Long term complications and Adverse effects associated with O2Vent Optima Oral Appliance and ExVent accessory in Obstructive Sleep Apnea Patients
Article Information
Sat Sharma MD1,2*, Antonella Conflitti CCPA1,2, Hilary Reiter DDS1, Ivan Valcarenghi DDS3, Barry Weinstein DDS4, Shideh Pejman DDS5, Brian Smith DDS6, Adam Teo DDS7, Bob Gibbons DDS8
1Centre for Sleep and Chronobiology, Toronto, Canada
2Windsor Sleep Disorders Clinic, Windsor, Canada
3Radiante Dental - Elmhurst (IL), USA
4Polo Park Dental Centre - Winnipeg (MB), Canada
5The Nimble Smile - North York (ON), Canada
6Burnham Dental - Peterborough (ON), Canada
7QLD Dental Sleep Therapy - Brisbane (QLD), Australia
8Future Dental - Brisbane (QLD), Australia
*Corresponding Author: Sat Sharma, Medical and Research Director, Centre for Sleep and Chronobiology, 301 – 295 College Street, Toronto, ON, Canada, M5T 1S2
Received: 06 March 2024; Accepted: 12 March 2024; Published: 22 March 2024
Citation: Sat Sharma, Antonella Conflitti, Hilary Reiter, Ivan Valcarenghi, Barry Weinstein, Shideh Pejman, Brian Smith, Adam Teo, Bob Gibbons. Long term complications and Adverse effects associated with O2Vent Optima Oral Appliance and ExVent accessory in Obstructive Sleep Apnea Patients. Dental Research and Oral Health. 7 (2024): 50-57.
Share at FacebookAbstract
Introduction: The ExVent is an accessory to the O2Vent Optima mandibular advancement device (MAD) and provides oral Expiratory Positive Airway Pressure (EPAP). Oral EPAP with the ExVent is designed to augment the OSA therapy provided by the O2Vent Optima. Long term complications and adverse effects associated with this combination therapy are not known, but require further study.
Methods: A retrospective survey was conducted of all patients who received O2Vent Optima MAD and ExVent in Canada and Australia since 2018. Data collected following consent included: demographics, duration of use, frequency of use, complications and adverse events.
Results: Out of the 480 subjects, 168 (35%) could be contacted and agreed to participate. 31 (18%) had stopped using the appliance. Out of 137 (81%) subjects, 118 (86%) were still using the ExVent Accessory, 92% medium strength (Yellow). 56% used morning aligner and 74% performed regular jaw exercises. After 4 weeks of the device use, excessive salivation was (12%, p<0.05), tooth and jaw pain were reported occasionally (8%, p<0.05), tooth movement was none (0%, p<0.05). Gum or tongue bruises (0%, p<0.05). TMJ pain/stiffness (5%, p<0.05). Change in bite or occlusion leading to discontinuation (0%, p<0.05), temporary chewing difficulties (24%, p<0.05) and dry mouth (0%, p<0.05). Difficulty to insert or remove ExVent valve (0%, p<0.05), difficulty breathing (0%, p<0.05), Malfunctioning or dislodgement of ExVent valve (0%, p<0.05). Participants reportedly valued their device (94%) and benefited with ExVent (100%).
Conclusion: Majority of the patients prescribed O2Vent Optima and ExVent accessory were compliant, demonstrated no significant complications and only minor adverse effects with the combination therapy.
Keywords
Obstructive sleep apnea; Mandibular advancement device; MAD; ExVent; Oral expiratory positive airway pressure; Sleep quality; O2Vent Optima
Article Details
Introduction
Obstructive sleep apnea (OSA) is a complicated chronic condition, which has emerged as a very relevant public health issue because of its high prevalence and long-term effects such as cardiovascular, metabolic, cognitive, and cancer-related alterations [1-6]. Current first-line treatment for OSA is continuous positive airway pressure (CPAP), which is highly effective but not well tolerated. Greater than 50% of patients with OSA on CPAP therapy report the use of CPAP devices for less than half the night or not at all [7-10]. MAD therapy often yields significant reductions in OSA severity [11-13]; however, despite being better tolerated by patients with OSA, MAD therapy remains less than optimal for greater than 50% of patients (residual apnea–hypopnea index [AHI]>5) [14-16]. Combination therapy with novel MAD O2Vent Optima and ExVent, an optional accessory that can be inserted into the O2Vent Optima MAD to provide upper airway support via oral expiratory positive airway pressure (EPAP) is promising [17-20]. Previous studies have demonstrated that the addition of an oral EPAP accessory, the ExVent, to the O2Vent Optima MAD effectively reduced respiratory events during sleep in patients with mild to moderate OSA [21,22].
Mild and “transient” side effects are commonly reported in the initial period of MAD therapy and include tooth pain, temporomandibular joint pain, myofascial pain, dry mouth, excessive salivation, and gum irritation [23-28]. The MAD side effects may be assigned to 1 of 6 groups [34]: (1) TMJ-related side effects such as transient morning jaw pain, persistent temporomandibular joint pain, tenderness in muscles of mastication, joint sounds; (2) intraoral tissue-related side effects such as soft tissue and tongue irritation, gingival irritation, excessive salivation/drooling, dry mouth; (3) cephalometric changes; (4) occlusal changes such as altered occlusal contacts/bite changes, incisor changes, decreased overjet and overbite, alterations in position of mandibular canines and molars, interproximal gaps; (5) damage to teeth or restorations such as tooth mobility, tooth fractures or damage to dental restorations; and (6) appliance issues such as appliance breakage, allergies to appliance material, gagging and Anxiety. Dental side effects related to long-term use of an oral appliance have been studied and published in retrospective studies but comprised small sample sizes. Furthermore, all studies except for two [30,31] evaluated the effects of an oral appliance that was nonadjustable and fixed the mandible in a predefined position at 50-75% of the maximum mandibular protrusion [32]. Therefore, the newer technologies that utilize additional mechanism to open the airway than just the mandibular protrusion, are likely to result in less side effects and require further study.
Long term complications and adverse effects associated with the combination therapy with O2Vent Optima and oral Expiratory Positive Airway Pressure (EPAP) ExVent are not known, but require further study. The objectives of the present retrospective study are to evaluate the long term complications and adverse effects associated with this combination therapy. A real-life survey of subjective improvements in patients prescribed combination therapy with O2Vent Optima MAD and ExVent was conducted to evaluate demographics, duration of use, frequency of use, complications and adverse events.
Methods
Device overview
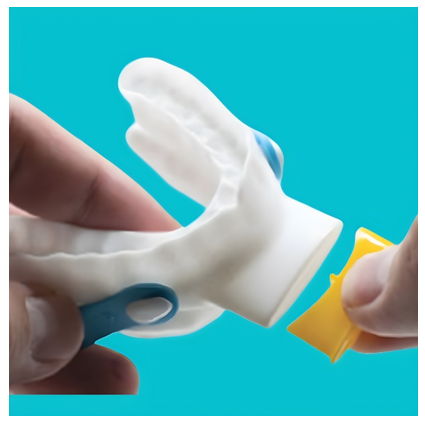
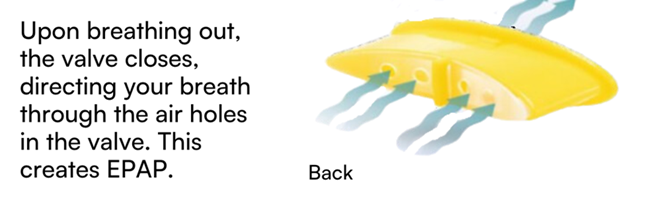
The ExVent is an oval-shaped, passive, flapper-type valve that can be inserted into the extended anterior airway inlet of the O2Vent Optima (Figure 1). When the patient is breathing through the airway, the valve fully opens during inspiration (Figure 2a) and closes upon expiration, with airflow directed through “holes” in the flapper valve, resulting in increased EPAP (Figure 2b). The ExVent is secured to the O2Vent Optima by a retention clip that allows for the easy removal of the ExVent accessory if desired. The ExVent is a single-patient, multiple-use device.
Study design
A retrospective survey was conducted of all patients who received O2Vent Optima MAD and ExVent in Canada and Australia since 2019. Data collected included: demographics, duration and frequency of use, daytime sleepiness, reported snoring, sleep satisfaction, morning and daytime functioning, daytime tiredness/fatigue and bed partner’s sleep interruption. The questionnaire consisted of questions that required binary responses and also questions with response on a 4-point Lickert’s scale. The manufacturer’s database of approximately 4,500 patients established in 2019, identified 480 patients who had previously agreed to participate in future surveys and had their contact information available. The study coordinator obtained a verbal consent to participate and the survey was conducted as Quality Improvement Project by the respective clinical facilities. The participants were previously diagnosed with mild to moderate OSA (defined as AHI 5-29) during a Level I polysomnographic (PSG) study and were prescribed and fitted with the novel Oral appliance O2Vent Optima and ExVent. The inclusion criteria included current use of O2Vent Optima therapy with or without the ExVent accessory. The subjects were excluded from participating in the study if they did not pursue oral appliance therapy, stopped therapy or had changed therapy to another device such as CPAP. The study participants were asked questions pertaining to their snoring, sleep quality, daytime functioning, overall satisfaction with therapy and their partner’s sleep quality. The study coordinator asked the participants to recall their sleep quality prior to initiating therapy with O2Vent Optima and ExVent, and their present status. The responses were then recorded for each item of the questionnaire, tabulated and analyzed.
Statistical analysis
All data are summarized descriptively. Categorical variables are summarized as frequency and percentage, and continuous variables are summarized as the number and mean, paired-sample t-tests were used to test for significant changes across time. Means and standard deviations were analyzed and were interpreted for the t-test analyses and significance was assumed at an alpha value of 0.05.
Results
O2Vent data repository from 2019 onwards was reviewed and individuals who were prescribed O2Vent Optima and ExVent and whose contact information was available were selected. Out of 480 evaluable, 168 (35%) responded and agreed to participate in the survey. 31 (18%) stopped using oral appliance and were not included in the survey. Out of 137 (81%) survey participants, 118 (86%) were still using ExVent Accessory, 92% medium strength (yellow color). Mean duration of O2Vent Optima and ExVent use was 2.7±0.9 years, the 91% participants used the device most nights and 86% used the device for >6 hours/night. 96% of the participants reported improvement in daytime sleepiness from moderate/severe to none/mild (p<0.05). (Table 1)
The subjects reported that 56% used morning aligner and 74% performed regular jaw exercises. After 4 weeks of the device use, excessive salivation was reported (12%, p<0.05), tooth and jaw pain were reported occasionally (8%, p<0.05), tooth movement was none (0%, p<0.05). Gum or tongue bruises were not reported (0%, p<0.05). TMJ pain/stiffness was reported (5%, p<0.05). Change in bite or occlusion leading to discontinuation was none (p<0.05), temporary chewing difficulties were seen in 24%, (p<0.05); however, did not lead to discontinuation of therapy. Dry mouth was not reported (0%, p<0.05). Difficulty to insert or remove ExVent valve (0%, p<0.05), difficulty breathing (0%, p<0.05), Malfunctioning or dislodgement of ExVent valve (0%, p<0.05). Participants reportedly valued their device (94%) and benefited with ExVent (100%). (Table 2)
|
Number of eligible patients to participate |
480 |
|
Numbers of patients could be contacted |
168 (35%) |
|
Patients who stopped using appliance |
31 (18%) |
|
Patients participated in the Survey |
137 (81%) |
|
Patients still using ExVent |
126 (92%) |
|
Duration of use (mean years) |
2.7±0.9 |
|
Mean use frequency (most nights) |
124 (91%) |
|
Average nightly use of device (>6 hrs.) |
117 (86%) |
|
ExVent accessory strength (Medium/yellow) |
126 (92%) |
|
Age (years) |
54.8±12.5 |
|
Sex (M/F) |
78/59 |
|
Previous CPAP use |
14 (10.2%) |
|
Baseline ESS |
12.6±2.1 |
|
Baseline AHI |
17.6±5.9 |
|
Baseline Severity of OSA |
|
|
Mild |
24% |
|
Moderate |
62% |
|
Severe |
14% |
|
Baseline Lowest SpO2 |
85±4.2% |
|
Mean Duration of O2Vent Optima Use (>6 hours/night) |
91±3.5% |
Table 1: Participant characteristics
|
Adverse Events |
On therapy with O2Vent Optima and ExVent |
|
Number of Responses out of a total of 137 |
|
|
I use a morning aligner after the OA use: |
|
|
None of the time |
26 |
|
Some of the time |
67 |
|
Most of the time |
44 |
|
I exercise my jaws and/or massage the jaw muscles: |
|
|
None of the time |
26 |
|
Some of the time |
79 |
|
Most of the time |
32 |
|
I developed excessive salivation 4 weeks after OAT use: |
|
|
None of the time |
121 |
|
Some of the time |
36 |
|
Most of the time |
0 |
|
I developed Tooth and or jaw pain 4 weeks after the OAT use: |
|
|
None of the time |
126 |
|
Some of the time |
11 |
|
Most of the time |
0 |
|
I noticed Tooth movement/s after the OAT use: |
|
|
No |
137 |
|
Yes |
0 |
|
I developed gum or tongue bruises after the OAT use: |
|
|
None of the time |
137 |
|
Some of the time |
0 |
|
Most of the time |
0 |
|
I developed TMJ pain or stiffness 4 weeks after the OAT use: |
|
|
None of the time |
130 |
|
Some of the time |
7 |
|
Most of the time |
0 |
|
I noticed change in my bite or occlusion after OA use: |
|
|
None of the time |
137 |
|
Some of the time |
0 |
|
Most of the time |
0 |
|
I noticed chewing difficulties after the OA use: |
|
|
None of the time |
134 |
|
Some of the time |
33 |
|
Most of the time |
0 |
|
I developed dry mouth after the OA use: |
|
|
None of the time |
137 |
|
Some of the time |
0 |
|
Most of the time |
0 |
|
I developed difficulty sleeping after the 4 weeks of OA use: |
|
|
None of the time |
137 |
|
Some of the time |
0 |
|
Most of the time |
0 |
|
I use ExVent accessory with OA: |
|
|
None of the time |
0 |
|
Some of the time |
10 |
|
Most of the time |
127 |
|
I found it difficult to insert or remove the ExVent valve: |
|
|
None of the time |
137 |
|
Some of the time |
0 |
|
Most of the time |
0 |
|
I use ExVent accessory and noticed difficulty breathing: |
|
|
None of the time |
137 |
|
Some of the time |
0 |
|
Most of the time |
0 |
|
The ExVent valve malfunctioned: |
|
|
None of the time |
137 |
|
Some of the time |
0 |
|
Most of the time |
0 |
|
I noticed accidental dislodgement of ExVent valve: |
|
|
None of the time |
137 |
|
Some of the time |
0 |
|
Most of the time |
0 |
|
I have benefited from the use of ExVent valve: |
|
|
Yes |
137 |
|
No |
0 |
Table 2: Sleep quality and daytime functioning with O2Vent Optima and ExVent therapy.
Discussion
Mandibular advancement devices (MAD), while effective in ameliorating the respiratory events of OSA, often cause alterations in occlusal (tooth) contacts and mandibular positioning as well as other side effects. The occlusal side effects from long-term therapy may result in poor patient compliance and patient drop-outs. The side effects resulting from MAD treatment are of short- and long-term nature. Short-term effects are generally mild, transient, and occur within 6 months of MAD application. Such effects, usually manageable by a sleep-trained dental professional, include excessive salivation, dry mouth, teeth discomfort, gums irritation, headaches, and temporomandibular joint and masticatory muscles discomfort [24-26]. The long-term side effects occurring beyond the 6 months after the initiation of treatment have a poor prognosis and are most often related to the occlusal changes [33,34].
Hence, knowledge of the possible side effects of these devices on occlusion is necessary for a successful treatment of OSA. Many studies have concluded that long-term therapy with mandibular advancements significantly decreased the overjet and overbite as compared to the baseline values [35]. Ringqvist et al. reported small and insignificant dental changes and no difference between any variables of the two controlled interventions studied [36]. Dental changes develop as a result of the MAD exerted forces on the upper and lower dental arches in order to maintain protrusion, and jaw resistant counter forces to persist in its initial position. A 21-year follow-up study on the monitoring of MAD side effects confirmed that there are significant and progressive dental changes with the prolonged use of MAD but skeletal or postural changes were insignificant [37]. A qualified MAD provider should be able to individually assess each patient, choose the best MAD and adjust it in order to evaluate and minimize its side effects.
The current literature, although rife with descriptions of the side effects, is lacking in the clarification of causative factors and methods to minimize these adverse effects. Available studies suggest that side effects may be related to the oral appliance design, materials, and extent of mandibular advancement. The long-term studies describe a progressive increase in occlusal side effects with ongoing use of OAT protrusion [32,33]. Our retrospective study showed that majority of the patients prescribed O2Vent Optima and ExVent accessory were compliant, demonstrated no significant complications and only minor adverse effects with the combination therapy. The TMJ pain and stiffness, and chewing difficulties persisted for more than 4 weeks; however, these did not lead to discontinuation of therapy. Specifically, the ExVent accessory was not associated with accidental dislodgement or breathing difficulties. Many of the MAD adverse events were associated with older bulky devices that preceded advances in the device design, use of novel medical grade biocompatible materials, smaller, lighter, durable and 3D printed devices designed from high resolution intraoral scans. The novel design and technology of O2Vent Optima incorporates an air channel that bypasses the soft palate thus obviating excessive nasal resistance common in OSA patients. Additionally, oral Expiratory Positive Airway Pressure (EPAP) provided by the ExVent prevents collapse of lateral pharyngeal walls and augments airway patency. Majority of patients in our study required no more than 50% mandibular protrusion for optimal therapy [21,22]. Therefore, it is plausible that less frequent side effects seen in our studywere secondary to the advanced device design and additional mechanisms (Air channel and ExVent) that enabled airway opening with less protrusion for optimal therapeutic effect.
The limitations of our study are that a retrospective design is prone to many biases and produces an inferior level of evidence compared to a prospective study. The inherent weaknesses of retrospective studies, such as participants may be recruited by convenience sampling thus not representative of all causing a selection bias, and also the recall bias apply to our study. Another drawback of our study is that patient drop-outs not captured in a retrospective design could be attributed to the discomfort and side effects caused by the appliance.
Conclusion
Mandibular advancement devices (MADs) are becoming increasingly recognized not only as an alternative to but also as an adjunct treatment modality for OSA. However, alterations in occlusion and other side effects with MAD therapy may result in poor patient compliance and discontinuation. Previous studies have demonstrated that combination therapy with novel MAD O2Vent Optima and ExVent, an optional accessory that can be inserted into the O2Vent Optima MAD effectively reduced respiratory events during sleep in patients with mild to moderate OSA. The present retrospective study demonstrated that majority of the patients treated with the combination therapy were compliant, demonstrated no significant complications and only minor adverse effects. Specifically, the ExVent accessory was not associated with accidental dislodgement or breathing difficulties. It is plausible that less frequent side effects seen in our study were secondary to the additional mechanisms built into the MAD that facilitated airway opening with less protrusion for desired therapeutic benefit.
Acknowledgments
None.
Funding details
Funding for this trial was provided by a research grant from the Centre for Sleep and Chronobiology, Toronto, Ontario, Canada
Declaration of interest
The authors report that they have no conflict of interest.
Authors contributions
S.S. conceptualised and prepared the original draft and wrote the manuscript; A.C., H.R., B.W., S.P., B.S., A.T. and B.G. performed data acquisition; S.S., A.C. and H.R. performed data analysis and interpretation; H.R. and I.V. revised the manuscript; S.S. acquired funding. All authors read and agreed to the final version of the manuscript.
Data availability
Data supporting the findings of this study are available upon request from the corresponding author [S.S.].
Ethics declarations
A verbal consent to participate in the survey was obtained and the study was conducted as Quality Improvement Project by the respective clinical facilities.
Consent to publish
The authors consent to the publication of this study, including their data.
References
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177 (2013): 1006-10014.
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328 (1993): 1230-1235.
- S Hla KM, Young T, Hagen EW, et al. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. Sleep 38 (2015): 677-684.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 182 (2010): 269-277.
- Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med 1 (2013): 329-338.
- Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 84 (2015): 1964-1971.
- Kushida CA, Morgenthaler TI, Littner MR, et al. American Academy of Sleep. Practice parameters for the treatment of snoring and Obstructive Sleep Apnea with oral appliances: an update for 2005. Sleep 29 (2006): 240-243.
- S Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 25 (2008): 173-178.
- Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 30 (2007): 711-719.
- Sawyer AM, Gooneratne NS, Marcus CL, et al. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev 15 (2011): 343-356.
- Kushida CA, Morgenthaler TI, Littner MR, et al. Practice Parameters for the Treatment of Snoring and Obstructive Sleep Apnea with Oral Appliances: An Update for 2005. Sleep 29 (2006): 240-243.
- Randerath WJ, Verbraecken J, Andreas S, et al. European Respiratory Society task force on non-CPAP therapies in sleep apnoea. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J 37 (2011): 1000-1028.
- Hoekema A, Stegenga B, De Bont LG. Efficacy and comorbidity of oral appliances in the treatment of obstructive sleep apnea-hypopnea: a systematic review. Crit Rev Oral Biol Med 15 (2004): 137-155.
- Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med 10 (2014): 215-227.
- Aarab G, Lobbezoo F, Hamburger HL, et al. Oral appliance therapy vs nasal continuous positive airway pressure in obstructive sleep apnea: a randomized, placebo-controlled trial. Respiration 81 (2011): 411-419.
- Blanco J, Zamarrón C, Abeleira PMT, et al. Prospective evaluation of an oral appliance in the treatment of obstructive sleep apnea syndrome. Sleep Breath 9 (2005): 20-25.
- Tong BK, Tran C, Ricciardiello A, et al. Efficacy of a novel oral appliance and the role of posture on nasal resistance in obstructive sleep apnea. J Clin Sleep Med 16 (2020): 483-492.
- Lavery D, Szollosi I, Czyniewski S, et al. Safety and efficacy of a novel oral appliance in the treatment of obstructive sleep apnea. J Dent Sleep Med 4 (2017): 57-63.
- Dutta R, Tong BK, Eckert DJ. Development of a physiological-based model that uses standard polysomnography and clinical data to predict oral appliance treatment outcomes in obstructive sleep apnea. J Clin Sleep Med 10 (2021): 69.
- Lai V, Tong B, Tran C, et al. Combination therapy with mandibular advancement and expiratory positive airway pressure valves reduces obstructive sleep apnea severity, Sleep 42 (2019): 119.
- Sharma S, Conflitti A, Reiter H, et al. Efficacy of the ExVent Accessory for the O2Vent Optima Oral Appliance in the Treatment of Obstructive Sleep Apnea. Adv Bioeng Biomed Sci Res 6 (2023): 131-137.
- Eckert DJ, White DP, Jordan AS, et al. Defining Phenotypic Causes of Obstructive Sleep Apnea. Identification of Novel Therapeutic Targets. American Journal of Respiratory and Critical Care Medicine 188 (2013): 996-1004.
- Ferguson KA, Ono T, Lowe AA, et al. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax 52 (1997): 362-368.
- Doff MH, Veldhuis SK, Hoekema A, et al. (2011) Long-term oral appliance therapy in obstruc- tive sleep apnea syndrome: a controlled study on temporomandibular side effects. Clin Oral Investig 12 (2011): 365.
- Lim J, Lasserson TJ, Fleetham J, et al. Oral appliances for obstructive sleep apnoea. Cochrane database of systematic reviews 4 (2004): 23.
- Tegelberg A, Wilhelmsson B, Walker-Engstrom ML, et al. Effects and adverse events of a dental appliance for treatment of obstructive sleep apnoea. Swed Dent J 23 (1999): 117-126.
- Mehta A, Qian J, Petocz P, et al. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med 163 (2001): 1457-1461.
- Sheats RD. Management of side effects of oral appliance therapy for sleep-disordered breathing: summary of American Academy of Dental Sleep Medicine recommendations. J Clin Sleep Med 16 (2020): 835.
- Walker-Engstrom ML, Ringqvist I, Vestling O, et al. A prospective randomized study comparing two different degrees of mandibular advancement with a dental appliance in treatment of severe obstructive sleep apnea. Sleep Breath 7 (2003): 119-130.
- Almeida FR, Lowe AA, Otsuka R, et al. Long-term sequellae of oral appliance therapy in obstruc- tive sleep apnea patients: part 2. Study-model analysis. Am J Orthod Dentofacial Orthop 129 (2006): 205-213.
- Ueda H, Almeida FR, Lowe AA, et al. Changes in occlusal contact area during oral appliance therapy assessed on study models. Angle Orthod 78 (2008): 866-872.
- Hamoda MM, Almeida FR, Pliska BT. Long-term side effects of sleep apnea treatment with oral appliances: nature, magnitude and predictors of long-term changes. Sleep Med 56 (2019): 184-191.
- Almeida FR, Lowe AA, Tsuiki S, et al. Long-term compliance and side effects of oral appliances used for the treatment of snoring and obstructive sleep apnea syndrome. J Clin Sleep Med 1 (2005): 143-152.
- Alessandri-Bonetti G, D’Anto V, Stipa C, et al. Dentoskeletal effects of oral appliance wear in obstructive sleep apnoea and snoring patients. Eur J Orthod 39 (2017): 482-488.
- Araie T, Okuno K, Minagi HO, et al. Dental and skeletal changes associated with long-term oral appliance use for obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev 41 (2018): 161-172.
- Ringqvist M, Walker-Engström ML, Tegelberg A, et al. Dental and skeletal changes after 4 years of obstructive sleep apnea treatment with a mandibular advancement device: a prospective, randomized study.
- Rose EC, Staats R, Virchow JC, et al. Occlusal and skeletal effects of an oral appliance in the treatment of obstructive sleep apnea. Chest 122 (2002): 871-877.