R, Sulz MC, Marti L, et al. better survival in right-sided versus left-sided stage I - III colon cancer patients. BMC Cancer 16 (2016): 554.
- Augustus GJ, Ellis NA. Colorectal Cancer Disparity in African Americans: Risk Factors and Carcinogenic Mechanisms. The American journal of pathology 188 (2018): 291-303.
