Local and Intravenous Anesthesia for Thoracic Endovascular Aortic Repair: 8 Years Experiences
Article Information
Masato Furui1, Shoji Sakaguchi2, Bunpachi Kakii1, Gaku Uchino1, Mai Asanuma1, Haruo Suzuki3, Takeshi Yoshida1
1Cardiovascular Surgery Department, Matsubara Tokushukai Hospital, 7-13-26 Amamihigashi, Matsubara-city Osaka, 580-0032, Japan
2Radiology Department, Matsubara Tokushukai Hospital, 7-13-26 Amamihigashi, Matsubara-city Osaka, 580-0032, Japan
3Cardiovascular Surgery Department, Kyoto Renaiss Hospital, 4-13, Suehiro-cho, Fukuchiyama, Kyoto, 620-0054, Japan
*Corresponding author: Masato Furui, Cardiovascular Surgery Department, Matsubara Tokushukai Hospital, 7-13-26 Amamihigashi, Matsubara-city Osaka, 580-0032, Japan.
Received: 14 December 2019; Accepted: 19 December 2019; Published: 02 January 2020
Share at FacebookAbstract
Background: General anesthesia is popular in the management of thoracic endovascular aortic repair (TEVAR). However, patients who undergo TEVAR are often elderly, with low activity or low respiratory function; therefore, less invasive anesthesia is considered. This study aimed to verify the safety and efficacy of local and intravenous anesthesia for TEVAR.
Methods and Results: From December 2010 to October 2017, 148 patients with an arch or descending aortic aneurysm underwent TEVAR in our hospital. Of these, 70 patients (38 males; mean age, 71±12 years) underwent TEVAR with local and intravenous anesthesia. We retrospectively examined the intraoperative difficulties due to patients’ movement, respiratory control, incidence of conversion to general anesthesia, and extension of procedure time by more than 2 minutes. Although 36 patients (51.4%) required additional sedation or analgesic administration due to patients’ movement or pain, the procedure time did not extend beyond 2 minutes for any patient. All procedures were completed without conversion to general anesthesia. A major complication, cerebral infarction, was noted in 1 patient (1.4%). For 5 patients (7.1%), the access route for TEVAR was narrow, and the iliac artery was dissected. This complication was easy to detect due to the patients’ perception of pain.
Conclusion: TEVAR with local and intravenous anesthesia was performed without difficulty and was advantageous due to the early detection of complications. This anesthetic management is less invasive and may be optimal for TEVAR in the elderly or other populations where full sedation is contraindicated.
Keywords
Masato Furui, Shoji Sakaguchi, Bunpachi Kakii, Gaku Uchino, Mai Asanuma, Haruo Suzuki, Takeshi Yoshida
Local and intravenous anesthesia articles, Thoracic endovascular aortic repair (TEVAR) articles, Aortic aneurysm articles
Article Details
1. Introduction
Thoracic endovascular aortic repair (TEVAR) is usually performed with general anesthesia because of the ease of patient management. However, risks of sore throat, hoarseness, or broken teeth are associated with intubation. Further, the induction of general anesthesia may pose additional risks for the elderly and for patients with severe cardiac or respiratory disease, such as chronic obstructive pulmonary disease [1]. For such patients, extubation can be difficult.
In contrast, there are several reports on the benefits and disadvantages of local and intravenous anesthesia in endovascular management [2-4]. It is simpler and easier to administer than general anesthesia and intubation is avoided. However, performing TEVAR can be more difficult under local and intravenous anesthesia due to patients’ movement or pain. We have performed TEVAR with local and intravenous anesthesia for eight years. Therefore, this study evaluated the perioperative experience with local and intravenous anesthesia in TEVAR as a less invasive management option.
2. Methods
2.1 Patients
From December 2010 to October 2017, 148 patients with an arch or descending aortic aneurysm underwent TEVAR in our hospital. With respect to anesthetic of choice, a total of 78 patients who needed debranching or who were unstable due to a ruptured aneurysm underwent TEVAR with general anesthesia. We retrospectively reviewed the remaining 70 patients (38 males; mean age, 71±12 years) who underwent TEVAR with local and intravenous anesthesia. This study was approved by the ethics committee of Matsubara Tokushukai Hospital, with approval number 180101. The requirement of individual informed consent was waived due to the retrospective nature of this study.
2.2 Procedures
Elective endovascular procedures were performed in a hybrid operating room under local and venous anesthesia (Figure 1). We first administered 0.03–0.06 mg/kg midazolam and then initiated administration of dexmedetomidine hydrochloride at a loading dose of 6 µg/kg/h for 10 minutes. Subsequently, we administered dexmedetomidine hydrochloride continuously at 0.2–0.7 µg/kg/h and used a 1% lidocaine injection at the incision line. Pentazocine hydrochloride or buprenorphine hydrochloride was administered, as needed, for pain. We aimed to achieve an anesthetic depth of Richmond agitation-sedation scale (RASS) score -1 or -2 for safety. Simple body movements were first managed with increased dose of dexmedetomidine. Midazolam (1 mg) was administered as necessary when the patient was unable to maintain the position.
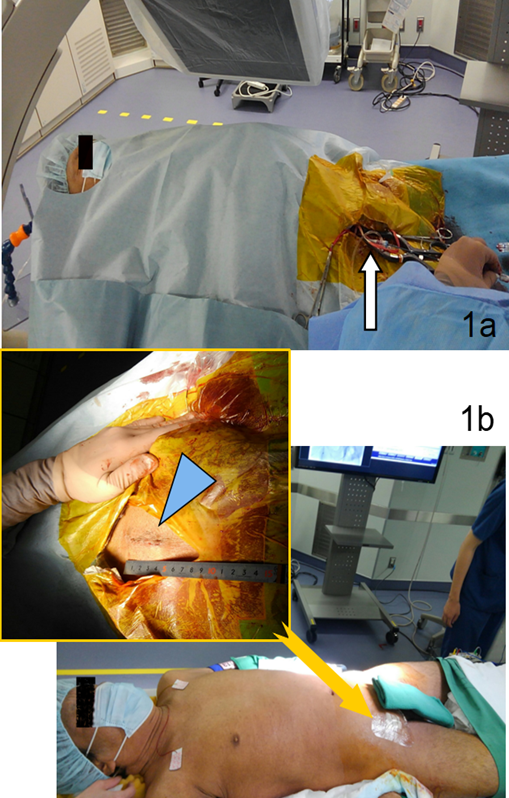
Figure 1: Operative images. (a) Intra-operative photograph of the patient under local and intravenous anesthesia in a hybrid operating room. The femoral artery was exposed using the cut-down technique (arrow). (b) Postoperative photograph showing the operative groin scar, which was less than 50 mm in length (arrowhead in enlarged view).
The access arteries for endograft delivery were exposed via a groin cut-down, and a guidewire was inserted through a dry sheath (Figure 1, Table 1). Additional wires (to determine the position) were inserted via a puncture on the other side of the groin.
|
Devices |
n (%), all = 70 |
|
Endograft typea |
|
|
Gore® TAG® |
48 (68.6%) |
|
Zenith TX2® |
14 (20.0%) |
|
Medtronic Valiant™ |
6 (8.6%) |
|
Relay® |
5 (7.1%) |
|
Othersb |
4 (5.7%) |
|
Sheath outer diameter |
|
|
18-Fr (6 mm) |
2 (2.9%) |
|
20-Fr (6.3 mm) |
23 (32.9%) |
|
22-Fr (7.3 mm) |
41 (58.6%) |
|
24-Fr (8 mm) |
4 (5.7%) |
Table 1: Endograft and sheath use
Fr; French
aMultiple types were used in 7 cases.
b2, Najuta, 1 homemade stent graft and 1 bare Powerlink® cuff stent were included.
The devices used include Gore® TAG® (Gore Medical, Flagstaff, AZ, USA), Zenith TX2® (Cook, Bloomington, IN USA), Medtronic Valiant™ (Medtronic, Santa Rosa, CA USA), Relay® (Bolton Medical, Sunrise, FL USA), and others. The delivery and deployment of the device was guided by angiographic landmarks and/or intravascular ultrasonography. The procedure was considered complete when a lack of major type I or III endoleaks was confirmed.
We examined the incidence of difficulty due to patients’ movement, blood pressure and respiratory control, conversion to general anesthesia, and procedure time extending beyond 2 minutes.
3. Results
Intra-operative events and postoperative complications are summarized in Table 2. Although 36 patients (51.4%) required additional sedation or analgesic administration due to patients’ movement or pain, the duration of interruption was within 2 minutes (64.7±25.0 seconds), accounting for 0.74–0.89% of the duration of the whole operative procedure. The patient experienced pain and moved at the time of cut-down, sheath insertion, or delivery of the device, but not at the time of deployment. All procedures were completed without conversion to general anesthesia. Though blood pressure was controlled without any issue, 2 patients (2.9%) required temporary biphasic positive airway pressure assistance for respiratory management.
|
Outcome |
n (%), all = 70 |
|
Access route injury |
5 (7.1%) |
|
Cerebral infarction |
1 (1.4%) |
|
Conversion to general anesthesia |
0 (0%) |
|
Difficult blood pressure management |
0 (0%) |
|
Difficult respiratory control |
2 (2.9%)a |
|
Pseudoaneurysm |
0 (0%) |
|
Seroma |
0 (0%) |
|
Time loss more than 2 minutes due to patients’ movements |
0 (0%) |
|
Time loss within 2 minutes due to patients’ movements |
36 (51.4%)b |
|
30-day mortality |
0 (0%) |
Table 2: Intra-operative events and postoperative complications
aManaged with biphasic positive airway pressure assistance; bManaged with additional sedation or analgesics
The major complication of cerebral infarction was observed in 1 patient (1.4%). For 5 patients (7.1%), the access route for TEVAR was narrow and the iliac artery was dissected. This access route injury was easily detected due to the patients’ perception of pain. The length of stay was 12±3 days (range, 7–19 days). The 30-day mortality was 0%. We confirmed the absence of pseudoaneurysms and seromas at the 1-, 3-, 6-, and 12-month follow-ups after the procedures.
4. Discussion
While there are positive reports on the use of local and intravenous anesthesia for endovascular aortic repair, general anesthesia is standard and more commonly used for TEVAR globally [3-9]. A few previous studies reported the advantages of local and intravenous anesthesia in TEVAR, such as shorter duration of hospital stay and reduced access-related complications [6, 8]. Although the duration of hospital stay was long because we checked the operative wound on the 7th postoperative day and performed routine enhanced computed tomography at 1 week postoperatively, our study could describe the actual medication process and the other advantages and problems associated with this procedure.
We have introduced less-invasive local and intravenous anesthesia from our earliest TEVAR procedures and have successfully accomplished TEVAR without conversion to general anesthesia as shown in Table 2. TEVAR could be performed without general anesthesia due to the administration of various drugs. Dexmedetomidine is a sedation drug with analgesic effects. It is very useful but is still inferior to general anesthesia with respect to the sedative and analgesic effects. Remifentanil and fentanyl are drugs used in anesthesia management; they must be prepared in advance and their administration must be strictly monitored in Japan. Therefore, drugs, such as midazolam, pentazocine hydrochloride, or buprenorphine hydrochloride which are easy to use were administered, as needed. Although 51.4% of all patients required these additional drugs, the time lost due to the interference from the patients only accounted for 0.74–0.89% of the total procedure time. Therefore, we think that this method is acceptable considering the time saved as intubation was avoided. Moreover, the patient experienced pain or moved only at the time of cut-down, sheath insertion, or delivery of the device; hence, device was deployed safely.
As a point of caution, dexmedetomidine may cause less respiratory depression, but bradycardia and hypotension can develop. In contrast, midazolam may cause more respiratory depression. In this study, 2 patients (2.9%) required non-invasive positive pressure ventilation (NPPV) because of shallow respiration due to over-sedation.
Access route injury on the incision for inserting devices was noted in 5 patients (7.1%). The patients complained of pain, which allowed rapid confirmation of the injury by angiography and subsequent quick repair by additional stenting. Although induced analgesia may be considered insufficient, this access route injury may not be detected until later if general anesthesia is used. Moreover, spinal cord ischemia can rarely occur after TEVAR. Fortunately we have never experienced this spinal cord ischemia in TEVAR under local and intravenous anesthesia, but the local and intravenous anesthesia also enables early detection of these complications and we can deal with them as soon as possible by methods previous study mentioned [10]. Therefore, this ease of detection is considered one of advantages of this type of management.
Some studies have reported that a puncture is more useful than cut-down artery access for local anesthesia [8, 9]. However, when the common femoral artery is narrow or has a thick sheath and a stent graft is used, the artery may be dissected, and the intima of the artery may sometimes form a flap under direct vision. Our TEVAR procedure applied a cut-down approach rather than percutaneous puncture because it allows the repair of these arterial injuries. Further, the cut-down technique has an advantage in hemostasis after removing the sheath because bleeding from the artery can be stopped directly.
There are rare reports of the development of pseudoaneurysms when using a cut-down technique [11, 12]. Mokaloski et al. reported a 6.4% incidence of the postoperative complication of pseudoaneurysm after TEVAR using their percutaneous fascial suture technique. In contrast, pseudoaneurysms or seromas were not detected within the follow-up period after TEVAR using our open access technique. Therefore, our TEVAR procedure involving local and intravenous anesthesia administration is safe and highly reproducible.
This study has some limitations. This was a retrospective study and included only a small number of patients. In addition, the follow-up period varied. Long-term follow-up data and more cases are required for future studies. This study focused on elective TEVAR, but we have also used local and intravenous anesthesia for emergency TEVAR. Data for emergency cases should also be assessed. Moreover, the complications associated with TEVAR under general anesthesia should also be compared with those of TEVAR under local and intravenous anesthesia in the future.
5. Conclusion
TEVAR under local and intravenous anesthesia is safely and easily performed. Since patients are only under light sedation, they can alert us to any pain or other concerns. This anesthetic management is less invasive and may be optimal for TEVAR in the elderly or other populations when full sedation is contraindicated.
Acknowledgements
We thank Mie Omae and Nozomi Ito (medical clerks in our hospital) for their assistance with data collection. We would like to thank Editage (www.editage.jp) for English language editing.
Conflict of Interest Statement
The authors declare no conflicts of interest.
References
- Jor O, Maca J, Kouta J, Gemrotova M, Vymazal T, Litschmannova M, et al. Hypotension after induction of general anesthesia: occurrence, risk factors, and therapy. A prospective multicenter observational study. J Anesth 32 (2018): 673–680.
- Eallard L, Djaiani G. Anaesthesia for vascular emergencies. Anaesthesia 68 (2013): 72–83.
- Henretta JP, Hodgson KJ, Mattos MA, Karch LA, Hurlbert SN, Sternbach Y, et al. Feasibility of endovascular repair of abdominal aortic aneurysm with local anesthesia with intravenous sedation. J Vasc Surg 29 (1999): 793–798.
- Van Orden K, Farber A, Schermerhorn ML, Goodney PP, Kalish JA, Jones DW, et al. Local anesthesia for percutaneous endovascular abdominal aortic aneurysm repair is associated with fewer pulmonary complications. J Vasc Surg 68 (2018): 1023–1029.
- Armstrong RA, Squire YG, Rogers CA, Hinchliffe RJ, Mouton R. Type of anesthesia for endovascular abdominal aortic aneurysm repair. J Cardiothorac Vasc Anesth 33 (2019): 462–471.
- Erkilic E, Kesimci E, Doger C, Gumus T, Yalcin A, Kanbak O. Anesthetic management and perioperative complications in endovascular interventions: the Turkish experience. Global J Anesthesiol 2 (2015): 006–011.
- Fort ACP, Rubin LA, Meltzer AJ, Schneider DB, Lichtman AD. Perioperative management of endovascular thoracoabdominal aortic aneurysm repair. J Cardiothorac Vasc Anesth 31 (2017): 1440–1459.
- van Dorp M, Gilbers M, Lauwers P, Van Schil PE, Hendriks JM. Local anesthesia for percutaneous thoracic endovascular aortic repair. Aorta 4 (2016): 78–82.
- Forcillo J, Duwayri YM, Jordan WD Jr, Leshnower BG. Awake thoracic endovascular aneurysm repair for aortic rupture: a case series. Semin Thorac Cardiovasc Surg 30 (2018): 36–39.
- Sueda T, Takahashi S. Spinal cord injury as a complication of thoracic endovascular aneurysm repair. Surg Today 48 (2018): 473-477.
- Lee WA, Brown MP, Nelson PR, Huber TS, Seeger JM. Midterm outcomes of femoral arteries after percutaneous endovascular aortic repair using the Preclose technique. J Vasc Surg 47 (2008): 919–923.
- Makaloski V, Kolbel T, Fiorucci B, Rohlffs F, Carpenter S, Law Y, et al. Fascial suture technique versus open femoral access for thoracic endovascular aortic repair. J Vasc Surg 69 (2019): 34–39.