Knowledge Distillation for COVID-19 Therapeutics
Article Information
Shiyao Zhaia,b, Yuhan Gaoc, Kailai Pengc, Qun Chenb, Qian Heb, Li Fangb, Linling Lid,
Bowen Zhongd, Yuhan Donge, Chenggang Yana,c, and Peiwu Qinb,f, Dongmei Yua*
aSchool of Mechanical, Electrical & Information Engineering, Shandong University, Weihai, Shandong Province, 264209, China
bCenter of Precision Medicine and Healthcare, Tsinghua-Berkeley Shenzhen Institute, Shenzhen, Guangdong Province, 518055, China
cDepartment of Automation, Hangzhou Dianzi University, Hangzhou, Zhejiang Province, 310000, China
dShenzhen Maternity and Child Healthcare Hospital, Affiliated to Southern Medical University, Shenzhen, Guangdong 518028, China
eDepartment of Information Science and Technology, Tsinghua Shenzhen International Graduate School, Shenzhen, Guangdong Province, 518055, China
fInstitute of Biopharmaceutical and Health Engineering, Tsinghua Shenzhen International Graduate School, Shenzhen, Guangdong Province, 518055, China
*Corresponding author: Dongmei Yu, School of Mechanical, Electrical & Information Engineering, Shandong University, Weihai, Shandong Province, 264209, China
Received: 19 January 2022; Accepted: 27 January 2021; Published: 10 February 2022
Citation:
Shiyao Zhai, Yuhan Gao, Kailai Peng, Qun Chen, Qian He, Li Fang, Linling Li, Bowen Zhong, Yuhan Dong, Chenggang Yan, and Peiwu Qin, Dongmei Yu. Knowledge Distillation for COVID-19 Therapeutics. Archives of Clinical and Biomedical Research 6 (2022): 153-183.
Share at FacebookAbstract
The novel coronavirus disease (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 is threatening the world's economy and healthcare with urgent therapeutic demand. Clinical and scientific studies have made promising progress in ameliorating and treating COVID-19. The unprecedented effort has brought several vaccines approved for emergency use, and prevalent vaccination is advocated globally. However, methods that can effectively clear the virus and terminate transmission are not available. The variants and uncontrolled circulations bring persistent challenges for therapeutics and vaccines. In this review, we discuss the ongoing treatments, the mechanism, and potential optimization of various therapeutics. We review the viral pathogenesis and epidemiology and provide insights for prospective therapeutics and vaccine evolution, which will help clinical doctors and researchers in this pandemic.
Keywords
COVID-19; SARS-CoV-2; Treatments; Therapeutics; Vaccine
COVID-19 articles; SARS-CoV-2 articles; Treatments articles; Therapeutics articles; Vaccine articles
Article Details
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019 as a Public Health Emergency of International Concern, spreading rapidly and leading to a global pandemic [1]. As of March 28, 2021, 126 million confirmed cases with 2.7 million deaths had been reported worldwide. Since World War II, the pandemic has demoralized and cracked down healthcare, economies, politics, and cultures unprecedentedly around the world. SARS-CoV-2 belongs to coronaviruses, a group of single-stranded RNA viruses with spherical shape including seven kinds of human disease-causing coronaviruses, such as SARS-CoV-1, Middle East respiratory syndrome coronavirus (MERS-CoV), etc [2]. The SARS-CoV-2 genome encodes structural proteins, such as spike protein (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N). The S protein on the viral surface initiates receptor binding; the E protein plays a role in virus assemblage and release; the M protein promotes membrane curvature and nutrient transport across cell membranes; the N protein binds the viral RNA genome and maintains its stability [3]. Diseases caused by SARS-CoV-2 range from asymptomatic, mild pneumonia to severe acute respiratory distress syndromes (ARDS), septic shock, and multiple organ dysfunction syndrome [4]. The general clinical manifestations are fever, cough, fatigue, sputum production, shortness of breath, sore throat, and headache [5]. Comorbidities are common, including hypertension, diabetes, cardiac arrhythmia, renal failure, heart failure, and chronic pulmonary disease. The susceptible population includes the elderly and people with certain underlying medical conditions. High viral load is associated not only with low immunity but also with high expression of the angiotensin-converting enzyme 2 (ACE2) receptor (the cell-entry receptor for SARS-CoV-2) in elderly patients [6]. Although World Health Organization (WHO) and many countries offer clinical guidelines for different severity of COVID-19, they have not yet shown safe and effective pharmaceutical products or measures. Without effective medicine, supportive therapeutics remains the mainstay of therapy including corticosteroids, respiratory support, nutritional support, and extracorporeal membrane oxygenation (ECMO) for salvage therapy. Recovered or recharged patients are still susceptible to a second infection, and the evolution rate of RNA viruses is dramatically high [7]. Traditional Chinese medicine (TCM), convalescent plasma, and immunotherapy have shown therapeutic benefits. Prevalent vaccination is advocated globally. More than seven different vaccines of SARS-CoV-2 across four platforms have been rolled out in the world. Until April 2nd, more than 640 million people have gotten the doses with over 1.8% of the population have been fully vaccinated. With tolerable side effects, such as pain, fever, fatigue, and headache, the vaccines have exhibited impressive efficacy [8]. However, the SARS-CoV-2 spike gene has accumulated mutations within the receptor-binding domain (RBD) and the N-terminal domain (NTD), the major targets of many antiviral drugs, vaccines, and immunotherapies, which can nullify the effects of treatments [9]. Therefore, the evolution of therapeutics and vaccines for the next generation is desired (Figure 1).
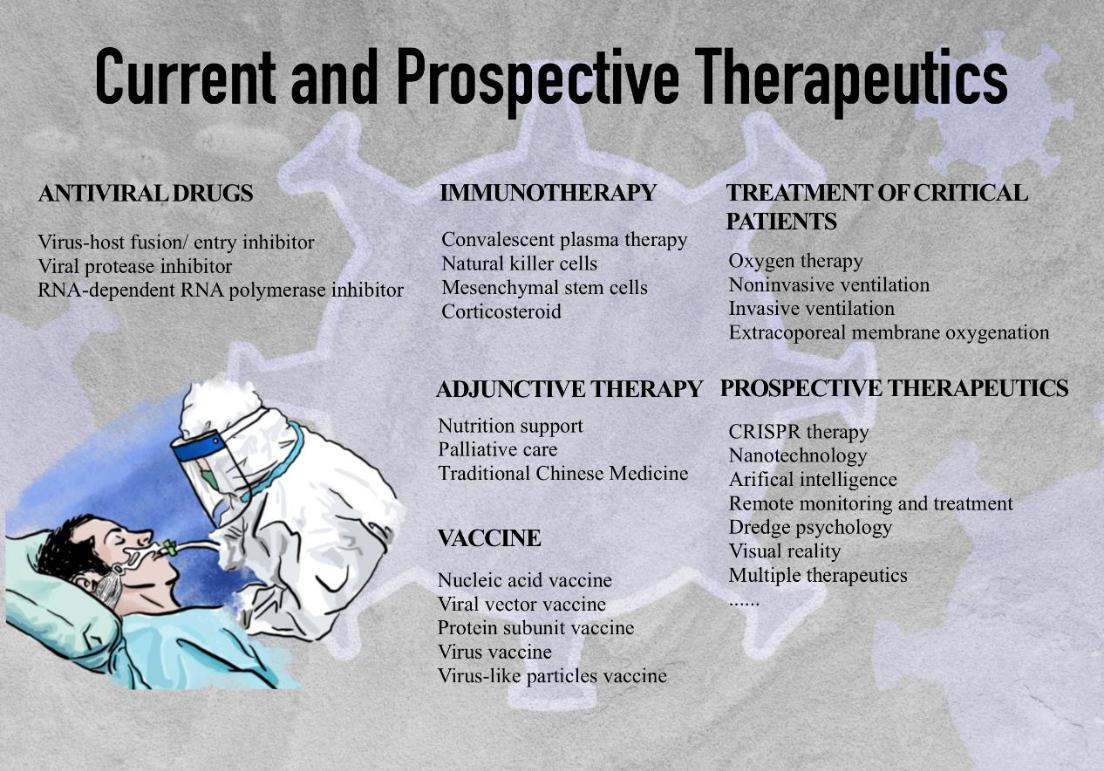
Figure 1: Current and prospective therapeutics
Antiviral Drugs
SARS-CoV-2 targets cells through the S1 subunit of the S protein that binds to the ACE2 receptor. After receptor binding, the virus enters the host cell via endocytosis. After inserting the genetic materials into the host genome, the coronavirus replicase-transcriptase complex is translated by the host cells. The virus then synthesizes the RNA genome via its RNA-dependent RNA polymerase (RdRp), and the structural proteins facilitate the completion of assemblage and release of viral particles. Key steps of the virus life cycle can be targeted by antiviral drugs for drug development (Figure 2) (Table 1).
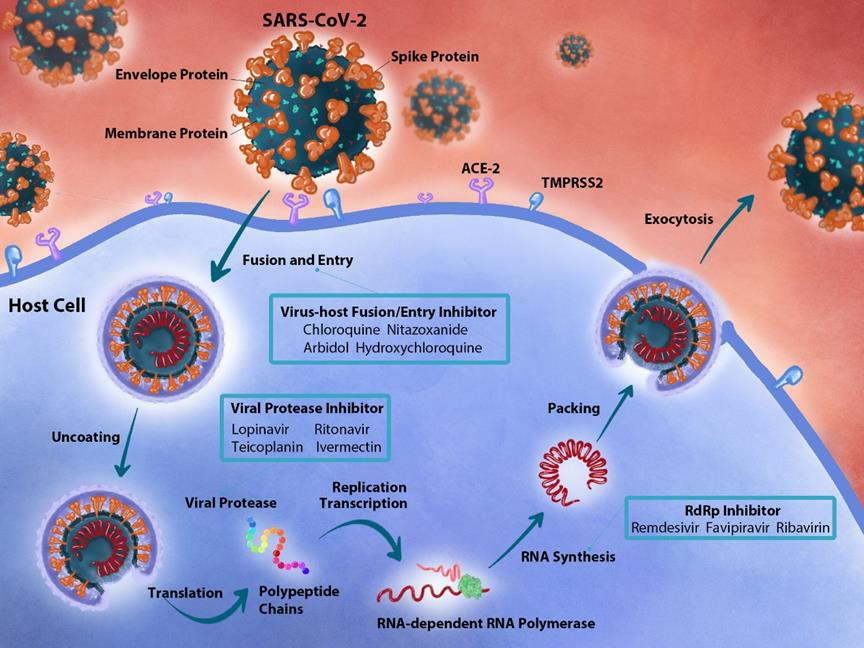
Figure 2: Overview of antiviral drugs in the context of the host pathway and SARS-CoV-2 replication mechanism. The SARS-CoV-2 virus particle enters host cells through the S protein that binds to the ACE-2 receptor. Once inside the cell, the virus encodes the replicase-transcriptase complex and synthesizes the viral protease. The RNA is then synthesized via RdRp. Viral drugs can act on the following three steps: (1) Chloroquine, nitazoxanide, arbidol, and hydroxychloroquine can act in the fusion and entry process. (2) Atazanavir, lopinavir, ritonavir, teicoplanin, and ivermectin can act as viral protease. (3) Remdesivir, favipiravir, and ribavirin can inhibit the synthesis of RdRp.
|
Name |
Chemical formula |
Dosage |
Adverse effects |
|
Chloroquine |
C18H26ClN3·2H3O4P |
Day 1-7: 500mg, bid |
Nausea, vomiting, and abdominal discomfort |
|
Hydroxychloroquine |
C18H28ClN3O5S |
Day 1: 400 mg, bid Day 2-5mg, bid |
|
|
Nitazoxanide |
C12H9N3O5S |
Day 1-7: 500mg, tid |
NA |
|
Arbidol |
C22H25BrN2O3S |
200 mg, tid |
NA |
|
Lopinavir |
C37H48N4O5 |
Day 1-14: 400mg Lopinavir and 100 mg Lopinavir, bid |
Gastrointestinal distress and hepatotoxicity |
|
Ritonavir |
C37H48N6O5S2 |
||
|
Ivermectin |
C48H74O14 |
NA |
NA |
|
Teicoplanin |
C77H77N9O31Cl2·R |
NA |
NA |
|
Remdesivir |
C27H35N6O8P |
Day 1: 200mg, IV Day 2-5 (or 10): 100 mg, IV |
Anemia, ecreased hemoglobin, and acute kidney injury |
|
Favipiravir |
C5H4FN3O2 |
Day 1: 1600mg, tid Day 2-14: 600mg, tid |
Teratogencity and embryotoxicity |
|
Ribavirin |
C8H12N4O5 |
A 4 g oral loading dose, followed by a 1•2 g oral dose every 8 hours |
Teratogencity and dose-dependent hematologic toxicity |
IV: intravenous; bid: bis in die; tid: ter in die
Table 1: Chemical information and clinical side effects of different antiviral drugs.
Generally, there are two paths toward viral drug development. One path is to attack the virus directly and interrupts its replication machinery or ability to attack host cells. However, the rapid emergence of protein variants enhanced the difficulty for drug development. The second path blocks host–viral interactions by protein–protein interactions (PPIs). A study detected 332 high-confidence SARS-CoV-2-human PPIs. 40% of SARS-CoV-2 proteins are connected to various biological processes including endomembrane compartments modification, vesicle trafficking, multiple innate immunes, translation, enzymes involved in ubiquitination regulation and so on [9]. The development of novel drugs is time-consuming, and repurposing existing drugs such as hydroxychloroquine (HCQ), chloroquine (CQ), remdesivir, ivermectin, and baricitinib shows good potential for COVID-19 treatment, which crucially helps in resolving outbreaks in times of urgent need. CQ has a potential action against COVID-19 at both entry and post-entry stages of COVID-19 infection, which inhibits terminal glycosylation of ACE2 [10]. HCQ and CQ can be engulfed in endosomes and lysosomes, leading to an increase in the pH of these cellular compartments, which in turn impedes membrane fusion and consequently disrupts several enzymes, including acid hydrolases, unmasks the heptad repeat subdomains of the S2 domain of the S glycoprotein, and inhibits post-translational modification of newly synthesized proteins. CQ prevents the phosphorylation of p38 mitogen-activated protein kinase and caspase-1, being an immunomodulatory agent that induces the inhibition of Interleukin-1 (IL-1), Interleukin-6 (IL-6), and Tumor necrosis factor-α (TNF-α) in immune cells [11]. Pharmacokinetics of HCQ, such as its long half-life and high lung concentration (500 times the blood concentration), is ideally suited for use as a prophylactic agent [12]. The QRS interval duration, a sodium channel-dependent ventricular conduction parameter, is increased in COVID-19 patients treated with HCQ [13]. HCQ is usually administered in COVID-19 together with QT-prolonging drugs, including azithromycin, which reinforces the treatment efficacy of HCQ in a cohort of 20 severe COVID-19 patients [14]. However, the combination of HCQ and azithromycin may increase the risk of 30-day cardiovascular mortality and heart failure. Moreover, the association of CQ/HCQ with melanin can cause ocular pigmentation, which may lead to eye damage [15]. ACE inhibitors or angiotensin receptor blockers could increase the expression of ACE2 in the respiratory tract, thereby increasing the risk of infection and complications from COVID-19. Miniproteins such as VH bind tightly to S and block its binding to its receptor ACE2 in order to prevent SARS-CoV-2 infection of mammalian Vero E6 cells. Antibodies trap S in a conformation that cannot bind to ACE2 to combat the virus and prevent the development of resistance for at least 5 months after infection [16]. Renin-angiotensin-aldosterone system (RAAS) inhibitors were predominantly ACE inhibitors and angiotensin receptor blockers, which could reduce the risk of severe complications or death due to COVID-19 [17]. Viral attachment and infection involve heparan sulfate-dependent enhancement of binding to ACE2. Manipulation of heparan sulfate or inhibition of viral adhesion by exogenous heparin presents new therapeutic opportunities [18]. The AP2-associated protein kinase 1 (AAK1) regulates the ACE-2 cell receptor, which is inhibited by Baricitinib [19]. Disruption of AAK1 interrupts the passage of the virus into cells and the intracellular assembly of virus particles [20]. Baricitinib inhibits AAK1 to prevent endocytosis and reduce virus assembly while impairs the IFN-mediated antiviral response and may contribute to the evolution of SARS-CoV-2 infection [21]. Baricitinib modulates the patients’ immune landscape that benefits patients with COVID-19 pneumonia22, which increases the levels of creatine kinase in Intensive Care Unit (ICU) and critically ill patients. Linoleic acid (LA) stabilizes a locked S conformation, resulting in reduced ACE2 interaction in vitro. In human cells, LA supplementation acts synergistically with the COVID-19 drug remdesivir, thereby suppressing SARS-CoV-2 replication [23]. Cleavage of S generates a polybasic Arg-Arg-Ala-Arg carboxyl-terminal sequence on S1, which conforms to a C-end rule motif, thereby binding to cell surface neuropilin-1 (NRP1) and NRP2 receptors [24]. The promotion of viral infection by NRP1 was inhibited by the addition of a soluble NRP1 or by an antibody mapping to the NRP1 binding pocket [25]. Teicoplanin, as a glycopeptide antibiotic, acts on an early stage of the viral life cycle by inhibiting the low-pH cleavage of the viral S protein via cathepsin L in the late endosomes, preventing the release of genomic viral RNA and the virus replication [26]. SARS-CoV-2 encodes two cysteine proteases, a 3 C-like protease (3CLpro) and a papain-like protease (PLpro) as potential therapeutic targets for drug discovery due to their critical role in viral entry and host cell invasion. Baicalin, herbacetin, and pectolinarin block the proteolytic activity of 3CLpro [27]. Atazanavir binds to the active site of SARS-CoV-2 3CLpro and inhibits this enzyme through zymographic studies [28]. Atazanavir and atazanavir/ritonavir prevented proinflammatory cytokine production in monocytes infected with SARS-CoV-2, as confirmed by low levels of lactate dehydrogenase and IL-6. Lopinavir (LPV), an inhibitor of 3CLpro, is used to treat HIV infection and has in vitro anti-coronavirus activity that may limit the spread of the virus in host cells. LPV is commonly administered in co-formulation with structurally related ritonavir (LPV/r), which is a mutagenic guanosine analog that inhibits cytochrome P450 metabolism of LPV and boosts LPV concentrations [29]. LPV/r–Interferon-β (IFN-β)–ribavirin combination therapy did show promising results for COVID-19 [30]. Ribavirin interferes with viral RNA synthesis and viral mRNA capping after undergoing metabolic activation of its active metabolite [31]. The phosphate prodrug PF-07304814 is a potent inhibitor of 3CLpro having in vitro antiviral activity against SARS-CoV-2 and is additive/synergistic in combination with remdesivir [32]. Twenty-two compounds from natural products and TCM shown activity against SARS-CoV 3CLpro [33]. Ribavirin, valganciclovir, β-thymidine, and natural products Platycodin D, Chrysin, and Neohesperidin, as potential precursors, show a high binding affinity with SARS Cov-2 PLpro, which aids in the processing of replicase polyproteins for viral replication. PLpro enzymes efficiently remove Interferon-stimulated gene product 15 (ISG15) and ubiquitin modifications, thereby attenuating inflammation and antiviral signaling upon viral infection [34]. RdRp replicates the genome of SARS-CoV-2 and transcribes the gene, which is the target of several nucleotide analog drugs, including remdesivir, favipiravir, ribavirin, galidesivir, and EIDD-2801 [35,36]. These inhibitors were originally derived from SARS-CoV-1 and can be transferred to SARS-CoV-2 due to 96% sequence similarity [37]. Following the host-dependent conversion to its active form by the cytochrome P450 enzyme (CYP), nucleoside triphosphate, Remdesivir incorporates into the RNA strand after fending off adenosine triphosphate, resulting in premature termination of RNA synthesis. Mutation hotspots at the catalytic site of RdRp challenge binding and effectiveness [38]. Remdesivir is not recommended for patients with creatinine clearance less than 30 mL/min due to the possibility of accumulation of the excipient sulfobutylether-β-cyclodextrin in patients with renal dysfunction [39]. Favipiravir is an antiviral drug approved for the treatment of a wide spectrum of RNA viruses, including coronaviruses, which inhibit RdRp and have been reported to exhibit activity against SARS-CoV-2 [40]. Scaffolds derived from peptide nucleic acid demonstrate excellent binding towards RdRp and excellent safety profile [41]. Host molecules such as EN-RAGE, TNFSF14, sphingosine kinase, importin-α/β, and their receptors, represent attractive therapeutic targets against COVID-19 [42]. Modulation of sphingosine kinase, sphingosine-1-phosphate (S1P), and the S1P receptor pathway may provide significant beneficial effects towards counteracting the life-threatening, acute and chronic complications associated with SARS-CoV-2 infection [43]. Ivermectin inhibits the importin-α/β receptor, which consists of a set of macrocyclic lactone isomers, and plays a significant role in the transfection process, thereby transmitting viral proteins to the nucleus of the host cell [44]. Ivermectin increases chloride channel permeability and binds to glutamate-gated chloride channels. Ivermectin inhibits the replication of SARS-CoV-2 in vitro, resulting in a 5000-fold reduction in avirulent viral RNA after 48 h in Vero-hSLAM cells [45]. When it was clear that the effectiveness of single drugs in the treatment of COVID-19 is less satisfactory, scientists proposed that drug cocktails may be more effective. Antiviral drug cocktails should be combined with information about their hydrophobic and hydrophilic properties in an attempt to avoid binding competition, increase drug cocktail efficiency, and reduce toxicity and other undesirable side effects [46]. Multifaceted therapeutics will not only enhance treatment efficacy but will also hinder resistance and adverse effects by simultaneously targeting multiple essential viral targets [47].
Immunotherapy
The impact of SARS-CoV-2 on the human immune process is to stimulate the host immune system to release cytokines by activating or damaging various immune cells, which in turn causes inflammation and immune dysfunction. The key immune populations included antibody-secreting cells, follicular T-helper cells, and activated (CD38+HLA-DR+) CD8+ and CD4+ T cells, as well as SARS-CoV-2−specific IgM and IgG antibodies [48]. Drugs regulate the immune system for therapeutic purposes by enhancing the corresponding innate response or by inhibiting the inflammatory response. Reprogramming the immune system is a stopgap measure for treatment when developing new antivirals and vaccines. The hypothetical mechanisms of the cytokine storm induced by SARS-CoV-2 infection and the possible drugs and targets for immunotherapy are illustrated in Figure 3.
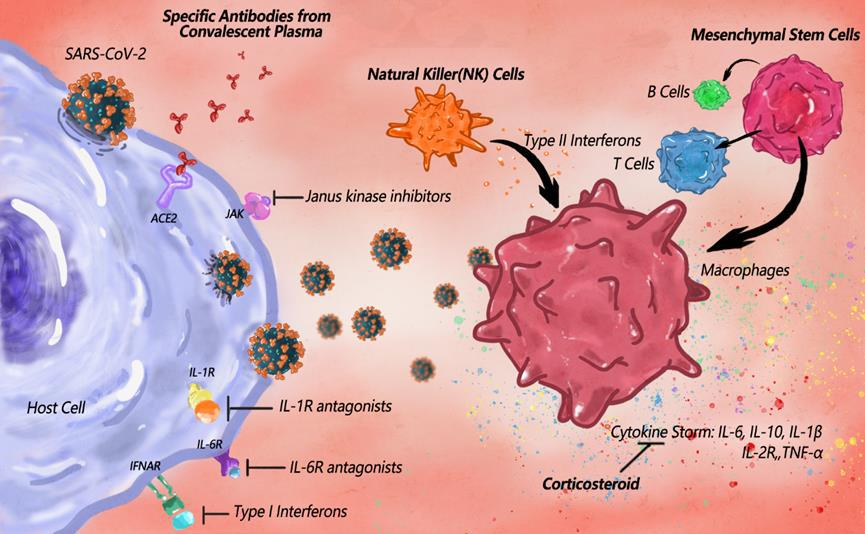
Figure 3: The hypothetical mechanisms of the cytokine storm induced by SARS-CoV-2 infection and the possible immune-therapeutics for COVID-19. A pro-inflammatory feedforward loop of cytokines on innate immune cells results in a cytokine storm. The COVID-19 cytokine storm may result from a combination of macrophage activation syndrome driven by IL-1β and immune dysregulation caused by IL-6. Increased cytokines include IL-6, IL-10, TNF, and inflammatory chemokines, as well as soluble forms of the IL-2 receptor α-chain, with corticosteroids potentially alleviating the cytokine storm. To combat cytokine storms, IL-1R and IL-6R antagonists, Janus kinase inhibitors and type I interferons are possible factors to specifically inhibit the pathway. Activated Natural Killer cells induce killing of target cells, whose cytotoxicity and proliferation can be promoted by interferons. Mesenchymal stem cells are capable of self-replicating and differentiating into immune cells, so as to show potential effectiveness in COVID-19 treatment.
The specific antibody, provided by COVID-19 convalescent plasma, affected the binding of ACE2-RBD, thereby becoming a possible clinical immunotherapy. Convalescent plasma therapy (CPT) uses plasma collected from convalescent patients to treat infected persons [49]. CPT confers passive immunity by providing antibodies and is active after administration. Immunoglobulins in CPT are recycled and excreted and are the first available therapy for an emerging infectious disease [50]. CPT been used in patients with SARS [51], MERS [52], influenza A H1N1 [53], or Ebola [54]. CPT is effective in treating severe and critically ill COVID-19 with the least severe side effects [55]. SARS-CoV-2−specific CD8+ T cells recovered from convalescent COVID-19 patients had an atypically high prevalence of stem cell memory, central memory, and naive phenotypes [48]. The ability to sort individual B cells from previously infected human patients and molecularly clone antibody genes from these B cells has led to an independent source of human antibodies [56]. The quality of the rehabilitation plasma determines the therapeutic approach. Thus, WHO has issued standard guidelines for convalescent plasma in the COVID-19 pandemic. The immune system of genetically humanized mice provides an efficient source of naturally selected fully human antibodies [57]. However, the sensitivity of B.1.35, the SARS-CoV-2 variants, to neutralizing antibodies from convalescent donors infected with the prototype lineage virus is 48% [58]. Natural Killer (NK) cells are an important component of the innate immune system and play a vital role in viral clearance and immunomodulation. NK cells are unconventional T lymphocytes whose T cell receptors recognize glycolipids that bind to the major histocompatibility complex class-I-like molecule, CD1d [59]. NK cells can recognize SARS-CoV-2 infected cells and clear SARS-CoV-2 by regulating the production of cytokines and chemokines [60]. The reduction in the number and function of NK cells leads to a decrease in the clearance of infected and activated cells, and an unchecked elevation of tissue-damaging inflammation markers in patients with severe COVID-19. Clinical trials are underway to evaluate the therapeutic effect of NK cells for the treatment and prevention of SARS-CoV-2 infection [61]. However, the actual efficacy depends on the timing of treatment [62], which is more suitable for the treatment of patients with mild COVID-19. Mesenchymal stem cells (MSCs) play an immunomodulatory role by secreting different regulatory cytokines [63], which prevent cytokine storm and improve pulmonary fibrosis and lung function [64]. MSCs regulate immune responses by converting T helpers (Th1) to Th2, activating M2 macrophages, and modulating the maturation of dendritic cells [65]. Adipose MSCs reduce the risk of complications and death of patients due to their strong anti-inflammatory and immunomodulatory capabilities, which can improve the microenvironment, promote neovascularization, and enhance tissue repair capabilities. Moreover, MSCs secrete a variety of bioactive factors, such as antimicrobial peptides and proteins (AMPs), indoleamine 2,3-dioxygenase, IL-17, and other molecules that can be applied for the regeneration of the respiratory tract in patients with COVID-19. MSCs secrete at least four AMPs: antibacterial peptide LL-37, human defensin-2, hepcidin, and lipocalin-2. These AMPs mediate the cell-killing process by killing cells, inhibiting the synthesis of essential proteins, DNA, and RNA of infected cells, and interacting with certain targets in infected cells, as well as playing an active regulatory role in the infection and inflammatory progress in patients with COVID-19. Patients treated with MSCs regained lung function and restored levels of cytokines and trophic factors, thus alleviating the suffering of COVID-19 patients [66]. Allergic reactions are serious complications caused by the improper application of MSCs [67]. Immuno-modulating therapeutics such as dexamethasone, IL-1 inhibitors, IL-6 inhibitors, IFN-β, Bruton’s tyrosine kinase inhibitors, and Janus kinase (JAK) inhibitors have raised hope in the treatment of COVID-19 [37]. Since IL-1 blocks the production of IL-6 and other proinflammatory cytokines, early blockade of the IL-1 receptor is therapeutic in acute hyperinflammatory respiratory failure in COVID-19 patients [68]. By competitively inhibiting IL-1α and IL-1β from binding to the IL-1 type I receptor, anakinra neutralizes the activity that pertains to these key mediators of autoinflammatory and/or immune processes induced by SARS-CoV-2 [69,70]. IL-6 signaling plays a crucial role in endothelial cell dysfunction during bacterial and viral cytokine release syndrome (CRS). IL-6 mediates production of hyperinflammatory cytokines and plasminogen activator inhibitor-1, indicating that IL-6 signaling blockade has potential as a therapy for CRS [71]. Tocilizumab is a recombinant monoclonal antibody directed against the IL-6 receptor (IL-6R), which inhibits IL-6-mediated signaling by binding to soluble and membrane-bound IL-6R, so as to ultimately cause effective downregulation of the immune system. Tocilizumab improves clinical symptoms and represses deterioration in patients with severe COVID-19 [72]. However, suppression of IL-6 may promote secondary bacterial infections and further complicate the disease course [73]. IFNs play an important role in the inhibition of viral replication, which is an important cytokine of the innate and adaptive immune system with three main types: I (α or β), II (γ), and III (λ). IFN signaling induces the expression of the tumor suppressor p53, which limits viral replication by causing cell cycle arrest of infected cells. IFN- λ protects against viral infections and increases the barrier functions of intestinal epithelial cells and endothelial cells. IFN-λ secreted by dendritic cells damages the lung epithelium, which increases susceptibility to lethal bacterial superinfections. IFN-λ mRNA from bronchoalveolar lavage fluid and naso-oropharyngeal samples correlated with disease morbidity in SARS-CoV-2– positive patients [74]. The location, timing, and duration of IFN exposure are critical parameters underlying the success or failure of therapeutics for viral respiratory infections. Optimal protection could be achieved by strongly inducing IFN-stimulated genes in the early stages of infection to curb viral replication, followed by timely down-regulation of IFN responses, thereby enabling efficient lung epithelial repair [75]. The detrimental activities of IFN-λ occur only after chronic exposure and in the presence of tissue damage [75]. Dexamethasone or methylprednisolone, an anti-inflammatory corticosteroid, reduces mortality and time to discharge in subjects with COVID-19 who require invasive mechanical ventilation or oxygen alone. Specifically, dexamethasone reduced the number of COVID-19-related deaths by 35% in patients in the ICU who require mechanical ventilation [76]. Dexamethasone binds to intracellular glucocorticoid receptors, which are ligand-dependent transcription factors expressed by most cells in the body. Patients with severe COVID-19 responded well to pulse therapy with high-dose methylprednisolone and intravenously human immunoglobulin [77]. In contrast, glucocorticoids cause widespread changes in cellular transcriptomes and processes, which act on T cells to dampen T cell receptor signaling and cytokine expression [78]. Immunosuppression by corticosteroids (both inhaled and systemic) impairs the induction of IFN-I responses in the context of COVID-19. Even the use of corticosteroids may lead to higher mortality, diabetes, avascular necrosis of the femoral head, and osteoporosis [79]. Deterioration in some patients after corticosteroid treatment may be due to the critical role of thymic stromal lymphopoietin in inducing corticosteroid resistance [80]. Low-dose dexamethasone treatment suppresses COVID-19-related immunopathology by complementing endogenous cortisol activity, while avoiding the adverse effects of high-dose glucocorticoid therapy [78]. The complement system is a key player in the innate immune response and relies on soluble pattern recognition molecules to act as a danger-sensing alert system. The classical pathway is triggered by antibodies, but also by acute-phase proteins such as C-reactive protein. Overwhelming complement activation may lead to destructive inflammation that damages the host. The complement inhibitor eculizumab prevents the cleavage of C5, and the conversion of nAbs to C5a exhibits beneficial effects in a subgroup of patients with aggravated COVID-19 by attenuating the over-activation of the inflammatory system [81]. Immunotherapy was inspired by the impact of SARS-CoV-2 on the human immune process, which at the same time was subject to the complexity of the immune process. A better understanding of the interplay between the humoral response and the viral adaptation will be important for us to design better immunotherapy for COVID-19. Melatonin acts as an antioxidant and anti-inflammatory agent against acute lung injury/ARDS induced by viral and bacterial infections. Melatonin can be beneficial in critically ill patients by reducing vascular permeability, inducing sedation, decreasing agitation, and improving sleep quality. Melatonin treatment significantly ameliorated myocardial injuries by improving myocarditis via repressing inflammation, which regulated the rate of autophagy and inhibited apoptosis [82].
Adjunctive Therapy
Adjunctive therapy is given to maximize the effectiveness of the treatment of COVID-19 in addition to the main treatment. TCM is an important adjunctive therapy to contain the pandemic history, which has been widely used in treating various infectious diseases such as SARS, H1N1, and Influenza A H5N1 [83-86]. TCM mitigates clinical symptoms, shortens fever and average hospitalization time, and slows down the transition from mild to severe phase [72,87]. Herbal medicines are tailored to each individual's syndromes. The quality of TCM raw materials and guidelines is critical to ensure the safety of patients [88]. However, their safety should be evaluated with caution because no peer-reviewed clinical trials of herbal drugs have been reported to date. According to TCM theory, COVID-19 is a "plague" belonging to the "epidemic of damp heat" or "epidemic of dampness toxin". Several Chinese herbal formulas have been used to combat acute infectious febrile diseases [89]. Chinese patent medicine (CPM) has the properties of portability, high bioavailability, rapid absorption, stable quality, and easy preservation [90]. CPM-Huoxiang Zhengqi capsules can improve gastrointestinal dysfunction and have an anti-inflammatory effect [91], which interferes with the SARS-CoV-2 replication. Jinhua Qinggan granule [92], Lianhua Qingwen (LH) capsule [93], and Shufeng Jiedu granule [94] can effectively relieve clinical symptoms such as fever, fatigue, cough, chest tightness, and shortness of breath. LH capsules have wide-spectrum antiviral effects and anti-inflammatory activities. The key components of the LH capsule such as Rhodiola, Honeysuckle, and Platanus can prevent SARS-CoV-2 from associating with ACE2 and eliminate lung inflammation [95]. The recovery rate of LH treatment was significantly higher (91.5% vs. 82.4%) and the recovery time from clinical symptoms was shorter in clinical trials without serious adverse reactions. Shuanghuanglian, containing honeysuckle and forsythia, is used routinely in traditional medicine to treat influenza and the common cold, which helps ward off or even cure COVID-1988. Screening potential active ingredients from traditional herbs is an alternative strategy when conventional drugs are ineffective. The miRNA MIR2911 in honeysuckle decoction can be absorbed by patients through drinking, and can effectively inhibit SARS-CoV-2 replication and accelerate negative conversion [96]. In addition to TCM, nutritional supplements and palliative care are also worthy of attention as adjunctive therapy. Nutrient deficiency prolongs hospitalization time and increases the risk of infection and other complications [97]. Critically ill patients are at higher nutritional risk in the ICU, which doubles the probability of a 28-day mortality rate than those at low nutritional risk [98]. Optimal nutrition involves careful management of glycaemic control, fluid balance, and gastrointestinal function [99]. Nutritional support facilities offer treatment and rehabilitation to avoid irreparable injury and a poor prognosis. Palliative care plays an essential role in responding to the extreme pressure caused by COVID-19 [100], which focuses on physical issues, psychological distress, spiritual distress, and social needs. Haloperidol and levomepromazine, as antipsychotic agents, manage delirium [101]. Regular physical activity is associated with a lower incidence of thromboembolism [102,103]. Palliative care supports healthcare workers to face the workplace stress and moral dilemmas created by COVID-19. Respect for the patient's preferences is the essential theme of palliative care [104]. The ideal situation is to provide each patient with life-sustaining treatment and comfortable care [105]. Family-centered care somewhat alleviates anxiety and depressive symptoms for the patient and family members after hospitalization [106].
Treatment of critically ill patients
COVID-19 primarily affected the respiratory system with some patients rapidly progressing to ARDS. Patients with severe COVID-19 respiratory failure may be accompanied by damage to the heart, kidneys, intestines, and other organs. Respiratory support is the main treatment for respiratory failure, including oxygen therapy, noninvasive ventilation, and invasive ventilation. Noninvasive positive-pressure ventilation (NIPPV) provides respiratory support and positive airway pressure by closely fitting the nasal mask to the face at all stages of the respiratory cycle with 100% FiO2. Excessive breathing of NIPPV may lead to spontaneous lung injury if patients attempt to control their breathing [107], which is associated with a higher mortality rate for patients with ARDS in the ICU [108]. Invasive ventilation utilizes intubation and mechanical ventilation for the treatment of severe respiratory failure [109]. Shortness of breath and pulmonary infiltration are determinants for intubation [110]. Mechanical ventilation, which keeps patients alive without healing the lungs until the coronavirus is defeated [111], should be avoided unless necessary [112]. High-frequency oscillatory ventilation may lead to lung injury [113]. Guidelines for lung-protective ventilation in ARDS suggest that: (1) tidal volume should be less than or equal to 6 ml/kg body weight; (2) respiratory rate should be less than or equal to 35 breaths/min; (3) plateau airway pressure should be less than or equal to 30 cm H2O; (4) positive end-expiratory pressure should be greater than or equal to 5 cm H2O [114]. In the most severe cases of ARDS, ECMO reduces mortality [115], which is a salvage treatment for patients with severe COVID-19. Oxygen is delivered and carbon dioxide is removed. The recharged blood returns to the patient through another vein or thick artery. ECMO has been successfully applied to patients with severe H1N1 influenza [116] to reduce the potential risk of ARDS, thereby rescuing ARDS patients with severe hypoxemia [117]. ECMO has been regarded as a rescue treatment for refractory hypoxemia after COVID-19 lung-protective ventilation by the WHO.
COVID-19 Vaccines
The development of effective vaccines is an unprecedented urgency for the containment of COVID-19 [118]. According to the WHO, as of April 28, 2021, there are 86 candidate vaccines in the clinical evaluation stage and 186 in the preclinical stage. Twelve vaccines have been approved by at least one country. However, the production and supply of these vaccines are still inadequate to cover the global population within a short period of time. The pressure and suspicion of vaccinal efficiency also emerge as a result of viral accumulated mutations. The vaccine platforms for COVID-19 include nucleic acid (DNA or RNA), viral vector (replicating or non-replicating), protein subunit, virus (live-attenuated or inactivated virus), and Virus-like Particles (VLPs) (Figure 4). The advantages and disadvantages of different schemes are illustrated in table 2.
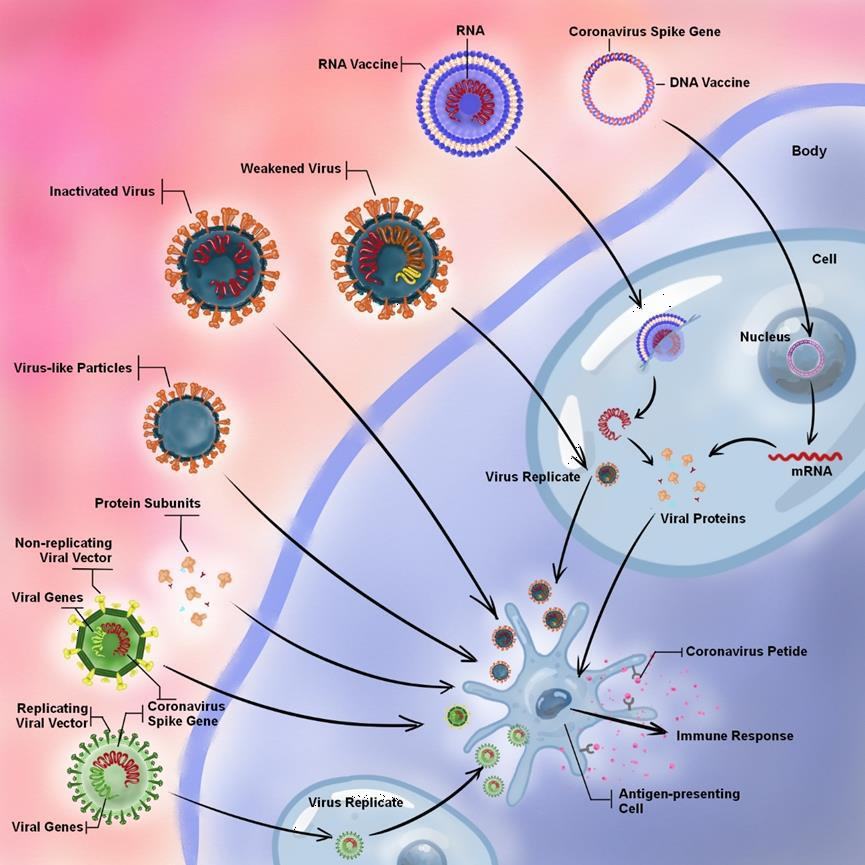
Figure 4: Types of vaccine platforms and their operating principles. The platforms include nucleic acid (DNA or RNA), viral vector (replicating or non-replicating), protein subunits, virus (weakened or inactivated virus), and Virus-like particles (VLPs). The VLPs, protein subunits, non-replicating viral vector, and inactivated virus are capable of directly stimulating the host immune response. However, virus replication in host cells is a prerequisite for replicating the viral vector and the weakened virus prior to the immune response. The nucleic acid is inserted into host cells and then produces copies of the viral protein, which stimulates the host immune response. In contrast to RNA vaccine, DNA vaccine also needs delivery device and host transcription machinery.
|
Advantages |
Disadvantages |
|
|
DNA vaccines |
No need to handle infectious virus; Appropriate immune response; Rapid manufacture; High thermal stability; ong-term stability |
No license and global using experience; Some DNA vaccines need specific delivery tools; Low risk for genomic integration in the host cell |
|
RNA vaccines |
No need to handle infectious virus; Strong early immune response; Rapid design and manufacture; No potential risk integration |
|
|
Protein Subunit vaccines |
No need to handle infectious virus; Strong immune response; Successful experience for other vaccines development; |
Need adjuvants to increase immunogenicity; Challenges for rapid production; |
|
Viral vector vaccines (replicating/non -replicating) |
No need to handle infectious virus; Extensive experience for safety and immunogenicity; Some clinical data for MERS-CoV |
The risk for immunocompromised individuals; Need to choose vector without vector immunity; The risk for oncogenesis chromosomal integration; Challenges for rapid manufacture |
|
Virus Vaccine (Live Attenuated) |
Traditional and proven platform; Several licensed human vaccines; Strong antibodies response; Simple formulation; Experience and existing infrastructure for largescale manufacturing |
High-level biosafety facilities; Risk for safety testing because of regaining virulence; Complicated and time-consuming for scale-up manufacture |
|
Virus Vaccine (Inactivated) |
Traditional and proven platform; Several licensed human vaccines; Strong antibodies response; Simple formulation; Experience and existing infrastructure for largescale manufacturing |
Handle of large amounts of infectious virus; High-level biosafety facilities; Risk for safety testing because of regaining virulence; Complicated and time-consuming for scale-up manufacture |
|
Virus Like Particles Vaccine |
No need to handle infectious virus; Successful experience for other viruses such as HPV, Influenza, and Zika; Cost-effective; Safe and no risk of infection |
Some vaccines need adjuvant; |
Table 2: Advantages and disadvantages of each vaccine platform
Nucleic Acid Vaccines
DNA- or RNA-based vaccines can be generated rapidly based on viral sequences and provide flexibility in antigen manipulation and rapid pathways to the clinic [119, 120], where nucleic acids encoding viral proteins are delivered to human cells. DNA vaccines expressing variants of the SARS-CoV-2 S protein developed humoral and cellular immune responses in animals [121]. Modern DNA vaccines have increased immunogenicity via codon optimization, the co-administration of immune-stimulatory cytokines, streamlined plasmid, and plasmid-free double-stranded DNA [122]. However, several reasons can fail the development of a DNA vaccine, such as the delivery device issue of INO-4800, which was effective in COVID-19 clinical trial I. There are 22 nucleic acids in clinical trials and two mRNA vaccines have been approved for emergency use [123]. mRNA-1273, an mRNA vaccine, encodes a SARS-CoV-2 S protein that stabilizes in the prefusion conformation and induces potent nAbs responses, as well as CD8+ T cell responses. CD8+ T cells eliminate SARS-CoV-2 by recognizing antigen-derived peptides displayed on the cell surface in combination with MHC class I molecules [124]. Volunteers produce a robust antibody response in a time- and dose-dependent manner after the first vaccine shot. The two-dose vaccine series had greater reactogenicity [125]. Phase III trial has been tested in more than 30,000 volunteers, and the final results showed vaccine efficacy of 94.1% against COVID-19 and 100% against severe COVID-19. The vaccine boosters may become an efficient way for solving the reduction of neutralization antibodies due to variants. Lipid nanoparticles act as immunogenic adjuvants to protect and deliver mRNA vaccines [126]. Another RNA vaccine-BNT62, developed by BioNTech and Pfizer, clinically shows that RBD-binding IgG concentrations and nAbs increase after two shots. The geometric mean neutralization titer was 1.9-4.6 times higher than that of a group of human COVID-19 convalescent sera at least 14 days after the PCR-positive test for SARS-CoV-2. Phase 2b/3 clinical trials demonstrated safety and effectiveness (NCT04368728), enrolling more than 43,000 participants with a 95% efficacy rate against COVID-19 beginning 28 days after the first dose. Neutralizing antibodies elicited by mRNA vaccines are capable of a fight against the B.1.1.7 variant, which contains the N501Y mutation with 53% increased transmissibility [127]. However, it showed approximately two-thirds less effective at neutralizing the B.1.351 variant [9].
Virus Vaccine
The virus vaccine contains two types of viruses, namely, inactivated vaccines and attenuated vaccines. Live attenuated vaccines are currently the most cost-effective health intervention available with a low incidence of adverse reactions, but requiring more than a single shot to achieve high seroconversion rates in humans [128]. Purified inactivated viruses are safe and effective in preventing diseases caused by viruses such as the influenza virus and poliovirus [129]. SARS-CoV-2 inactivated vaccine is made similarly to the polio vaccine by growing and killing the virus in the laboratory. Sinovac’s vaccine, CoronaVac, showed well tolerance and induced humoral responses against SARS-CoV-2 with a seroconversion rate above 90% [130], which showed varied efficacy between 50.65% and 83.5% based on trials from Brazil, Turkey, and Indonesia, and got nearly 20 countries’ approvals. Two inactivated vaccines, BBIBP-CoV and Vero cell, from Sinopharm, acquired China’s approvals. Both are tolerable and immunogenic in healthy people in phase 1/2 trial [131,132]. BBIBP-CoV has been approved in 30. Meanwhile, Vero cell had 71.25% efficiency. BBV152 is an inactivated SARS-CoV-2 vaccine approved by India, formulated with a toll-like receptor (TLR) 7/8 agonist molecule (IMDG) adsorbed to alum (Algel) [133], which neutralize the B.1.1.7 variant [134].
Protein Subunit Vaccine
The protein subunit vaccine is a novel technology and has been used in clinical applications with the generation of commercial profits, such as licensed vaccines for influenza, hepatitis, and papillomavirus. The whole coronavirus protein, shell protein, or protein fragments that stimulate the immune response are candidates for the protein subunit vaccine. As an increasing number of S glycoprotein-based vaccine candidates are being developed, their detailed glycan analysis offers a route to compare immunogenic integrity and will be important for monitoring the manufacture of serological test kits. A potent adjuvant will be necessary for protein-based vaccines to induce TH1 immune reactions. Sequential administration of multiple different pathogen-like proteins is a promising strategy to elicit nAbs through vaccination. However, it remains unclear how best to design/administer these proteins [135]. There are two protein subunit vaccines that have been approved for emergency use, including the ZF2001 and EpiVacCorona. The ZF2001 against COVID-19 by using a dimeric form of the RBD from the spike protein as the antigen. In phase 1 and 2 clinical trials, ZF2001 appeared to be well tolerated and immunogenic. The 25 μg antigen with the three-dose schedule brought 97% seroconversion rates of neutralizing antibodies and is undergoing the phase 3 trial for large-scale evaluation of ZF2001's safety and efficacy. However, its three-dose schedule is more time consuming for mass immunization [136]. EpiVacCorona is an antigens-based vaccine that relies on chemically synthesized peptide antigens of SARS-CoV-2, conjugated to a carrier protein and adsorbed on an aluminum-containing adjuvant. This vaccine contributes to the development of protective immunity following two intramuscular administrations spaced 21-28 days apart, achieving 94% efficacy in clinical trials, whose phase 3 clinical trial is ongoing.
Viral Vector Vaccines
The viral gene is encoded in targeted vectors that can produce coronavirus proteins in the host. Adenovirus (Ad), measles, and vesicular stomatitis are common viral vectors with weakened virulence to ensure safety. Viral vector vaccines include two categories: replicating and non-replicating viral vectors, and four non-replicating vaccines have been approved in several countries against COVID-19. The AZD1222 is a non-replicating Ad vector vaccine from a chimpanzee viral vector (ChAdOx1) containing full-length S, with a tissue plasminogen activator leader sequence, shares the same modality with the vaccines for Zika, tuberculosis, influenza, Chikungunya, and other viruses. Ad vector vaccine achieves humoral responses to SARS-CoV-2 S glycoprotein, as well as T-cell responses. ChAdOx1is a weakened cold adenovirus that can safely produce temporary side effects such as fever, fatigue, and injection site pain [137]. A single vaccination of ChAdOx1 can effectively prevent lung damage without immune-enhancing diseases in rhesus macaques [138], which safely induces humoral and cellular responses to SARS-CoV-2 [139]. The AZD1222 was 70% effective in preventing symptomatic COVID-19 in phase 3 trial interim results and 83 countries have approved it [140]. In addition, a two-dose regimen of the ChAdOx1 nCoV-19 vaccine did not show protection against mild-to-moderate COVID-19 caused by the B.1.351 variant [127]. However, despite the European Medicines Agency concluded the AZD1222 is safe and not associated with an increased overall risk of thromboembolic events, Germany, France, Italy, and other countries had suspended the use of the vaccine tentatively. Another non-replicating Ad vector vaccine Ad5-nCoV (Convidicea), has been approved in China, Hungary, Mexico, and other countries. The Ad5 vector vaccine platform is highly efficient with a well-established delivery system and it is safe in gene therapy and vaccination against Ebola and MERS. The Ad5-nCoV consists of the S antigen itself, combined with an immunogenic adjuvant to trigger a strong sustained response of T-cell and neutralizing antibodies. This vaccine showed tolerability and immunogenicity at 28 days post-vaccination [141]. A single dose induces cellular or humoral immune responses and has mild to moderate side effects. Phase III clinical trials are underway in Russia, Argentina, Chile, Mexico, and Pakistan. The interim analysis data of the phase III clinical trial shows that this vaccine has overall efficacy of 65.28% at preventing all symptomatic COVID-19 diseases and 90.07% at preventing severe diseases 28 days after single-dose vaccination. Russia approved the world's first public COVID-19 vaccine named Gam-COVID-Vac (Sputnik V) on August 11, 2020, which is an Ad vector vaccine. This vaccine showed a good safety profile and induced strong humoral and cellular immune responses in participants in phase 1/2 clinical trials [142]. According to interim data of the phase 3 trial, Gam-COVID-Vac showed 91.6% efficacy against COVID-19 and was well tolerated in a large cohort. There were some mild-to-moderate side effects and no major side effects [143]. More than 50 countries have approved Gam-COVID-Vac for emergency use. Attempts to improve the effectiveness and duration of protection include new formulations, scheduling options, oral vaccines, and vaccine types [144]. Ad26.COV2-S vaccine is similar to Sputnik V that consists of Ad26 vector expressing spike protein in a stabilized conformation. This vaccine experienced the pause and resume of clinical trials, and its phase 3 trial interim results reported 66% efficacy against COVID-19. However, the protection level varied with geography: effectiveness was 72% in the USA, 64% in South Africa, and 61% in Latin America, indicating different circulating SARS-CoV-2 strains and the vaccine failure risk due to SARS-CoV-2 variants. Thirty-seven countries have approved Ad26.COV2-S vaccine.
Virus-Like Particles
VLPs vaccines are in the preclinical phase, similar to viruses in some characteristics, and consist of repetitive structural proteins from infectious viruses. VLPs are not infectious in host cells because they have no genetic material [145]. These VLPs can easily reach the lymph node and spleen to activate T and B cells, which improves safety and manufacturing compared to traditional vaccines. The ideal adjuvant facilitates particle transfer and release into lymphoid organs and induces the innate immune system [146]. VLP has been validated in vaccine development for HBV, hepatitis E virus, and human papillomavirus [147], and the plant-based VLP vaccine for COVID-19 is proceeding in phase 3 clinical trials in Canada and America. The duration of vaccine-induced immunity is unclear, and waning of antibody response results in loss of immune protection. A lower immune response may be found in the older and pregnant women population, considering this population as an important target population for a COVID-19 vaccine [148]. Vaccine-enhanced diseases (VED), including antibody-dependent enhancement (ADE) [149] and vaccine-associated enhanced respiratory disease [150], could result in clinically more severe secondary infections [151], and the ineffective vaccines may exacerbate disease through ADE. Currently, most vaccines require the administration of two doses separated by 14-28 days, and the engineering of potent vaccines becomes necessary to clarify the concerns of single-dose potency and long-term immunity [152]. VED reduction results in avoiding provoking host response systems such as inflammation, complement, and coagulation in COVID-19 [153]. Global collaboration is essential for speeding up the development, manufacture, and equitable distribution of new vaccines [154]. The COVAX (the COVID-19 Vaccines Global Access Facility) is part of the Access to COVID-19 Tools (ACT) Accelerator, a global collaboration of WHO and partners. In their plan, two billion doses will be distributed by the end of 2021 and this will help vaccinate 20% of the world populations and terminate the acute phase of the pandemic.
Perspective
Compared with the SARS-CoV-1 outbreak in 2003, an unprecedented level of scientific engagement from all over the world, novel therapies are coming up in various areas contributing to the therapeutics of COVID-19. After distilling the knowledge of COVID-19 therapeutics, we propose three future research directions for COVID-19 therapeutics transferrable to other potential pandemics: applying precision therapeutics such as CRISPR, immunotherapy, or traditional Chinese medicine as adjuvant therapy, leveraging AI, multi-omics, or nanotechnology, etc. on the new drug or vaccine development, and tracking the recovery of COVID-19 patients including the mental status via a disease tracking database. The utilization of CRISPR's gene-editing capabilities was initially used to treat genetic diseases. A potential therapy based on CRISPR technology against the SARS-CoV-2 and other RNA viruses was proposed in the early phase of COVID-19 outbreak. A flexible and efficient approach, termed Prophylactic Antiviral CRISPR in huMAN cells (PAC-MAN), targets RNA in the laboratory using CRISPR/Cas13d technology to chew up the SARS-CoV-2 RNA genome precisely, hence inhibiting the virus replication [155]. This strategy has the therapeutic potential, providing options to combat viruses that are likely to evolve and develop resistance rapidly. It is still a significant challenge for human clinical trials' approval until settling a stable in vivo delivery of PAC-MAN to the target organ. The performance of TCM in the treatment of COVID-19 was very conspicuous. Various compounds from different herbs in Qingfei Paidu Tang may interfere with the COVID-19 disease process through multiple signaling pathways, such as Toll-like receptor activation and the IFN response induced by a viral infection. Integrative Chinese-Western medicine improves the clinical symptoms in patients with mild or moderate COVID-19 [156,157]. However, their safety should be evaluated with caution. In addition, the establishment of suitable animal models should greatly broaden our understanding of SARS-CoV-2 transmission and pathogenesis and accelerate the development of countermeasures against COVID-19 [158]. Nanotechnology plays a vital role in advancing COVID-19 treatment and vaccine development. Suitable nanocarriers offer the opportunity to address limitations and make therapeutics safer and more effective. Nanotechnology is a viable approach to reducing toxicity, improving bioavailability and solubility, and delivering multiple drugs to realize combination therapies. For vaccine development, nanoparticles can encapsulate or adsorb antigens and release them in a controlled manner. For example, with RNA or DNA vaccines, the naked nucleic acid will be degraded by nucleases, while nanocarriers can prevent biological inactivation, provide a controlled release and targeted cellular delivery. Surface modification of nanoparticles can also improve the stability of the vaccine and reduce systemic toxicity. Simultaneously, the use of vaccine adjuvant nanoparticles can bring additional protection to specific populations, such as the elderly, while improving the safety and effectiveness of the immune response. We cannot ignore the considerable contribution created by AI in the COVID-19, including contact tracing, auxiliary diagnosis, clinical monitoring, adjuvant treatment, and new drug development. Artificial intelligence (AI) has been a huge supplement to drug repositioning, which aided in the search for potential drug repositioning candidates in treating COVID-19 [159]. A pre-trained deep learning model for drug repurpose predicts that atazanavir has an inhibitory potency with Kd of 94.94 nM against the SARS-CoV-2 proteinase, followed by efavirenz (199.17 nM), ritonavir (204.05 nM), and dolutegravir (336.91 nM) [160]. Meanwhile, AI is a powerful tool for treatment optimization and vaccine development based on the epidemic spread, pandemic sequelae, and virus mutation. Harnessing machine learning to study the mechanism of drug interactions is helpful to develop the antiviral drug. A more comprehensive circRNA-miRNA-mRNA targeting network analysis will help identify infection through RNA-mediated host-virus interaction and ascertain new antiviral agents' targets. The prediction of RNA secondary structure may help reveal druggable targets. The untargeted metabolomics platform can further understand the pathomechanism by identifying correlations between metabolites related to COVID-19. Besides, the mathematical model that integrates virology and immunology can improve understanding of the immune-pathogenesis of SARS-CoV-2 and help optimize antiviral evaluation in future outbreaks [161]. Overcoming the COVID-19 is a protracted battle. Even if the patients have overcome the COVID-19 infections, there are still other sequelae, whether it is physical or psychological. The multidisciplinary and collaborative methods that combine respiratory medicine with sports science, engineering, and digital technology will help develop more effective support and therapeutics [162]. Remote monitoring and treatment reduce the possibility of cross-infection by guiding patients with mild or asymptomatic infections and triages chronic patients to ensure that adequate medical resources are available for patients with life-threatening conditions. Remote monitoring provides the feasibility of remote clinical trials. Besides, remote psychological care has become the main measure for treating patients' psychological problems and the general population under the COVID-19 pandemic. Virtual reality (VR) simulates physical locations in palliative care [163], which can bring remote palliative and psychological care and comfort to patients and their families in real time [164]. VR is the auxiliary equipment for disease therapeutics and doctor/patient training. VR provides safe training opportunities for doctors and nurses to effectively improve the success rate and reduce infectious risks in intubation and ECMO operation. By projecting critical structures such as major vessels, nerves, or other vital tissues directly onto the patient, AR can improve safety and reduce the time required to complete the procedure [165]. With the AR platform's assistance, telesurgical technologies can help safeguard both patients and surgical teams against viral transmission by reducing the number of healthcare professionals. Multiple therapeutics are available for COVID-19. However, therapeutics that can effectively clear the virus and terminate transmission are not available, and supportive therapeutics is the main clinical approach to COVID-19. Large-scale cohort clinical 3 trials were expedited by the international collaboration to evaluate its therapeutics and safety. Further investment and research into the mechanism of viral pathogenesis, the collective effects of multiple treatments, and complications will provide insight and guidance for the development of COVID-19 therapeutics.
Author Contributions
All authors are involved in discussion and writing of the manuscript. All authors have read and agreed to the published version of the manuscript. † These authors contributed equally to this work.
Funding Sources
This work is supported by National Natural Science Foundation of China (31970752), Science, Technology, and Innovation Commission of Shenzhen Municipality (JCYJ20190809180003689, JSGG20200225150707332, JSGG20191129110812), and Shenzhen Laboratory Open Funding (SZBL2020090501004).
Declaration of interests
The authors declare no conflict of interest.
References
- Zhou M, Zhang X, Qu J, et al. Coronavirus disease 2019 (COVID-19): a clinical update. Front Med 14 (2020): 126-135.
- Su S, Wong G, Shi W, et al. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol 24 (2016): 490-502.
- Cao YC, Deng QX, Dai SX. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: An evaluation of the evidence. Travel Med Infect Dis 35 (2020): 101647.
- Zha L, Li S, Pan L, et al. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19). Med J Aust 212 (2020): 416-420.
- Guan W, Ni Z, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China 382 (2020): 1708-1720.
- Sirima SB, Richert L, Chene A, et al. PRIMVAC vaccine adjuvanted with Alhydrogel or GLA-SE to prevent placental malaria: a first-in-human, randomised, double-blind, placebo-controlled study. Lancet Infect 20 (2020): 585-597.
- Islam MR, Hoque MN, Rahman MS, et al. Genome-wide analysis of SARS-CoV-2 virus strains circulating worldwide implicates heterogeneity. Sci Rep 10 (2020): 14004.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 383 (2020): 2603-2615.
- Garcia-Beltran WF, Lam EC, St. Denis K, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021.
- Thome R, Lopes SC, Costa FT, et al. Chloroquine: modes of action of an undervalued drug. Immunol Lett 153 (2013): 50-57.
- Nakagami H, Morishita R, Yamamoto K, et al. Phosphorylation of p38 mitogen-activated protein kinase downstream of bax-caspase-3 pathway leads to cell death induced by high D-glucose in human endothelial cells. Diabetes 50 (2001): 1472-1481.
- Tilangi P, Desai D, Khan A, et al. Hydroxychloroquine prophylaxis for high-risk COVID-19 contacts in India: a prudent approach. Lancet Infect Dis 20 (2020): 1119-1120.
- Funck-Brentano C, Nguyen LS, Salem JE, et al. Retraction and republication: cardiac toxicity of hydroxychloroquine in COVID-19. Lancet 396 (2020): e2-e3.
- Maisonnasse P, Guedj J, Contreras V, et al. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature 585 (2020): 584-587.
- Hashem AM, Alghamdi BS, Algaissi A, et al. Therapeutic use of chloroquine and hydroxychloroquine in COVID-19 and other viral infections: A narrative review. Travel Med Infect Dis 35 (2020): 101735.
- Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 370 (2020): 1227-1230.
- Chan KK, Dorosky D, Sharma P, et al. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 369 (2020): 1261-1265.
- Clausen TM, Sandoval DR, Spliid CB, et al. SARS-CoV-2 Infection depends on cellular heparan sulfate and ACE2. Cell 183 (2020): 1043-1057 e15.
- Low ZY, Farouk IA, Lal SK, Drug Repositioning: New Approaches and Future Prospects for Life- Debilitating Diseases and the COVID-19 Pandemic Outbreak. Viruses 12 (2020).
- Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 395 (2020): e30-e31.
- Favalli EG, Biggioggero M, Maioli G, et al. Baricitinib for COVID-19: a suitable treatment? Lancet Infect Dis 20 (2020): 1012-1013.
- Bronte V, Ugel S, Tinazzi E, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. The Journal of Clinical Investigation 130 (2020).
- Toelzer C, Gupta K, Yadav SKN et al. Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science 370 (2020): 725-730.
- Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370 (2020): 856-860.
- Kielian M. Enhancing host cell infection by SARS-CoV-2. Science 370 (2020): 765-766.
- Baron SA, Devaux C, Colson P, et al. Teicoplanin: an alternative drug for the treatment of COVID-19? Int J Antimicrob Agents 55 (2020): 105944.
- Jo S, Kim, S, Kim DY, et al. Flavonoids with inhibitory activity against SARS-CoV-2 3CLpro. J Enzyme Inhib Med Chem 35 (2020): 1539-1544.
- Simsek Yavuz S, Ünal S. Antiviral treatment of COVID-19. Turk J Med Sci 50 (2020): 611-619.
- Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 59 (2004): 252-256.
- Maciorowski D, Idrissi SZE, Gupta Y, et al. A Review of the Preclinical and Clinical Efficacy of Remdesivir, Hydroxychloroquine, and Lopinavir-Ritonavir Treatments against COVID-19. SLAS Discov 25 (2020): 1108-1122.
- Faheem BK, Sekhar K, Kunjiappan S, et al. Druggable targets of SARS-CoV-2 and treatment opportunities for COVID-19. Bioorg Chem 104 (2020): 104269.
- Boras B, Jones RM, Anson BJ, et al. Discovery of a Novel Inhibitor of Coronavirus 3CL Protease as a clinical candidate for the potential treatment of COVID-19. BioRxiv (2020).
- Choudhry N, Zhao X, Xu D, et al. Chinese therapeutic strategy for fighting COVID-19 and potential small-molecule inhibitors against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). J Med Chem 63 (2020): 13205-13227.
- Klemm T, Ebert G, Calleja DJ, et al. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. EMBO J 39 (2020): e106275.
- Sheahan TP, Sims AC, Zhou S, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med 12 (2020).
- Yin W, Mao C, Luan X et al. Inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 368 (2020): 1499-1504.
- Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 34 (2020): 327-331.
- Tchesnokov EP, Gordon CJ, Woolner E, et al. Template-dependent inhibition of coronavirus RNA-dependent RNA polymerase by remdesivir reveals a second mechanism of action. J Biol Chem 295 (2020): 16156-16165.
- McCreary EK, Angus DC. Efficacy of Remdesivir in COVID-19. JAMA 324 (2020): 1041-1042.
- Pujari R, Thommana MV, Ruiz MB, et al. Therapeutic options for COVID-19: A Review. Cureus 12 (2020): e10480.
- Sahu B, Behera SK, Das R, et al. Design and in-silico screening of Peptide Nucleic Acid (PNA) inspired novel pronucleotide scaffolds targeting COVID-19. Curr Comput Aided Drug Des (2020).
- Arunachalam PS, Wimmers F, Mok CKP, et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 369 (2020): 1210-1220.
- McGowan EM, Haddadi N, Nassif NT, et al. Targeting the SphK-S1P-SIPR Pathway as a potential therapeutic approach for COVID-19. Int J Mol Sci 21 (2020).
- Wagstaff KM, Sivakumaran H, Heaton SM, et al. Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J 443 (2012): 851-6.
- Caly L, Druce J, Catton MG, et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 178 (2020): 104787.
- Perisic O. Recognition of Potential COVID-19 drug treatments through the study of existing protein-drug and protein-protein structures: An analysis of kinetically active residues. Biomolecules 10 (2020).
- George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Resp Med 8 (2020): 807-815.
- Habel JR, Nguyen THO, Van de Sandt CE, et al. Suboptimal SARS-CoV-2-specific CD8(+) T cell response associated with the prominent HLA-A*02:01 phenotype. Proc Natl Acad Sci 117 (2020): 24384-24391.
- Garraud O, Heshmati F, Pozzetto B, et al. Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow. Transfus Clin Biol 23 (2016): 39-44.
- Zhou D, Duyvesteyn HME, Chen CP, et al. Structural basis for the neutralization of SARS-CoV-2 by an antibody from a convalescent patient. Nat Struct Mol Biol 27 (2020): 950-958.
- Yeh K M, Chiueh T S, Siu L K, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother 56 (2005): 919-922.
- Arabi Y M, Hajeer A H, Luke T, et al. Feasibility of Using Convalescent Plasma Immunotherapy for MERS-CoV Infection, Saudi Arabia. Emerg Infect Dis 22 (2016): 1554-1561.
- Hun I F, To K K, Lee C K, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis 52 (2011): 447-456.
- Kraft C S, Hewlett A L, Koepsell S, et al. The Emory Serious Communicable Diseases, the use of TKM-100802 and Convalescent Plasma in 2 Patients With Ebola Virus Disease in the United States. Clin Infect Dis 61 (2015): 496-502.
- Syal K. COVID-19: Herd immunity and convalescent plasma transfer therapy. J Med Virol (2020).
- Hansen J, Baum A, Pascal K E, et al, Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 369 (2020): 1010-1014.
- Wibmer C K, Ayres F, Hermanus T, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma Nat Med (2021).
- Meng J, Xiao G, Zhang J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect 9 (2020): 757-760.
- Market M, Angka L, Martel A Bet al. Flattening the COVID-19 Curve With Natural Killer Cell Based Immunotherapies. Front Immunol 11 (2020): 1512.
- Slater H. FDA Accepts IND for NK Cell Therapy CYNK-001 to Treat Patients with COVID-19 (2020).
- Mostafa H H, Vogel P, Srinivasan A, et al Dynamics of Sendai Virus Spread, Clearance, and Immunotherapeutic Efficacy after Hematopoietic Cell Transplant Imaged Noninvasively in Mice. J Virol 92 (2018).
- Weiss A R R, Dahlke M H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front Immunol 10 (2019): 1191.
- Kavianpour M, Saleh M, Verdi J. The role of mesenchymal stromal cells in immune modulation of COVID-19: focus on cytokine storm. Stem Cell Res Ther 11 (2020): 404.
- Mallis P, Michalopoulos E, Chatzistamatiou T, et al. Mesenchymal stromal cells as potential immunomodulatory players in severe acute respiratory distress syndrome induced by SARS-CoV-2 infection. World J Stem Cells 12 (2020): 731-751.
- Khorshidi M, Zarezadeh M, Emami M, et al. Promising impacts of mesenchymal stem cell therapy in treatment of SARS-CoV-2 (COVID-19). Heart Lung 49 (2020): 745-748.
- Liu C, Xu X, Han L, et al. LRCH1 deficiency enhances LAT signalosome formation and CD8(+) T cell responses against tumors and pathogens. Proc Natl Acad Sci U S A 117 (2020): 19388-19398.
- Cauchois R, Koubi M, Delarbre D, et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci U S A 117 (2020): 18951-18953.
- Yalcin, A. D.; Yalcin, A. N., Future perspective: biologic agents in patients with severe COVID-19. Immunopharmacol Immunotoxicol (2020): 1-7.
- Bakare L S, Allen J M. COVID-19 Therapeutics: Making Sense of It All. AACN Adv Crit Care 31 (2020): 239-249.
- Kang S, Tanaka T, Inoue H, et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci U S A 117 (2020): 22351-22356.
- Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 117 (2020): 10970-10975.
- Gou X, Yuan J, Wang H, et al. IL-6 During Influenza-Streptococcus pneumoniae Co-Infected Pneumonia- A Protector. Frontiers in Immunology 10 (2020).
- Grajales-Reyes G E, Colonna M. Interferon responses in viral pneumonias. Science 369 (2020): 626-627.
- Major J, Crotta S, Llorian M, et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science 369 (2020): 712-717.
- Lammers T, Sofias A M, van der Meel R,et al. Dexamethasone nanomedicines for COVID-19. Nat Nanotechnol 15 (2020): 622-624.
- Sheianov M V, Udalov Y D, Ochkin S S, et al. Pulse Therapy With Corticosteroids and Intravenous Immunoglobulin in the Management of Severe Tocilizumab-Resistant COVID-19: A Report of Three Clinical Cases. Cureus 12 (2020): e9038.
- Cain D W, Cidlowski J A. After 62 years of regulating immunity, dexamethasone meets COVID-19. Nat Rev Immunol 20 (2020): 587-588.
- Ni Y N, Chen G, Sun J, et al. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care 23 (2019): 99.
- Doggrell S A. Remdesivir, a remedy or a ripple in severe COVID-19?. Expert Opin Investig Drugs 29 (2020): 1195-1198.
- Holter J C, Pischke S E, de Boer E, et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci U S A 117 (2020): 25018-25025.
- Bahrampour Juybari K, Pourhanifeh M H, Hosseinzadeh A, et al. Melatonin potentials against viral infections including COVID-19: Current evidence and new findings. Virus Res 287 (2020): 198108.
- Wang C, Cao B, Liu Q Q, et al. Oseltamivir compared with the Chinese traditional therapy maxingshigan-yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann Intern Med 155 (2011): 217-225.
- Wang J, Xiong X. Current situation and perspectives of clinical study in integrative medicine in china. Evid Based Complement Alternat Med (2012): 268542.
- Organization W H. SARS: Clinical Trials on Treatment Using a Combination of Traditional Chinese Medicine and Western Medicine (2020).
- Zhou Z, Li X, Liu J, et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res 25 (2015): 39-49.
- Zhang D, Zhang B, Lv J T, et al. The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence. Pharmacol Res 157 (2020): 104882.
- Yang Y C. Use of herbal drugs to treat COVID-19 should be with caution. Lancet 395 (2020): 1689-1690.
- Xingjiang X, Chun-Tao C, Francesca B, et al. Evidence-Based TAM Classic Herbal Formula: From Myth to Science (2017): 1-3.
- Kang T, Dou D, Xu L. Establishment of a quality marker (Q-marker) system for Chinese herbal medicines using burdock as an example. Phytomedicine 54 (2019): 339-346.
- He Y H, Zhao H Y, Liu Z L, et al. Effects of huoxiangzhengqi liquid on enteric mucosal immune responses in mice with Bacillus dysenteriae and Salmonella typhimurium induced diarrhea. World J Gastroenterol 12 (2006): 7346-7349.
- Can D. Clinical observation of novel coronavirus infected pneumonia treated by Jinhua Qinggan Granule. Journal of Traditional Chinese Medicine (2020).
- Xin L R W W L. 63 suspected cases with Novel coronavirus pneumonia treated with Lianhua Qingwen Granules: a clinical observation. Journal of Traditional Chinese Medicine (2020).
- Q X. Clinical value analysis of Shufeng Jiedu Capsule combined with abidol in treating mild cases of novel coronavirus pneumonia. Journal of Emergency in Traditional Chinese Medicine (2020).
- Niu M W R, Wang Z. Rapid screening model and application of anti-new coronavirus TCM prescription based on clinical experience and molecular docking technology. China Journal of Chinese Materia Medical (2020).
- Zhou L K Z Z, Jiang X M, Zheng Y, et al Absorbed plant MIR2911 in honeysuckle decoction inhibits SARS-CoV-2 replication and accelerates the negative conversion of infected patients. Cell Discov 6 (2020): 54.
- Alberda C, Gramlich L, Jones N, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intens Care Med 35 (2009): 1728-1737.
- Zhang P, He Z, Yu G, et al. The modified NUTRIC score can be used for nutritional risk assessment as well as prognosis prediction in critically ill COVID-19 patients. Clin Nutr (2020).
- Chapple L S, Fetterplace K, Asrani V, et al. Nutrition management for critically and acutely unwell hospitalised patients with coronavirus disease 2019 (COVID-19) in Australia and New Zealand. Nutr Diet 77 (2020): 426-436.
- Williams N K, Bamert R S, Patel O, et al Dissecting Specificity in the Janus Kinases: The Structures of JAK-Specific Inhibitors Complexed to the JAK1 and JAK2 Protein Tyrosine Kinase Domains. J Mol Biol 387 (2009): 219-232.
- Praveen D, Puvvada R C, MV A. Janus kinase inhibitor baricitinib is not an ideal option for management of COVID-19. Int J Antimicrob Agents 55 (2020): 105967.
- Lim S, Bae J H, Kwon H S, et al. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol 17 (2021): 11-30.
- Ritchey K C, Foy A, McArdel E, et al. Reinventing Palliative Care Delivery in the Era of COVID-19: How Telemedicine Can Support End of Life Care. Am J Hosp Palliat Care 37 (2020): 992-997.
- Usher K, Bhullar N, Jackson D. Life in the pandemic: Social isolation and mental health. J Clin Nurs 29 (2020): 2756-2757.
- Papadimos T J, Marcolini E G, Hadian, M, et al. Ethics of Outbreaks Position Statement. Part 2: Family-Centered Care. Crit Care Med 46 (2018): 1856-1860.
- Watanabe M. The COVID-19 Pandemic in Japan. Surg Today 50 (2020): 787-793.
- Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med 195 (2017): 438-442.
- Bellani G, Laffey J G, Pham T, et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome. Insights from the LUNG SAFE Study. Am J Respir Crit Care Med 195 (2017): 67-77.
- Yuan X, Mu J S, Mo G X, et al. Respiratory support for severe 2019-nCoV pneumonia suffering from acute respiratory failure: time and strategy 43 (2020): 177-180.
- Henderson W R, Chen L, Amato M B P, et al. Fifty Years of Research in ARDS. Respiratory Mechanics in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 196 (2017): 822-833.
- Tobin M J. Basing Respiratory Management of COVID-19 on Physiological Principles. Am J Respir Crit Care Med 201 (2020): 1319-1320.
- Tobin M J. Advances in mechanical ventilation. N Engl J Med 344 (2001): 1986-1996.
- Ramsey C D, Funk D, Miller R R, et al. Ventilator management for hypoxemic respiratory failure attributable to H1N1 novel swine origin influenza virus. Crit Care Med 38 (2010): e58-e65.
- Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev (2013): CD003844.
- Goligher E C, Tomlinson G, Hajage D, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome and Posterior Probability of Mortality Benefit in a Post Hoc Bayesian Analysis of a Randomized Clinical Trial. Jama 320 (2018): 2251-2259.
- Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 302 (2009): 1888-1895.
- Aretha D, Fligou F, Kiekkas P, et al. Extracorporeal Life Support: The Next Step in Moderate to Severe ARDS-A Review and Meta-Analysis of the Literature. Biomed Res Int 2019 (2019): 1035730.
- Krause P, Fleming T R, Longini I, et al. World Health Organization Solidarity Vaccines Trial Expert, G., COVID-19 vaccine trials should seek worthwhile efficacy. Lancet 396 (2020): 741-743.
- Dowd K A, Ko S Y, Morabito K M, et al. Rapid development of a DNA vaccine for Zika virus. Science 354 (2016): 237-240.
- Pardi N, Hogan M J, Pelc R S, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543 (2017): 248-251.
- Yu J, Tostanoski L H, Peter L, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 369 (2020): 806-811.
- Gaudinski MR, Houser KV, Morabito K M, et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. The Lancet 391 (2018): 552-562.
- Cohen J. Vaccine designers take first shots at COVID-19. Science 368 (2020): 14-16.
- Cosma G L, Lobby J L, Fay E J, et al. Kinetically distinct processing pathways diversify the CD8(+) T cell response to a single viral epitope. Proc Natl Acad Sci U S A 117 (2020): 19399-19407.
- Anderson EJ, Rouphael N G, Widge A T, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med (2020).
- Suk J S, Xu Q, Kim N, et al. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 99 (2016) 28-51.
- Madhi S A, Baillie V, Cutland C L, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. New England Journal of Medicine (2021).
- Mura M, Tournier J N. Chikungunya vaccine: a single shot for a long protection?. Lancet Infect Dis 20 (2020): 1111-1112.
- Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A 117 (2020): 9490-9496.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 21 (2021): 181-192.
- Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis 21 (2021): 39-51.
- Xia S, Duan K, Zhang Y, et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA 324 (2020): 951-960.
- Ella R, Vadrevu K M, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. The Lancet Infectious Diseases (2021).
- Lam C, Yi D, Guo M, et al. Automated Detection of Diabetic Retinopathy using Deep Learning. AMIA Jt Summits Transl Sci Proc (2018): 147-155.
- Sprenger K G, Louveau J E, Murugan P M, et al. Optimizing immunization protocols to elicit broadly neutralizing antibodies. Proc Natl Acad Sci U S A (2020): 20077-20087.
- Yang S, Li Y, Dai L, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis (2021).
- Folegatti P M, Bittaye M, Flaxman A, et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis 20 (2020): 816-826.
- Van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature (2020).
- Folegatti PM, Ewer K J, Aley P K, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396 (2020): 467-478.
- Voysey M, Clemens S A C, Madhi S A, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet (2021): 99-111.
- Zhu F C, Li Y H, Guan X H, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 395 (2020): 1845-1854.
- Logunov D Y, Dolzhikova I V, Zubkova O V, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 396 (2020): 887-897.
- Logunov D Y, Dolzhikova I V, Shcheblyakov D V, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397 (2021): 671-681.
- Bines J E, Kotloff K L. Next-generation rotavirus vaccines: important progress but work still to be done. Lancet Infect Dis 20 (2020): 762-764.
- Mohsen M O, Speiser D E, Knuth A, et al. Virus-like particles for vaccination against cancer. Wiley Interdiscip Rev Nanomed Nanobiotechnol 12 (2020): e1579.
- Mohsen M O, Augusto G, Bachmann M F. The 3Ds in virus-like particle based-vaccines: "Design, Delivery and Dynamics". Immunol Rev 296 (2020): 155-168.
- Mohsen M O, Zha L, Cabral-Miranda G, et al. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin Immunol 34 (2017): 123-132.
- Dashraath P, Nielsen-Saines K, Madhi S A, et al. COVID-19 vaccines and neglected pregnancy. Lancet 396 (2020): E22-E22.
- Mascola J R, Mathieson B J, Zack P M, et al. Summary report: workshop on the potential risks of antibody-dependent enhancement in human HIV vaccine trials. AIDS Res Hum Retroviruses 9 (1993): 1175-1184.
- Polack F P. Atypical measles and enhanced respiratory syncytial virus disease (ERD) made simple. Pediatr Res 62 (2007): 111-115.
- Saad-Roy C M, Wagner C E, Baker R E, et al. Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years. Science (2020).
- Tan X, Letendre J H, Collins J J, et al. Synthetic biology in the clinic: engineering vaccines, diagnostics, and therapeutics. Cell 184 (2021): 881-898.
- Sambhara S, McElhaney J E. Immunosenescence and influenza vaccine efficacy. Vaccines for Pandemic Influenza 333 (2009): 413-429.
- Krammer F. SARS-CoV-2 vaccines in development. Nature 586 (2020): 516-527.
- Abbott T R, Dhamdhere G, Liu Y, et al. Development of CRISPR as an Antiviral Strategy to Combat SARS-CoV-2 and Influenza. Cell 181 (2020): 865-876.e12.
- Wang M, Wu T, Zuo Z, et al. Evaluation of current medical approaches for COVID-19: a systematic review and meta-analysis. BMJ Support Palliat Care (2020).
- Chan K W W V T, Tang S C W. COVID-19: An Update on the Epidemiological, Clinical, Preventive and Therapeutic Evidence and Guidelines of Integrative Chinese-Western Medicine for the Management of 2019 Novel Coronavirus Disease. Am J Chin Med 48 (2020): 737-762.
- Gu H, Chen Q, Yang G, et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science 369 (2020): 1603-1607.
- Young B E, Fong S W, Chan Y H, et al. Effects of a major deletion in the SARS-CoV-2 genome on the severity of infection and the inflammatory response: an observational cohort study. Lancet 396 (2020): 603-611.
- Beck B R, Shin B, Choi Y, et al. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput Struct Biotechnol J 18 (2020): 784-790.
- Madelain V, Baize S, Jacquot F, et al. Ebola viral dynamics in nonhuman primates provides insights into virus immuno-pathogenesis and antiviral strategies. Nat Commun 9 (2018): 4013.
- Van Dorn A. COVID-19 and readjusting clinical trials. Lancet 396 (2020): 523-524.
- Roland K, Markus M. COVID-19 pandemic: palliative care for elderly and frail patients at home and in residential and nursing homes. Swiss Med Wkly (2020).
- Ferguson L, Barham D. Palliative Care Pandemic Pack: A Specialist Palliative Care Service Response to Planning the COVID-19 Pandemic. J Pain Symptom Manage 60 (2020): e18-e20.
- Vavra P, Roman J, Zonca P, et al. Recent Development of Augmented Reality in Surgery: A Review. J Healthc Eng 2017 (2017): 4574172.
- AlMazeedi S M, AlHasan A, AlSherif O M, et al. Employing augmented reality telesurgery for COVID-19 positive surgical patients. Br J Surg 107 (2020): e386-e387.
