Ketamine in a Patient with Comorbid Anorexia and MDD
Article Information
Esther Dechant, Brian Boyle, Rachel A Ross*
Department of Psychiatry, McLean Hospital, Harvard Medical School, Belmont, United States
*Corresponding author: Rachel A Ross, Department of Psychiatry, McLean Hospital, Harvard Medical School, 115 Mill St Mailman Research Building - mailstop 339, Belmont, MA 02478, United States
Received: 30 July 2020; Accepted: 12 August 2020; Published: 09 September 2020
Citation:
Esther Dechant, Brian Boyle, Rachel A Ross. Ketamine in a Patient with Comorbid Anorexia and MDD. Journal of Women’s Health and Development 3 (2020): 373-375.
Share at FacebookKeywords
Anorexia nervosa, Ketamine, Psychopharmacology
Anorexia nervosa articles Anorexia nervosa Research articles Anorexia nervosa review articles Anorexia nervosa PubMed articles Anorexia nervosa PubMed Central articles Anorexia nervosa 2023 articles Anorexia nervosa 2024 articles Anorexia nervosa Scopus articles Anorexia nervosa impact factor journals Anorexia nervosa Scopus journals Anorexia nervosa PubMed journals Anorexia nervosa medical journals Anorexia nervosa free journals Anorexia nervosa best journals Anorexia nervosa top journals Anorexia nervosa free medical journals Anorexia nervosa famous journals Anorexia nervosa Google Scholar indexed journals Ketamine articles Ketamine Research articles Ketamine review articles Ketamine PubMed articles Ketamine PubMed Central articles Ketamine 2023 articles Ketamine 2024 articles Ketamine Scopus articles Ketamine impact factor journals Ketamine Scopus journals Ketamine PubMed journals Ketamine medical journals Ketamine free journals Ketamine best journals Ketamine top journals Ketamine free medical journals Ketamine famous journals Ketamine Google Scholar indexed journals Psychopharmacology articles Psychopharmacology Research articles Psychopharmacology review articles Psychopharmacology PubMed articles Psychopharmacology PubMed Central articles Psychopharmacology 2023 articles Psychopharmacology 2024 articles Psychopharmacology Scopus articles Psychopharmacology impact factor journals Psychopharmacology Scopus journals Psychopharmacology PubMed journals Psychopharmacology medical journals Psychopharmacology free journals Psychopharmacology best journals Psychopharmacology top journals Psychopharmacology free medical journals Psychopharmacology famous journals Psychopharmacology Google Scholar indexed journals psychological articles psychological Research articles psychological review articles psychological PubMed articles psychological PubMed Central articles psychological 2023 articles psychological 2024 articles psychological Scopus articles psychological impact factor journals psychological Scopus journals psychological PubMed journals psychological medical journals psychological free journals psychological best journals psychological top journals psychological free medical journals psychological famous journals psychological Google Scholar indexed journals co-morbid articles co-morbid Research articles co-morbid review articles co-morbid PubMed articles co-morbid PubMed Central articles co-morbid 2023 articles co-morbid 2024 articles co-morbid Scopus articles co-morbid impact factor journals co-morbid Scopus journals co-morbid PubMed journals co-morbid medical journals co-morbid free journals co-morbid best journals co-morbid top journals co-morbid free medical journals co-morbid famous journals co-morbid Google Scholar indexed journals clinical report articles clinical report Research articles clinical report review articles clinical report PubMed articles clinical report PubMed Central articles clinical report 2023 articles clinical report 2024 articles clinical report Scopus articles clinical report impact factor journals clinical report Scopus journals clinical report PubMed journals clinical report medical journals clinical report free journals clinical report best journals clinical report top journals clinical report free medical journals clinical report famous journals clinical report Google Scholar indexed journals Death articles Death Research articles Death review articles Death PubMed articles Death PubMed Central articles Death 2023 articles Death 2024 articles Death Scopus articles Death impact factor journals Death Scopus journals Death PubMed journals Death medical journals Death free journals Death best journals Death top journals Death free medical journals Death famous journals Death Google Scholar indexed journals Anorexia Nervosa articles Anorexia Nervosa Research articles Anorexia Nervosa review articles Anorexia Nervosa PubMed articles Anorexia Nervosa PubMed Central articles Anorexia Nervosa 2023 articles Anorexia Nervosa 2024 articles Anorexia Nervosa Scopus articles Anorexia Nervosa impact factor journals Anorexia Nervosa Scopus journals Anorexia Nervosa PubMed journals Anorexia Nervosa medical journals Anorexia Nervosa free journals Anorexia Nervosa best journals Anorexia Nervosa top journals Anorexia Nervosa free medical journals Anorexia Nervosa famous journals Anorexia Nervosa Google Scholar indexed journals psychiatric illness articles psychiatric illness Research articles psychiatric illness review articles psychiatric illness PubMed articles psychiatric illness PubMed Central articles psychiatric illness 2023 articles psychiatric illness 2024 articles psychiatric illness Scopus articles psychiatric illness impact factor journals psychiatric illness Scopus journals psychiatric illness PubMed journals psychiatric illness medical journals psychiatric illness free journals psychiatric illness best journals psychiatric illness top journals psychiatric illness free medical journals psychiatric illness famous journals psychiatric illness Google Scholar indexed journals psychosocial articles psychosocial Research articles psychosocial review articles psychosocial PubMed articles psychosocial PubMed Central articles psychosocial 2023 articles psychosocial 2024 articles psychosocial Scopus articles psychosocial impact factor journals psychosocial Scopus journals psychosocial PubMed journals psychosocial medical journals psychosocial free journals psychosocial best journals psychosocial top journals psychosocial free medical journals psychosocial famous journals psychosocial Google Scholar indexed journals
Article Details
1. Case Report
MDD (Major Depressive Disorder) is common in patients with Anorexia Nervosa (AN); an estimated 39.1% to 49.5% of patients with AN have a lifetime comorbid MDD. However, standardized mortality ratio for AN is higher than any other psychiatric illness, and suicide is the second leading cause of death [1]. Depression is complicated in patients with AN due to the deleterious impact of AN on social, academic, psychological and emotional functioning. Furthermore, the physical effects of malnutrition mimic or exacerbate depression. In addition, patients with AN often have trauma, SUD, anxiety disorders, and psychosocial stresses that complicate their cases and worsen their moods [2, 3]. Because of these common co-morbidities, treating AN goes beyond weight restoration and re-establishing regular eating, and maintenance of recovery requires treatment of the psychological symptoms of AN and comorbid conditions that persist after weight restoration. Multiple randomized controlled trials have established that ketamine can quickly and effectively treat MDD and suicidality in adults, but none have looked at its use in patients with co-morbid eating disorders [4].
Here we present a case of a 29 year old patient with a history of AN since adolescence, with co-morbid MDD, with borderline and narcissistic personality features, and high risk for suicide, who showed initial but not sustained improvement in response to treatment with ketamine. The patient’s eating disorder began in adolescence in the setting family stress and loss. Initially she restricted intake and lost weight rapidly due to excessive physical activity. These symptoms worsened further in the setting of an emotionally abusive relationship in young adulthood. She reported symptoms of self-hatred and feelings of worthlessness, though she declined symptoms of PTSD. She acknowledged food restriction as a method of self-soothing. The patient was hospitalized three times for AN within the two years prior to this presentation, each time with improved symptoms and weight restoration, but with minimal time to relapse after discharge. She was diagnosed with MDD and was hospitalized for a fourth time after a suicide attempt. She had multiple full medication trials with SSRIs, atypical antidepressants, augmentation with second generation antipsychotics, and full dose lithium + lamotrigine with minimal effect on mood. She received bilateral ECT which was helpful while hospitalized, but not on maintenance. She presented to Klarman Eating Disorders Center at McLean Hospital in the summer of 2019 after an initial course of ketamine, through which her depression and SI persisted, as did her disordered eating behavior.
The patient’s BMI on admission was 16.9 (47.5kg), so we postponed ketamine treatment in favor of improving malnutrition. All laboratory values (BMP, CBC, thyroid hormone, amylase, LFT) were normal. Residential treatment for three weeks focused on improving sleep, weight restoration, normalizing eating, stopping compulsive exercise, and intensive trauma informed therapy (individual, family, group, art) to target low self-esteem and coping. Lithium at 600 mg was added to Abilify 5 mg, Cymbalta 60 mg, Trazodone 50 mg with no improvement in mood or suicidality. Lamictal 400mg daily was discontinued. Upon reaching a BMI of 18.3 (54.3kg), the patient began ketamine treatment. She received nine treatments consisting of racemic ketamine IV dosed at 0.5mg/kg actual body weight, infused over 40 minutes. The first eight treatments occurred twice weekly; the ninth treatment occurred one full week following the eighth. The patient’s response was demonstrated by subjective report during clinical visit interviews, as well as scores on validated self-reported instruments that included the 16-item Quick Inventory of Depressive Symptomatology (QIDS) [5] and 24-item Behavior and Symptom Identification Scale (Basis-24) [6], all obtained on the day of treatment, prior to infusion. After treatment 3 (BW 54.1kg), the patient reported no active thoughts of suicidality, but continued to have passive ideation and wish to be dead.
After treatment 8 (BW 57.8kg), clinical report shows no active or passive thoughts of suicidality. The following week, there was an acute, significant external stressor, that led to a resurgence of suicidal thoughts requiring a step up from residential unit to inpatient level of care. Though this indicates a short-lived nature of the response to ketamine, the initial improvement was significant, and achieved more quickly than prior response with ECT alone. Figure 1 shows the improvement in symptoms over the course of ketamine treatment, with the most significant drop in scores occurring after three doses, concurrent with clinician notes indicating a change from active to passive suicidal thoughts. Based on this case, we suggest that ketamine can produce symptom improvement during continuous use, including decreased suicidality, in patients with comorbid AN and MDD, and we hypothesize that the treatment may be more effective after weight restoration, but it is unclear if the effect can be maintained any more than other anti-depressant treatments for patients with complex presentations of eating disorders and co-morbid MDD.
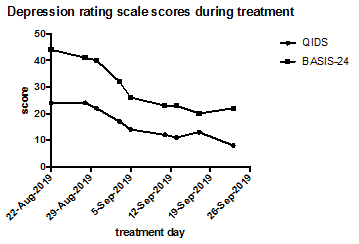
Figure 1: Depression rating scale scores for QIDS and BASIS-24 during treatment with ketamine. The patient received 9 doses of ketamine over the course of 5 weeks, and depression was seen to improve markedly during this time.
References
- Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders: A meta-analysis of 36 studies. Arch Gen Psychiatry 68 (2011): 724-731.
- Hudson JI, Hiripi E, Pope HG, Kessler RC. The Prevalence and Correlates of Eating Disorders in the National Comorbidity Survey Replication. Biol Psychiatry 61 (2007): 348-358.
- Udo T, Grilo CM. Psychiatric and medical correlates of DSM-5 eating disorders in a nationally representative sample of adults in the United States. Int J Eat Disord 52 (2019): 42-50.
- Newport DJ, Div M, Carpenter LL, Mcdonald WM, Potash JB, Tohen M, et al. Evidence-Based Psychiatric Treatment Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression The APA Council of Research Task Force on Novel Biomarkers and Treatments. Am J Psychiatry 172 (2015): 950-966.
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): A Psychometric Evaluation in Patients with Chronic Major Depression. Biol Psychiatry 54 (2003): 573-583.
- Eisen SV, Normand SL, Belanger AJ, Spiro A, Esch D. The revised behavior and symptom identification scale (BASIS-R): Reliability and validity. Med Care 42 (2004): 1230-1241.
