Left Ventricular Outflow Tract Obstruction due to Primary Cardiac Tumors
Article Information
Alex Nantsios1*, Alex Zapolanski2, Juan Grau2, Habib Jabagi2
1Division of Cardiac Surgery, University of Ottawa Heart Institute, Ontario, Canada
2Division of Cardiac Surgery, Valley Health System, Ridgewood, New Jersey, USA
*Corresponding Author: Alex Nantsios, Division of Cardiac Surgery, University of Ottawa Heart Institute, Ontario, Canada
Received: 22 March 2022; Accepted: 20 April 2022; Published: 06 May 2022
Citation: Alex Nantsios, Alex Zapolanski, Juan Grau, Habib Jabagi. Left Ventricular Outflow Tract Obstruction due to Primary Cardiac Tumors. Archives of Clinical and Medical Case Reports 6 (2022): 334-341.
Share at FacebookAbstract
Left Ventricular Outflow (LVOT) masses are rare and raise concern for infective endocarditis vegetations, thrombus, and cardiac tumors. When these masses arise in the LVOT, they can lead to symptoms of LVOT obstruction and thromboembolism. The most common cause of a cardiac tumor is cardiac myxoma, occurring in 0.02% of the population, and almost exclusively arises in the atria [1]. We present two cases of large cardiac masses of different pathology arising in the left ventricle causing LVOT obstruction.
Keywords
Left Ventricular Outflow; Infective endocarditis; Cardiac tumor; Myxoma
Left Ventricular Outflow articles; Infective endocarditis articles; Cardiac tumor articles; Myxoma articles
Left Ventricular articles Left Ventricular Research articles Left Ventricular review articles Left Ventricular PubMed articles Left Ventricular PubMed Central articles Left Ventricular 2023 articles Left Ventricular 2024 articles Left Ventricular Scopus articles Left Ventricular impact factor journals Left Ventricular Scopus journals Left Ventricular PubMed journals Left Ventricular medical journals Left Ventricular free journals Left Ventricular best journals Left Ventricular top journals Left Ventricular free medical journals Left Ventricular famous journals Left Ventricular Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Infective endocarditis articles Infective endocarditis Research articles Infective endocarditis review articles Infective endocarditis PubMed articles Infective endocarditis PubMed Central articles Infective endocarditis 2023 articles Infective endocarditis 2024 articles Infective endocarditis Scopus articles Infective endocarditis impact factor journals Infective endocarditis Scopus journals Infective endocarditis PubMed journals Infective endocarditis medical journals Infective endocarditis free journals Infective endocarditis best journals Infective endocarditis top journals Infective endocarditis free medical journals Infective endocarditis famous journals Infective endocarditis Google Scholar indexed journals Cardiac tumor articles Cardiac tumor Research articles Cardiac tumor review articles Cardiac tumor PubMed articles Cardiac tumor PubMed Central articles Cardiac tumor 2023 articles Cardiac tumor 2024 articles Cardiac tumor Scopus articles Cardiac tumor impact factor journals Cardiac tumor Scopus journals Cardiac tumor PubMed journals Cardiac tumor medical journals Cardiac tumor free journals Cardiac tumor best journals Cardiac tumor top journals Cardiac tumor free medical journals Cardiac tumor famous journals Cardiac tumor Google Scholar indexed journals Ultra Sound articles Ultra Sound Research articles Ultra Sound review articles Ultra Sound PubMed articles Ultra Sound PubMed Central articles Ultra Sound 2023 articles Ultra Sound 2024 articles Ultra Sound Scopus articles Ultra Sound impact factor journals Ultra Sound Scopus journals Ultra Sound PubMed journals Ultra Sound medical journals Ultra Sound free journals Ultra Sound best journals Ultra Sound top journals Ultra Sound free medical journals Ultra Sound famous journals Ultra Sound Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Radiology articles Radiology Research articles Radiology review articles Radiology PubMed articles Radiology PubMed Central articles Radiology 2023 articles Radiology 2024 articles Radiology Scopus articles Radiology impact factor journals Radiology Scopus journals Radiology PubMed journals Radiology medical journals Radiology free journals Radiology best journals Radiology top journals Radiology free medical journals Radiology famous journals Radiology Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Myxoma articles Myxoma Research articles Myxoma review articles Myxoma PubMed articles Myxoma PubMed Central articles Myxoma 2023 articles Myxoma 2024 articles Myxoma Scopus articles Myxoma impact factor journals Myxoma Scopus journals Myxoma PubMed journals Myxoma medical journals Myxoma free journals Myxoma best journals Myxoma top journals Myxoma free medical journals Myxoma famous journals Myxoma Google Scholar indexed journals
Article Details
1. Case Reports
1.1 Case 1
A healthy 57-year-old female was found to have a murmur and underwent Transthoracic Echocardiogram (TTE) demonstrating a large mass attached to the LV and part of the Mitral Valve (MV), prolapsing into the LVOT. When sent to hospital for further workup, the patient denied symptoms of heart failure, infection, and constitutional symptoms. There was no history of transient ischemic attack, stroke, or migraines. Upon examination, she was euvolemic with a 2/6 systolic ejection murmur along the right sternal border. Repeat TTE revealed a pedunculated 2×3 cm mass in the inferior left ventricle (LV), attached to the anterior MV leaflet, causing intermittent LVOT obstruction. Peak and mean LVOT gradients were 29 and 13 mmHg respectively. Due to the size and obstructive pathology of the mass, the patient was taken for urgent surgical removal. Intraoperative Transesophageal Echocardiogram (TEE) confirmed a large pedunculated mass arising from the LV, with attachment to the anterior MV leaflet, prolapsing into the LVOT, and causing a maximum LVOT gradient of 28.9 mmHg. Biventricular size and function were normal with no other valvulopathy noted. She subsequently underwent sternotomy and was placed on Cardiopulmonary Bypass (CPB) via central cannulation. The heart was arrested with antegrade and retrograde cardioplegia. A transverse aortotomy was performed 2 cm above the sinotubular junction and the mass was immediately visible prolapsing through the AV. The mass was mobilized, revealing attachments to the LV septum and not the MV. The septal base of the tumor was then ablated using cryoablation to ensure tumor free margins. Postoperative TEE confirmed no residual mass with no significant AV or LVOT gradients, and normal AV function. Immunohistochemical studies confirmed cardiac myxoma (factor VII, CD34+ve, CD31+ve, calretinin, pancytokeratin, and vimentin) with septal muscle the tumor base.
1.2. Case 2
A 79-year-old female, with a history of smoking, hypertension, hypothyroidism, stage III chronic kidney disease, and a stable meningioma presented to the emergency department with an episode of syncope without prodrome. ECG revealed sinus bradycardia (HR 40 bpm) on arrival. MRI brain showed stable meningioma, with no acute infract or hemorrhage. TTE confirmed a mass attached to the anterior LV septal wall with a peak intra-cavitary gradient of 100 mmHg during systole due to almost complete obstruction of the LVOT by this tumor. Cardiac MRI demonstrated a 1.2 × 1.2 cm hypermobile mass in the LV with possible attachment to the anteroseptal wall via a stalk (Figure 4). Further assessment with CT cardiac confirmed this mass was attached to the anterior wall of the LV. In the setting of syncope, electrophysiology consultation was obtained that ruled out any conduction abnormalities or sick sinus syndrome, syncopal episode appear to be obstructive in nature. With no other cause identified, the patient was taken surgical excision. She underwent sternotomy and placed on CPB. The mass was accessed through the aorta, and two separate tumors were identified, one at the level of the interventricular septum approximately 4 cm from the aortic valve and the other 1 at the very apex of the left ventricle. They were both resected with margins. A limited LV myectomy was utilized to ensure negative margins, the apical tumor was resected with margins as well through the aortic valve. Pathology workup revealed the mass to be a papillary fibroelastoma with negative margins (Figure 6).
2. Discussion
Cardiac neoplasms are often referred to as “great mimickers”, as they have no pathognomonic features, and can be associated with variable presentations. Cardiac masses involving the LVOT are rare, and often suspicious for thrombus, vegetation, or primary cardiac mass. The differential of primary cardiac neoplasm in the LV includes: fibroma, rhabdomyoma, lipoma or lymphoma. Herein, we present two unusual cases of patients with cardiac masses arising from the LV causing symptoms associated with LVOT obstruction as their initial clinical presentation.
2.1. Imaging
Echocardiography remains the cornerstone for diagnostic evaluation of suspected cardiac masses [2], providing rapid morphological characterization and dynamic identification of their hemodynamic consequences. Diagnostic TEE may be useful in determining mass etiology, particularly in ruling out thrombus. Prompt echocardiographic characterization is important, as thrombus and cardiac masses often have similar morphology, but very different management strategies. The use of contrast echocardiography to identify tumor vascularity or tissue Doppler imaging to assess mobility can be helpful to make this distinction [2]. Additionally, three-dimensional TEE may further provide information about concomitant attachments, morphology, and functional impact. The gold-standard technique in the work-up of cardiac tumors is cardiac MRI (CMR) [3]. CMR may provide precise information about tumor extent, location, and tissue characterization, with the ability to differentiate fat, fluid, and blood. CMR should therefore be used to rule out thrombus or vegetation when suspected. Additionally, CMR has a high diagnostic accuracy for predicting malignancy of masses based on tissue characteristics of the tumor, such as large size (>5cm), irregular border, or invasion through tissue planes, tissue heterogeneity on T1 and T2 weight images or presence of pericardial effusion [4, 5] (Figure 4). Cardiac computed tomography (CT) is an alternative imaging modality to assess cardiac masses, given its wide availability. Similar to CMR, CT provides information about tumor size, location, morphology, and tissue characterization. However, CT generally offers lower resolution and tissue characterization compared to CMR and echocardiography. Compared the other modalities, CT has superior detection for the presence of calcification, which can be suggestive of fibroma or hemangioma, and can further assess contrast enhancement of the mass, indicating tumor vascularization [6]. CT acquisition is also important for surgical planning, including assessment of the coronary arteries [5], involvement of pericardium or great vessels, the best surgical approach to the tumor, and optimal cannulation strategy. Cine CT imaging with steady-state free-precession (SSFP) sequence can further assess the mobility of a mass, in addition to evaluating its impact on cardiac function [2] (Figure 5).
2.2. Cardiac Myxomas
Cardiac myxoma is the most common cause of cardiac neoplasm, almost exclusively arising in the atria [1]. Myxomas are usually asymptomatic and indolent. Consequently, the most common initial presentation is thromboembolism [7]. Alternatively, patients may be symptomatic with heart failure due to obstructive mass effect or valve function impairment [1]. There are two pathological phenotypes of myxoma as originally described by St. John et al., Papillary myxomas (characterized by an irregular exterior and are associated with neurologic symptoms and embolic phenomena) and solid myxomas (characterized by smooth borders, and often present with heart failure) [8]. On CMR, myxomas usually appear as isodense or hypodense on T1, hyperintense on T2 suggesting high water density, and typically enhance with gadolinium [2]. Complete surgical resection is indicated in patients with LV myxoma, for the prevention of embolic complications [9].
2.3. Cardiac Papillary Fibroelastoma
Papillary fibroelastoma most commonly occur on the downstream surfaces of the aortic and mitral valves [1], and rarely on endocardial surfaces. The incidence of papillary fibroelastoma is 0.3%, with a predilection for the left heart [10]. Although papillary fibroelastomas are benign neoplasms, patients typically present with cerebral embolic complications. Pathologically, they are characterized by an avascular collagenous core containing mucopolysaccharide and elastin [1]. On TEE they have a shimmering appearance due to vibration of its finger-like projections. T1 and T2 MRI weighted images usually appear with similar intensity to the myocardium, reflecting their fibroelastic composition with uniform intermediate signal intensity [5]. CT characterization of fibroelastoma are challenging due to their small size. Surgical excision is indicated for tumors to reduce the risk of stroke irrespective of size or symptomatic status [11].
2.4. Surgical Management
Cardiac masses can lead to LVOT obstruction due to mass effect. This may lead to symptoms as LVOT gradients increase, and may predispose to sudden cardiac death [12], as gradients become severe. The high pressure of the LVOT during systole may also increase the risk of systemic embolization (reported up to 50% in LV myxomas), which further supports the urgent need for surgical intervention [13]. In general, the transaortic approach is the preferred method to access masses in the LVOT. For adequate visualization of tumors more distally near the LV apex, access through the left atrial groove or the LV apex may be required to visualize and mobilize the mass completely without disruption, this is more morbid to the heart than a transaortic approach.
2.5. Prognosis
Prognosis of cardiac myxomas is excellent following surgical excision [14], with a mortality of <1% [15]. Intracardiac recurrence occurs at a rate of 3% in sporadic myxomas, and 12-22% in familial cases (Carney’s complex) [16]. Local recurrence of myxoma may result from incomplete surgical removal or undiagnosed multicentric primary lesion [14,16] Shah et al showed that ventricular tumors, younger patients, and smaller tumors were at higher risk of recurrence [17]. Cryoablation of the myxoma stalk has been previously described as a technique to theoretically decrease recurrence, however, this has yet to be validated [18]. Most authors advocate that wide endocardial resection with clear margins should be performed when there is little risk of excising additional tissue [17] in order to reduce recurrence. In this report two surgeons used two different approaches to secure negative margins.
3. Conclusion
In summary, although cardiac myxomas or papillary fibroelastoma are a rare cause of mass in the LV, particularly in the LVOT, they must be considered a differential along with thrombus or vegetation. This highlights the importance of multimodal diagnostic imaging to confirm a correct diagnosis before any intervention is undertaken. Given the high incidence of thromboembolic complications or potential of LVOT obstruction, prompt diagnosis and surgical excision are advised. There are different approaches to get access to these tumors arising in the interventricular septum. We recommend a transaortic approach as the least morbid way to provide negative margins after excision. In any oncological operation where tumoral tissue is removed a negative margin resection is the most paramount goal a surgeon must achieve.
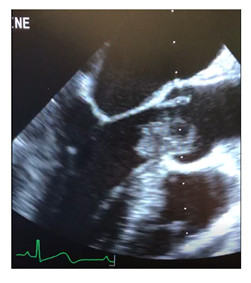
Figure 1: Intraoperative transesophageal echocardiography in midesophageal long axis demonstrating left ventricular outflow tract myxoma.
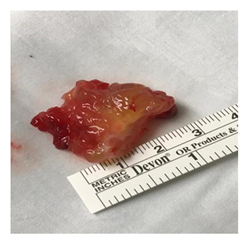
Figure 2: Gross appearance of left ventricular outflow tract myxoma attached to interventricular septum.
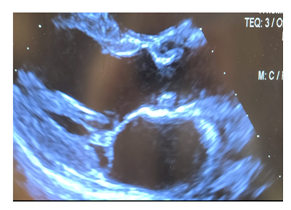
Figure 3: Postoperative transthoracic echocardiogram in parasternal long axis post-surgical resection demonstrating cardiac myxoma involving the left ventricular outflow tract.
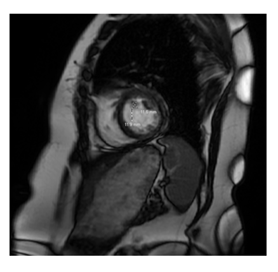
Figure 4: Cardiac MRI demonstrating globular 1.2 x 1.2 cm left ventricular papillary fibroelastoma attatched to anterospetal wall.
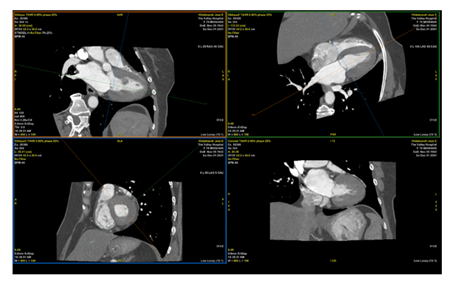
Figure 5: CT Cardiac demonstrating left ventricular papillary fibroelastoma measuring 12 x 13 mm, attached to proximal anteroseptal, mid and distal anterior wall.
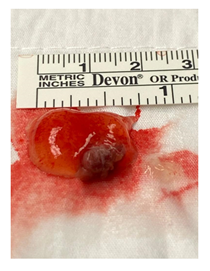
Figure 6: Gross appearance of left ventricular papillary fibroelastoma.
References
- Tyebally S, Chen D, Bhattacharyya S, et al. Cardiac Tumors: JACC CardioOncology State-of-the-Art Review. JACC CardioOncol 2 (2020): 293-311.
- Auger D, Pressacco J, Marcotte F, et al. Cardiac masses: an integrative approach using echocardiography and other imaging modalities. Heart 97 (2011): 1101-1109.
- Hundley WG, Bluemke DA, Finn JP, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol 55 (2010): 2614-2662.
- Fussen S, De Boeck BW, Zellweger MJ, et al. Cardiovascular magnetic resonance imaging for diagnosis and clinical management of suspected cardiac masses and tumours. Eur Heart J 32 (2011): 1551-1560.
- Motwani M, Kidambi A, Herzog BA, et al. MR imaging of cardiac tumors and masses: a review of methods and clinical applications. Radiology 268 (2013): 26-43.
- Kassop D, Donovan MS, Cheezum MK, et al. Cardiac Masses on Cardiac CT: A Review. Curr Cardiovasc Imaging Rep 7 (2014): 9281.
- Swartz MF, Lutz CJ, Chandan VS, et al. Atrial myxomas: pathologic types, tumor location, and presenting symptoms. J Card Surg 21 (2006): 435-440.
- St John Sutton MG, Mercier LA, Giuliani et al. Atrial myxomas: a review of clinical experience in 40 patients. Mayo Clin Proc 55 (1980): 371-376.
- Velez Torres JM, Martinez Duarte E, Diaz-Perez JA, et al. Cardiac Myxoma: Review and Update of Contemporary Immunohistochemical Markers and Molecular Pathology. Adv Anat Pathol 27 (2020): 380-384.
- Grolla E, Dalla Vestra M, Zoffoli G, et al. Papillary fibroelastoma, unusual cause of stroke in a young man: a case report. J Cardiothorac Surg 12 (2017): 33.
- Tamin SS, Maleszewski JJ, Scott CG, et al. Prognostic and Bioepidemiologic Implications of Papillary Fibroelastomas. J Am Coll Cardiol 65 (2015): 2420-2429.
- Norrish G. 2 The relationship between left ventricular outflow tract gradient and suuden cardiac death in childhood hypertrophic cardiomyopathy. Heart 106 (2020): A2-A2.
- Thongcharoen P, Laksanabunsong P, Thongtang V. Left ventricular outflow tract obstruction due to a left ventricular myxoma: a case report and review of the literature. J Med Assoc Thai 80 (1997): 799-806.
- Singhal P, Luk A, Rao V, et al. Molecular basis of cardiac myxomas. Int J Mol Sci 15 (2014): 1315-1337.
- Karabinis A, Samanidis G, Khoury M, et al. Clinical presentation and treatment of cardiac myxoma in 153 patients. Medicine (Baltimore) 97 (2018): 12397.
- Shinfeld A, Katsumata T, Westaby S. Recurrent cardiac myxoma: seeding or multifocal disease? Ann Thorac Surg 66 (1998): 285-288.
- Shah IK, Dearani JA, Daly RC, et al. Cardiac Myxomas: A 50-Year Experience with Resection and Analysis of Risk Factors for Recurrence. Ann Thorac Surg 100 (2015): 495-500.
- Rathore KS, Hussenbocus S, Stuklis R, et al. Novel strategies for recurrent cardiac myxoma. Ann Thorac Surg 85 (2008): 2125-2126.
