Investigation of Snoring and Obstructive Sleep Apnea Using Portable Polysomnography in Patients with Temporomandibular Disorder
Article Information
Yeon-Hee Lee1*, Q-Schick Auh1, and Eun-Jae Chung2
1Department of Orofacial Pain and Oral Medicine, Kyung Hee University Dental Hospital, Kyung Hee Medical center, Kyung Hee University, Seoul, Korea
2Otorhinolaryngology-Head & Neck Surgery, SNUCM Otorhinolaryngology-Head & Neck Surgery, Seoul National University Hospital Otorhinolaryngology-Head & Neck Surgery, Seoul, Korea
*Corresponding Author:Yeon-Hee Lee, Department of Orofacial Pain and Oral Medicine, Kyung Hee University Dental Hospital, Kyung Hee Medical center, Kyung Hee University, Seoul, Korea
Received: 21 September 2022; Accepted 26 September 2022; Published: 30 September 2022
Citation:
Yeon-Hee Lee, Q-Schick Auh, and Eun- Jae Chung. Investigation of Snoring and Obstructive Sleep Apnea Using Portable Polysomnography in Patients with Temporomandibular Disorder. Dental Research and Oral Health 5 (2022): 063-073.
Share at FacebookAbstract
Objective: To investigate snoring and obstructive sleep apnea (OSA) in patients with temporomandibular disorder (TMD) using portable polysomnography and identify sex-based differences in clinical features and sleep-related results
Methods: Seventy consecutive patients (44 female; mean age, 46.6918.18 years) with myofascial pain-associated TMD, diagnosed based on the criteria for TMD Axis I, were enrolled. Sleep quality and quantity were measured using portable polysomnography. Clinical characteristics were investigated using well-structured standardized reports on clinical signs and symptoms, questionnaires, and clinical examination by TMD specialists.
Results: Among 70 TMD patients, 50.0% had OSA and 15.7% had snoring, with no sex-based differences. The mean Mallampati scores for OSA prediction (2.69±1.12 vs. 1.70±0.82, p<0.001), mean body mass index (BMI) (24.94±1.78 vs. 22.02±2.24, p<0.001), and ratio of overweight patients (57.7 vs. 11.4%) with BMI ≥25 were significantly higher in males than in females (all p<0.001). Conversely, the mixed sleep apnea index was significantly higher in females than in males (0.81±0.80 vs. 0.44±0.54, p=0.022). Female sex was associated with the absence of snoring (OR=0.146, p=0.022). Based on the area under curve (AUC) value for snoring prediction, Mallampati score was the strongest predictor (AUC>0.932, p<0.001), followed by BMI, overweight, and obstructive sleep apnea index (AUC>0.8, all p<0.001).
Conclusions: Our results support the necessity of investigating sex-based differences when examining sleep problems, including snoring and OSA, in TMD patients. Mallampati scoring could be a useful tool for physical examination prior to polysomnography. Sleep and bio
Keywords
Snoring; Obstructive sleep apnea, Mallampati score, Overweight, Polysomnography, Temporomandibular disorder
Snoring articles; Obstructive sleep apnea articles; Mallampati score articles; Overweight articles; Polysomnography articles; Temporomandibular disorder articles
Snoring articles Snoring Research articles Snoring review articles Snoring PubMed articles Snoring PubMed Central articles Snoring 2023 articles Snoring 2024 articles Snoring Scopus articles Snoring impact factor journals Snoring Scopus journals Snoring PubMed journals Snoring medical journals Snoring free journals Snoring best journals Snoring top journals Snoring free medical journals Snoring famous journals Snoring Google Scholar indexed journals Obstructive sleep apnea articles Obstructive sleep apnea Research articles Obstructive sleep apnea review articles Obstructive sleep apnea PubMed articles Obstructive sleep apnea PubMed Central articles Obstructive sleep apnea 2023 articles Obstructive sleep apnea 2024 articles Obstructive sleep apnea Scopus articles Obstructive sleep apnea impact factor journals Obstructive sleep apnea Scopus journals Obstructive sleep apnea PubMed journals Obstructive sleep apnea medical journals Obstructive sleep apnea free journals Obstructive sleep apnea best journals Obstructive sleep apnea top journals Obstructive sleep apnea free medical journals Obstructive sleep apnea famous journals Obstructive sleep apnea Google Scholar indexed journals Mallampati score articles Mallampati score Research articles Mallampati score review articles Mallampati score PubMed articles Mallampati score PubMed Central articles Mallampati score 2023 articles Mallampati score 2024 articles Mallampati score Scopus articles Mallampati score impact factor journals Mallampati score Scopus journals Mallampati score PubMed journals Mallampati score medical journals Mallampati score free journals Mallampati score best journals Mallampati score top journals Mallampati score free medical journals Mallampati score famous journals Mallampati score Google Scholar indexed journals Overweight articles Overweight Research articles Overweight review articles Overweight PubMed articles Overweight PubMed Central articles Overweight 2023 articles Overweight 2024 articles Overweight Scopus articles Overweight impact factor journals Overweight Scopus journals Overweight PubMed journals Overweight medical journals Overweight free journals Overweight best journals Overweight top journals Overweight free medical journals Overweight famous journals Overweight Google Scholar indexed journals Polysomnography articles Polysomnography Research articles Polysomnography review articles Polysomnography PubMed articles Polysomnography PubMed Central articles Polysomnography 2023 articles Polysomnography 2024 articles Polysomnography Scopus articles Polysomnography impact factor journals Polysomnography Scopus journals Polysomnography PubMed journals Polysomnography medical journals Polysomnography free journals Polysomnography best journals Polysomnography top journals Polysomnography free medical journals Polysomnography famous journals Polysomnography Google Scholar indexed journals Temporomandibular disorder articles Temporomandibular disorder Research articles Temporomandibular disorder review articles Temporomandibular disorder PubMed articles Temporomandibular disorder PubMed Central articles Temporomandibular disorder 2023 articles Temporomandibular disorder 2024 articles Temporomandibular disorder Scopus articles Temporomandibular disorder impact factor journals Temporomandibular disorder Scopus journals Temporomandibular disorder PubMed journals Temporomandibular disorder medical journals Temporomandibular disorder free journals Temporomandibular disorder best journals Temporomandibular disorder top journals Temporomandibular disorder free medical journals Temporomandibular disorder famous journals Temporomandibular disorder Google Scholar indexed journals Obstructive sleep apnea articles Obstructive sleep apnea Research articles Obstructive sleep apnea review articles Obstructive sleep apnea PubMed articles Obstructive sleep apnea PubMed Central articles Obstructive sleep apnea 2023 articles Obstructive sleep apnea 2024 articles Obstructive sleep apnea Scopus articles Obstructive sleep apnea impact factor journals Obstructive sleep apnea Scopus journals Obstructive sleep apnea PubMed journals Obstructive sleep apnea medical journals Obstructive sleep apnea free journals Obstructive sleep apnea best journals Obstructive sleep apnea top journals Obstructive sleep apnea free medical journals Obstructive sleep apnea famous journals Obstructive sleep apnea Google Scholar indexed journals Dental Hospital articles Dental Hospital Research articles Dental Hospital review articles Dental Hospital PubMed articles Dental Hospital PubMed Central articles Dental Hospital 2023 articles Dental Hospital 2024 articles Dental Hospital Scopus articles Dental Hospital impact factor journals Dental Hospital Scopus journals Dental Hospital PubMed journals Dental Hospital medical journals Dental Hospital free journals Dental Hospital best journals Dental Hospital top journals Dental Hospital free medical journals Dental Hospital famous journals Dental Hospital Google Scholar indexed journals
Article Details
Introduction
Temporomandibular disorder (TMD) is a collective term for conditions characterized by pain and dysfunction of the temporomandibular joint (TMJ), masticatory muscles, and the surrounding structures. Symptoms of TMD include TMJ pain, masticatory muscle pain, TMJ noise, mouth-opening limitation, headache, sleep problems, and accompanying psychological deterioration [1]. The prevalence of TMD varies widely with the study methodology, ranging between 5% and 87% [2,3]. Female patients have TMD signs and symptoms more frequently than male patients, and the male-to-female ratio is approximately 1:2 [4]. With a complex etiology, TMD is not easily identified and resolved and hence becomes chronic [5]. To successfully diagnose and treat patients with TMD, they must be comprehensively identified in their respective biopsychosocial models. Sex is a crucial factor for pain; hormonal system, immunity, psychological axis, bodily changes with age, and pain control mechanisms can differ with it [6]. Thus, patients can have different signs and symptoms depending on their sex.
Ninety percent of patients with TMD experience poor sleep quality [7]. In 2014, the Diagnostic Criteria for TMD (DC/TMD) was published as a diagnostic tool for 12 common TMDs, including arthrogenous and myogenous TMD, and TMD attributed to TMD [8]. Currently, DC/TMD is the most widely used diagnostic criterion for TMD in clinical and research fields worldwide. DC/TMD is composed of dual axes: Axis I deals with the physical part, and Axis II deals with the psychosocial part, without consideration for sleep. Although sleep deterioration is supposedly substantial in TMD patients [9], the type and magnitude of sleep problems are not clearly known because of the numerous methodological limitations. Laboratory-based polysomnography (PSG) is considered the gold standard for objectively measuring sleep but is impractical for home utilization [10]. Portable PSG devices are an alternative to laboratory-based PSG, allowing objective sleep measurements in a home environment. In this study, we investigated sleep and its sex-based differences in patients with TMD using a portable PSG.
Obstructive sleep apnea (OSA) is a representative sleep disorder that involves a significant decrease or cessation of airflow in the presence of breathing effort. OSA is an upper airway dysfunction during sleep characterized by increased respiratory effort secondary to snoring and/or greater upper airway resistance and pharyngeal collapse [11]. At an apnea-hypopnea index (AHI) of ≥5 events/h, the prevalence ranges from 9% to 38% in the general population [12]. OSA affects both men (15%) and women (5%), and its prevalence is higher in men [13]. Sex-based differences exist in OSA signs and symptoms; male sex, a higher body mass index (BMI) and waist-to-hip ratio, greater neck circumference, arterial hypertension, older age, smoking, and snoring are closely related to OSA [14]. The common clinical complaints of OSA patients and their sleep partners are daytime sleepiness, snoring, gasping, and choking [15]. In the pathophysiological mechanism, there may be a marked decrease (hypopnea) or absence (apnea) of airflow in the nose and/or mouth, which is usually accompanied by oxyhemoglobin desaturation, and is usually terminated by a brief micro-arousal. Recurrent episodes of apnea lead to a decrease in slow-wave sleep and rapid eye movement (REM) sleep, ultimately leading to sleep disturbances and fragmentation [16]. OSA is a risk and/or persistence factor for chronic pain including TMD [17,18]. However, few studies have addressed snoring, a common symptom of OSA, in patients with TMD. Measuring periodic snoring at home is a simple and useful method for predicting OSA [19].
The first goal of this study was to objectively investigate sleep in patients with TMD using portable PSG. TMD and OSA are also reported to be comorbid conditions. The purpose of this study was to conduct an in-depth investigation of snoring, which may be the representative and early symptom of OSA, in patients with TMD. We hypothesized that the signs and symptoms of both diseases and the increase in BMI or accompanying collapsibility of the upper airway (obstructiveness) may differ depending on sex and have varying relationships with TMD. Finally, we intended to examine the relationship between sleep-related parameters and their clinical characteristics in TMD patients and investigate whether there are sex-based differences.
Materials and Methods
Participants
The research protocol was reviewed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Kyung Hee University Dental Hospital (KHD IRB no.1804–2). Written informed consent was obtained from all participants. Informed consent was obtained to publish de-identified images of one participant who performed portable PSG in an online, open-access publication.
To investigate this research purpose, we conducted an observational study in clinical practice. A total of 70 consecutive patients (44 female; mean age, 46.69±18.18 years) with myofascial pain-associated TMD were included. We identified patients with TMD who visited the Department of Orofacial Pain and Oral Medicine at Kyung Hee University Dental Hospital of Seoul from October 1, 2018, to May 31, 2022. The patients were diagnosed with TMD using DC/TMD for TMD Axis I [8].
The inclusion criteria for patients with TMD were as follows: completed a set of routine TMJ assessments, performed portable PSG, completed sleep reports and constructive questionnaires, and no other treatment of the current episode, except medication. The exclusion criteria were as follows: serious injuries such as facial fractures and unstable multiple traumas, previous injury, neurological disorder unrelated to the trauma, musculoskeletal disorder before the injury, rheumatism, psychological problems, and pregnancy.
To assess the impact of sex on the distribution of demographics, clinical factors for signs and symptoms of TMD, and the presence of snoring and OSA, all variables were compared between the male and female TMD groups.
TMD classification and clinical evaluation
Clinical evaluation procedures included an oral examination, interview, panoramic radiography, and a comprehensive questionnaire on DC/TMD Axis I diagnostic algorithms for TMD. Myofascial pain was diagnosed based on the DC/TMD Axis I algorithm.
Characteristics of pain
Patients reported the duration of symptoms related to the masticatory muscles and adjacent structures based on the number of days elapsed since the first experience of symptoms related to TMD. TMD-related pain was scored subjectively by patients, ranging from 0 (no pain at all) to 10 (worst pain imaginable), using the visual analog scale (VAS).
Contributing factors for TMD
We investigated self-reported parafunctional activities, including bruxism, using the Oral Behavior Checklist [20]. Self-assessment of sleep problems, headaches, psychological distress, and tinnitus have also been reported. Self-reported macrotrauma experience was evaluated using the dichotomous question, "Do you have any macrotrauma experience associated with current TMD?" Each parameter was recorded as a binary answer (yes or no) for all patients.
Body mass index
Self-reported weight and height were used to assess overweight and obesity, based on the body mass index (BMI). To obtain BMI, the patient's weight (kg) was divided by the square of the height (m). Major adult BMI categories are underweight (<18.5 kg/m2), normal weight (18.5–24.9), overweight (25–29.9), and obese (≥30) [21]. None of the participants in this study were obese.
Mallampati score
The Mallampati score (Mallampati classification) is a simple test, possibly a sensitive predictor of OSA [22]. The Mallampati score is based on inspection of the upper airway. The patient was instructed to open the mouth, while protruding the tongue, to their best possible limit. Scoring depends on the visibility of anatomical structures in the oropharynx, and Mallampati scores are distributed as follows (Figure 1): 1, the soft palate and entire uvula are visible; 2, the soft palate, hard palate, and upper portion of the uvula are visible; 3, the soft palate; hard palate; and base of the uvula are visible; 4, only the hard palate is visible. For every 1-point increase in the Mallampati score, the odds of having OSA, increased by more than 1.5 folds [23].
Class 1: Faucial/tonsillar pillars, uvula, and soft palate are all visible. Class 2: Partial visibility of the faucial/tonsillar pillars, uvula, and soft palate. Class 3: Base of the uvula, soft palate, and hard palate are visible. Class 4: Only the hard palate is visible.
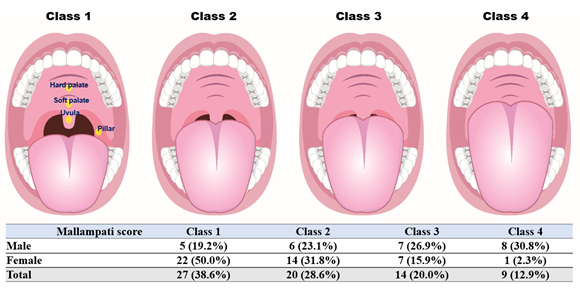
Figure 1: Distribution of Mallampati score according to sex in TMD patients.
Portable PSG index for snoring and OSA
We identified and diagnosed OSA using Alice OneNight (Philips, Amsterdam, The Netherlands). Alice OneNight home sleep testing is a portable level 3 PSG device used to test sleep apnea at home. It includes oxygen saturation (saturation of percutaneous oxygen (SpO2), finger probe, and oximetry board Nonin), pulse rate (from oximeter probe), airflow (pressure-based airflow with detection of snoring through a nasal cannula and thermistor), thoracic and abdominal effort (inductance plethysmography), and body position (Figure 2). The patient installed and operated this device on their body for more than 1 day, and the operator selected representative data for 1 day without data loss and used them for analysis.
The presence of snoring, total time spent snoring, percentage of snoring time (ratio of total time spent snoring to total sleep time), total number of snoring episodes, and average snoring episode time were obtained and analyzed. The respiratory event index (REI) was used to predict OSA. REI represents the number of apneas and hypopneas detected by the portable monitoring device per hour of elapsed recording time. The central apnea index (CAI) and obstructive apnea index (OAI) were added, and each index was used to calculate the number of events per hour. The number of events per hour of mixed sleep apnea was also determined. Mixed sleep apnea is a combination of obstructive and central sleep apnea [24]. Apnea was defined as a 90% reduction in airflow for at least 10 s, and hypopnea was defined as a ≥30% reduction in airflow for at least 10 s, associated with a ≥3% reduction in oxygen saturation. OSA was defined as an REI ≥5/h, and while the normal REI was <5/ h) [25]. Sleep studies were considered acceptable for data analysis if none of the following criteria were met: (1) portable monitoring device with elapsed time <2 h or (2) poor-quality PSG recording (defined as a substantial portion of the PSG not interpretable for the scoring of sleep and respiratory events).
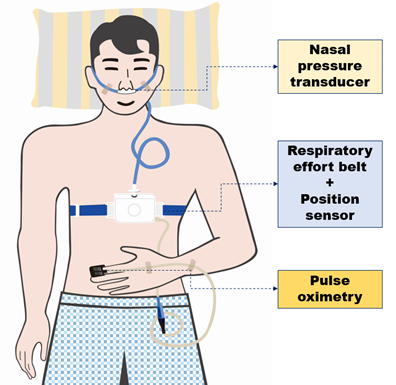
Figure 2: Schematic diagram of a study participant equipped with portable polysomnography
ROC, receiver operating characteristic; OSA, obstructive sleep apnea; BMI, body mass index; AUC, area under ROC curve; REI, respiratory event index; OAI, obstructive apnea index; CAI, central apnea index; SpO2, saturation of percutaneous oxygen.
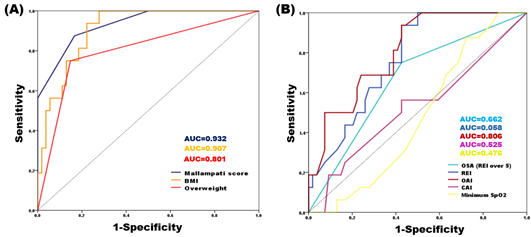
Figure 3: ROC analysis of significant predictors of snoring. (A) Factors related to Mallampati score, (B) factors related to OSA
Data analysis
Descriptive statistics are presented as percentages, means, and standard deviations (SDs) for continuous variables. The student’s t-test for non-normally distributed variables was used to compare the male and female TMD groups. Differences in the means of continuous variables between the independent groups were examined using the student’s t-test. The chi-square test with Bonferroni correction was used to determine the equality of the proportions. The Spearman’s correlation test was used to analyze bivariate correlations between the categorical and continuous variables. The Spearman’s correlation coefficient was expressed as Spearman’s rho (r), with an absolute value closer to 1, indicating a stronger correlation. The performance of the classification model at the defined threshold was demonstrated by plotting a receiver operating characteristic (ROC) curve, and the area under the ROC curve (AUC) value was calculated for each model. AUC values were interpreted as AUC=0.5 (no discrimination), 0.6≥ AUC >0.5 (poor discrimination), 0.7≥ AUC >0.6 (acceptable discrimination), 0.8≥ AUC >0.7 (excellent discrimination), and AUC >0.9 (outstanding discrimination) [26]. Kappa statistics were used to measure the degree of agreement (kappa coefficient) between two examiners who evaluated and rated the same subjects. Multiple logistic regression analysis was performed with snoring as a dependent variable and demographics, clinical characteristics of TMD, and other PSG parameters as independent variables. We also investigated whether there were sex-based differences in the OSA-related factors, examined using portable PSG. Odds ratios (ORs) with 95% confidence intervals (CIs) and p-values were calculated. Statistical significance was set at a two-tailed p-value of <0.05. Data were analyzed using IBM SPSS Statistics for Windows (version 20.0; IBM Corp., Armonk, NY, USA).
Results
Demographics and clinical characteristics of TMD patients
Demographic and clinical factors are shown in table 1. In this study, 44 female (62.9%, mean age: 46.61±17.73 years) and 26 male (37.1%, mean age: 46.81±19.27 years) TMD patients were enrolled, and there was no significant difference in age and sex. The male to female ratio was 1:1.69. BMI (male vs. female, 24.94±1.78 vs. 56.30±4.25, p<0.001), ratio of overweight (57.7% vs. 11.4%, p<0.001), and Mallampati score (2.69±1.12 vs. 1.70±0.82, p<0.001) differed according to sex, with the values being significantly higher in male than in female patients.
|
Male TMD patients (n=26) |
Female TMD patients (n=44) |
||
|
mean ± SD or n (%) |
mean ± SD or n (%) |
p-value |
|
|
Demographics |
|||
|
Age (years) a |
46.81 ± 19.27 |
46.61 ± 17.73 |
0.966 |
|
Sex |
26 (37.1%) |
44 (62.9%) |
|
|
TMD index |
|||
|
VAS (0-10) a |
5.96 ± 2.03 |
5.76 ± 2.02 |
0.114 |
|
Symptom duration (days) a |
593.65 ± 1054.75 |
829.05 ± 1613.69 |
0.509 |
|
BMI |
|||
|
Weight (kg) a |
76.15 ± 4.09 |
56.30 ± 4.25 |
<0.001*** |
|
Height (m) a |
1.75 ± 0.59 |
1.59 ± 0.46 |
<0.001*** |
|
BMI (kg/m2) a |
24.94 ± 1.78 |
22.02 ± 2.24 |
<0.001*** |
|
Overweight (BMI?25) b |
15 (57.7%) |
5 (11.4%) |
<0.001*** |
|
Mallampati score for the prediction of OSA |
|||
|
Mallampati score (1-4) a |
2.69 ± 1.12 |
1.70 ± 0.82 |
<0.001*** |
TMD: temporomandibular disorder, BMI: body mass index, VAS: visual analogue scale, OSA: obstructive sleep apnea, SD: standard deviation.
a: The results were obtained via t-test. b: The results were obtained from chi-square test. A p-value <0.05 was considered significant. ***: p-value<0.001.
Table 1: Demographics and clinical characteristics of TMD patients
Distribution of contributing factors for TMD
Among the representative contributing factors of TMD, only the presence of bruxism differed between sexes. Bruxism was found in 3.8% of male and 29.5% of female patients with TMD (p=0.012). The most common contributing factors for TMD in both male and female patients were headache (male, 53.8%; female, 54.5%), followed by psychological distress (male, 50.5%; female, 52.3%), and sleep problems (male, 34.6%; female, 36.4%) (Table 2).
|
Male TMD patients (n=26) |
Female TMD patients (n=44) |
||
|
mean ± SD or n (%) |
mean ± SD or n (%) |
p-value |
|
|
Contributing factor |
|||
|
Bruxisma |
1 (3.8%) |
13 (29.5%) |
0.012* |
|
Sleep problema |
9 (34.6%) |
16 (36.4%) |
1 |
|
Headachea |
14 (53.8%) |
24 (54.5%) |
1 |
|
Psychological distressa |
13 (50.0%) |
23 (52.3%) |
1 |
|
Tinnitusa |
2 (7.7%) |
11 (25.0%) |
0.111 |
|
Macrotraumab |
7 (26.9%) |
6 (15.9%) |
0.356 |
TMD: temporomandibular disorder, A p-value <0.05 was considered significant. *: p-value<0.05, **: p-value<0.01, ***: p-value<0.001.
a: The results were obtained from chi-square test. b: The results were obtained from Fisher’s exact test and Bonferroni correction. c: The results were obtained via t-test.
Table 2: Distribution of contributing factors for TMD
OSA diagnosis with portable PSG
Considering SpO2, the duration of SpO2 <90% was significantly higher in female than in male TMD patients (0.15±0.33 min vs. 0.53±1.12 min, p=0.043). In addition, the mixed sleep apnea index was significantly higher in female than in male TMD patients (0.81±0.80 vs. 0.44±0.54, p<0.022). There was no difference between the REI (8.84±10.41 vs. 9.21±8.39, p>0.05) and OSA presence rate based on REI ≥5 (54.5 % vs. 42.3%, p>0.05) in female and male TMD patients (Table 3).
Snoring investigated by portable PSG
Total sleep time and that spent snoring did not differ significantly between male and female patients (295.57±188.42 min vs. 255.03±184.77 min, p=0.382 and 5.18±5.43 min vs. 3.61±3.89 min, p=0.205, respectively). In addition, the total number of snoring episodes and average snoring episode time did not differ significantly between male and female patients (16.98±41.88 vs. 16.85±34.27, p=0.806 and 23.71±14.22 s vs. 19.43±13.27 s, p=0.214, respectively). The presence of snoring was observed in 15.4% of male patients and 13.6% of female patients (p=0.398). Interestingly, the prevalence of snoring was lower than that of OSA in both sexes (Table 4).
|
Male TMD patients (n=26) |
Female TMD patients (n=44) |
||
|
mean ± SD or n (%) |
mean ± SD or n (%) |
p-value |
|
|
Saturation of oxygen |
|||
|
Lowest SpO2 (%)a |
81.50 ± 24.21 |
94.36 ± 1.98 |
0.634 |
|
Mean SpO2 (%)a |
94.65 ± 0.89 |
94.65 ± 0.89 |
0.404 |
|
SpO2 < 90% (min)a |
0.15 ± 0.33 |
0.53 ± 1.12 |
0.043* |
|
Sleep apnea index |
|||
|
Mixed sleep apnea (events/hour)a |
0.44 ± 0.54 |
0.81 ± 0.80 |
0.022* |
|
OAI (events/hour)a |
5.31 ± 6.76 |
4.83 ± 5.16 |
0.755 |
|
CAI (events/hour)a |
0.59 ± 0.60 |
1.41 ± 4.06 |
0.195 |
|
REI (events/hour)a |
8.84 ± 10.41 |
9.21 ± 8.39 |
0.876 |
|
Prediction of OSA |
|||
|
Normal (REI < 5)b |
15 (57.7%) |
20 (45.5%) |
0.458 |
|
OSA (REI ? 5)b |
11 (42.3%) |
24 (54.5%) |
TMD: temporomandibular disorder, PSG: polysomnography, SpO2: saturation of percutaneous oxygen, OSA: obstructive sleep apnea, OAI, obstructive apnea index, CAI, central apnea index, REI: respiratory event index.
a: The results were obtained via t-test. b: The results were obtained from chi-square test. A p-value <0.05 was considered significant. *: p-value<0.05.
Table 3: Investigation of OSA with portable polysomnography
|
Male TMD patients (n=26) |
Female TMD patients (n=44) |
||
|
mean ± SD or n (%) |
mean ± SD or n (%) |
p-value |
|
|
Portable PSG index |
|||
|
Total sleep time (min)a |
295.57 ± 188.42 |
255.03 ± 184.77 |
0.382 |
|
Total spent snoring (min)a |
5.18 ± 5.43 |
3.61 ± 3.89 |
0.205 |
|
Percentage of snoring time (%)a |
1.74 ± 2.88 |
1.42 ± 2.11 |
0.398 |
|
Total number of snoring episodesa |
16.98 ± 41.88 |
16.85 ± 34.27 |
0.806 |
|
Average snoring episode time (sec)a |
23.71 ± 14.22 |
19.43 ± 13.27 |
0.214 |
|
The presence of snoringb |
5 (15.4%) |
0.398 |
TMD: temporomandibular disorder, PSG: polysomnography, SpO2: saturation of percutaneous oxygen. a: The results were obtained via t-test. b: The results were obtained from chi-square test. A p-value <0.05 was considered significant.
Table 4: Investigation of snoring by portable polysomnography
Multiple logistic regression analysis with clinical factors for predicting snoring
Among several factors, including age, sex, and contributing factors for TMD, female sex and macrotrauma history significantly predicted the presence of snoring. When compared to male patients, female patients showed a 0.146-fold decrease in the incidence of snoring (OR=0.146, p=0.022). In addition, macrotrauma history was found to increase the prediction of snoring by 6.165 times (OR=6.165, p=0.034). The presence of bruxism, sleep problems, headache, psychological distress, and tinnitus, along with age and VAS score, were not significant predictors of snoring (Table 5).
|
Predicting snoring in TMD patients |
||||||
|
OR |
95% CI Lower |
95% CI Upper |
B |
SE |
p-value |
|
|
Age [ref.=under average value] |
1.22 |
0.147 |
10.123 |
0.199 |
1.08 |
0.854 |
|
Female [ref.=male] |
0.146 |
0.028 |
0.757 |
-1.926 |
0.84 |
0.022* |
|
VAS [ref.=under average value] |
0.177 |
0.032 |
0.987 |
-1.734 |
0.888 |
0.051 |
|
Bruxism [ref.=none] |
1.841 |
0.457 |
7.412 |
0.61 |
0.711 |
0.39 |
|
Sleep problem [ref.=none] |
0.56 |
0.13 |
2.404 |
-0.58 |
0.744 |
0.435 |
|
Headache [ref.=none] |
0.169 |
0.014 |
2.097 |
-1.781 |
1.286 |
0.166 |
|
Psychological distress [ref.=none] |
0.657 |
0.1 |
4.332 |
-0.421 |
0.963 |
0.662 |
|
Tinnitus [ref.=none] |
0.297 |
0.07 |
1.256 |
-1.213 |
0.735 |
0.099 |
|
Macrotrauma [ref.=none] |
6.165 |
1.143 |
33.27 |
1.819 |
0.86 |
0.034* |
|
Constant |
1.442 |
- |
- |
0.366 |
0.911 |
0.688 |
TMD: temporomandibular disorder, VAS: visual analogue scale, OR: odds ratio, CI: confidence interval, SE: Standard error, B: logistic regression coefficient
a: The results were obtained via t-test. b: The results were obtained from chi-square test. A p-value <0.05 was considered significant. *: p-value<0.05
Table 5: Multiple logistic regression analysis for predicting snoring in TMD patients
Factors correlating with the presence of snoring in TMD patients
The presence of snoring showed a positive correlation with the Mallampati score in both male (r=0.818, p=0.001) and female (r=0.501, p=0.001) patients, and the degree of correlation expressed by Spearman's r was greater in male patients. In addition to the Mallampati score, significant positive correlations were observed between OAI (r=0.788, p=0.001), REI (r=0.750, p=0.001), and snoring (Table 6), exclusively in the male patients with TMD.
|
Parameters |
Male TMD patients (n=26) |
Female TMD patients (n=44) |
|
|
The presence of snoring |
The presence of snoring |
||
|
Mallampati score |
Spearman's r |
0.818 |
0.501 |
|
p-value |
0.001** |
0.001** |
|
|
OAI |
Spearman's r |
0.788** |
0.195 |
|
p-value |
0.001** |
0.204 |
|
|
CAI |
Spearman's r |
0.055 |
0.057 |
|
p-value |
0.789 |
0.711 |
|
|
REI |
Spearman's r |
0.75 |
0.155 |
|
p-value |
0.001** |
0.315 |
TMD: temporomandibular disorder, OSA: obstructive sleep apnea, OAI, obstructive apnea index, CAI, central apnea index, REI: respiratory event index.
Table 6: Factors correlated with the presence of snoring in TMD patients
The results were obtained from Spearman’s correlation analysis. Spearman's r indicates the correlation between two factors (the range of Spearman's r: -1 to 1). The larger the absolute value, the stronger the correlation. In this table, red indicates positive correlation and green indicates negative correlation. A p-value <0.05 was considered significant. **: p-value<0.01.
ROC analysis predicting snoring in TMD patients
For the total sample, the significance of AUC values was investigated using the ROC analysis (Table 7). Mallampati score (AUC=0.932, p<0.001) and BMI (AUC=0.907, p<0.001) strongly predicted the presence of snoring at an outstanding discrimination level (AUC>0.9) in patients with TMD. Overweight (BMI ≥25) (AUC=0.801, p<0.001), OAI (AUC=0.806, p<0.001), and REI (AUC=0.768, p=0.01) were also significant predictors with excellent discrimination levels for the presence of snoring (Figure 3). Therefore, snoring was correlated with Mallampati score, BMI, and risk of OSA.
|
AUC |
SD |
p-value |
95% CI |
||
|
Lower |
Upper |
||||
|
Clinical factors |
|||||
|
Mallampati score |
0.932 |
0.033 |
<0.001*** |
0.867 |
0.997 |
|
BMI |
0.907 |
0.035 |
<0.001*** |
0.84 |
0.975 |
|
Overweight (BMI?25) |
0.801 |
0.069 |
<0.001*** |
0.665 |
0.937 |
|
Polysomnography factors |
|||||
|
Minimum SpO2 |
0.476 |
0.071 |
0.774 |
0.337 |
0.616 |
|
OAI |
0.806 |
0.055 |
<0.001*** |
0.698 |
0.914 |
|
CAI |
0.525 |
0.086 |
0.758 |
0.357 |
0.694 |
|
REI |
0.768 |
0.058 |
0.001** |
0.655 |
0.881 |
|
Snoring (REI > 5) |
0.662 |
0.076 |
0.05 |
0.514 |
0.81 |
TMD: temporomandibular disorder, ROC: Receiver operating characteristic, AUC: area under the ROC curve, SD: standard deviation, CI: confidence interval, BMI: body mass index, OAI, obstructive apnea index, CAI, central apnea index, REI: respiratory event index, SpO2: saturation of percutaneous oxygen.
The results were obtained by the ROC curve analysis. A p-value <0.05 was considered significant. **: p-value<0.01, ***: p-value<0.001.
Table 7: ROC analysis predicting snoring in TMD patients
Discussion
In the present study, for the first time, clinical characteristics, snoring, and OSA were investigated in patients with TMD using portable PSG, and sex-based differences were comprehensively investigated in terms of their mean values, distribution, and relationships. While Mallampati score, BMI, and overweight ratio were significantly higher in male than in female TMD patients, OSA was more common than snoring in TMD patients; 50.0% of TMD patients had OSA and 15.7% had snoring, but no sex-differences were found in the prevalence of OSA and snoring. In both sexes, Mallampati score was significantly positively correlated with snoring; it was the strongest predictor of snoring, and the AUC value of 0.932 was indicative of its outstanding discrimination value. In addition, there were significant positive correlations between OAI, REI, and presence of snoring in male patients with TMD, while no correlation was observed among female patients. Furthermore, sleep in patients with TMD was also examined for PSG parameters with sex-based differences, and the mixed sleep apnea index was significantly higher in female than in male TMD patients. The results demonstrate that the causes of sleep problems, including snoring and OSA, and the correlation with BMI and other clinical characteristics may differ between female and male TMD patients.
In this study, Mallampati score was an important anatomical factor for predicting snoring in both male and female patients with TMD. It is a measure of the density of soft tissue in the oropharynx and usually increases with increasing obesity and BMI 27. A high Mallampati score is associated with sleep problems in the upper airway, including snoring and OSA 28. According to Nuckton et al., the Mallampati score may be useful as an independent predictor of OSA, which is consistent with the subsequent overnight PSG results 22. The mixed sleep apnea index was higher in female than in male TMD patients. In our results, there were significant positive correlations between OAI, REI, and presence of snoring, only in male TMD patients. Thus, Mallampati scoring could be a useful tool for the physical examination of patients prior to PSG 22 by predicting the presence of snoring in patients with TMD, while a high score could correlate with the presence of OSA in male patients.
The mixed sleep apnea index was higher in female than in male TMD patients. No previous studies have examined sex-based differences in mixed sleep apnea in TMD patients. Unlike OSA, central sleep apnea is defined as a lack of continuous breathing effort during airflow [29]. However, this distinction is sometimes rather difficult because there is a significant overlap in the etiology and pathophysiology of OSA and central sleep apnea [30]. Therefore, the concepts of mixed sleep apnea, complex sleep apnea, or sleep apnea spectrum have emerged. Mixed sleep apnea is a combination of both obstructive and central sleep apnea symptoms [31]. Although the pathogenesis of OSA and central sleep apnea is not fully understood, several anatomical and non-anatomical factors are considered to interact. The key factors include the interaction of upper airway obstruction, unstable central ventilation modulating factors, and an individual's systemic conditions/characteristics [32]. Withdrawal of behavioral control over ventilation during sleep and blunt chemoresponsiveness to changes in arterial CO2 (PaCO2) and oxygen (PaO2), changes in lung volume, and ventilation due to sleep states (REM vs. non-REM) result in greater variability in PaCgO2 levels [33]. If the ventilatory response to the stimulus is exaggerated, PaCO2 can easily fall below the apnea threshold, causing central apnea. In this study, female sex was a predictor of a decrease in the incidence of snoring. The prevalence and characteristics of sleep apnea in female patients vary throughout their life span as they go through different life stages: puberty, reproductive years, pregnancy, and postmenopausal state [34]. Additionally, male and female patients have different pathophysiological factors for sleep apnea, such as upper airway anatomy, chemoreflexes, sex hormones, and the ability to recognize sleep disorders. Further studies on snoring and sleep apnea, considering these parameters in patients with TMD, are needed.
Based on the results of portable PSG, snoring was strongly correlated with OSA and REI scores in male patients with TMD. In addition, AUC values suggest that OAI and REI were major predictors of snoring. Despite not affecting sleep architecture, snoring adversely affects sleep efficiency [35]. Although snoring is considered a representative symptom of OSA, there has been no evaluation of snoring in patients with TMD. Decreased sleep efficiency may interfere with patients’ daily activities, contributing to an increase in their sensitivity to pain [36]. In this study, the clinical pain index, VAS, had no significant relationship with snoring; additional studies on TMD pain index and snoring are needed to clarify these findings. There was no significant sex-based difference in the prevalence of snoring (female, 15.4% vs. male, 13.6%, p=0.398) and OSA (female, 54.5% vs. male, 42.3%, p=0.458) in TMD patients based on portable PSG results. A bidirectional association between OSA and TMD has been proposed, which is evident from its high prevalence [37]. OSA has been reported as a common comorbidity in 37% of chronic pain patients and 28.6% of TMD patients [18]. The development and progression of snoring and OSA are closely related to obesity, measured as an increase in BMI [38]. In this study, overweight and increased BMI were observed more frequently in male than in female TMD patients. Patients with obesity (BMI ≥30) were not observed in this TMD study, and 57.7% of male and 11.4% of female patients were overweight (BMI ≥25). Considering 63% of patients to be overweight and 26% as obese [39], Korean patients with TMD have a lower percentage than American patients. The link between overweight, snoring, and OSA in TMD patients, particularly in male patients, may be due to underlying metabolic alterations and elevations in systemic inflammation.
This study had several limitations. First, patients with TMD who participated in this study willingly agreed to perform portable PSG at home, and there may be a bias depending on the patient's propensity. Therefore, the mean age (46.69±18.18 years) of patients with TMD in this study is higher than that in our previous studies [40,41]. Second, the sample size was limited to represent the characteristics of typical TMD patients. As a result of this single-institution study, a multicenter, large-sample study was planned. Third, additional studies that consider blood tests, psychological questionnaires, and TMD pain indices are needed to support the results of this study. Additional sleep studies using laboratory-based PSG may further reinforce these findings. The significance and strength of this study is that snoring and OSA in TMD patients were objectively investigated using a portable PSG, and sex-based differences in clinical patterns and sleep-related results were uniquely identified.
Acknowledgments
None.
Author contributions statement
Y.-H. L. wrote the manuscript. Y.-H. L. and Q.-S.A. contributed to data acquisition and analysis. Y.-H.L. and Q.-S.A. contributed to data interpretation, and Y.-H. L. contributed to figures. Y.-H.L. and E.-J.J. provided their expertise and contributed to the revisions. All the authors have read and agreed to the published version of the manuscript.
Data availability statement
Owing to the sensitivity of patient data, the KHU-IRB will discuss any requests before disclosure.
Competing interest statement
The authors declare no competing interests.
Funding
This research was supported by the National Research Foundation of Korea Grant (NRF/2020R1F1A1070072), and by a Korea Medical Device Development Fund grant, funded by the Korean government (Ministry of Science and ICT, Ministry of Trade, Industry and Energy, Ministry of Health & Welfare, Republic of Korea, Ministry of Food and Drug Safety). Project Number: KMDF_PR_20200901_0023, 9991006696, was obtained by Y.-H.L., and funded by the Korean government and Kyung Hee University in 2021 (KHU-20211863).
References
- Lee YH, Lee KM, Auh QS, et al. Magnetic Resonance Imaging-Based Prediction of the Relationship between Whiplash Injury and Temporomandibular Disorders. Front Neurol 8 (2017): 215.
- Ryalat, S. Prevalence of temporomandibular joint disorders among students of the university of jordan. J Clin Med Res 1 (2009): 158-164.
- Kmeid E, Nacouzi M, Hallit S, et al. Prevalence of temporomandibular joint disorder in the Lebanese population, and its association with depression, anxiety, and stress. Head & Face Medicine 16 (2020): 19-25.
- Bagis B, Ayaz EA, Turgut S, et al. Gender difference in prevalence of signs and symptoms of temporomandibular joint disorders: a retrospective study on 243 consecutive patients. Int J Med Sci 9 (2012): 539-544.
- List, T. & Jensen, R. H. Temporomandibular disorders: Old ideas and new concepts. Cephalalgia 37 (2017): 692-704.
- Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 111 (2013): 52-58.
- Yatani H, Studts J, Cordova M, et al. Comparison of sleep quality and clinical and psychologic characteristics in patients with temporomandibular disorders. J Orofac Pain 16 (2002): 221-228.
- Schiffman, E. et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J Oral Facial Pain Headache 28 (2014): 6-27.
- Sommer I, Lavigne G, Ettlin DA. Review of self-reported instruments that measure sleep dysfunction in patients suffering from temporomandibular disorders and/or orofacial pain. Sleep Med 16 (2015): 27-38.
- Van de Water AT, Holmes A, Hurley DA. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography- a systematic review. J Sleep Res 20 (2011): 183-200.
- Fogel RB, Malhotra A, White DP. Sleep. 2: pathophysiology of obstructive sleep apnoea/hypopnoea syndrome. Thorax 59 (2004): 159-163.
- Senaratna CV. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev 34 (2017): 70-81.
- Young T. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328 (1993): 1230-1235.
- Pataka A. Gender Differences in Obstructive Sleep Apnea: The Value of Sleep Questionnaires with a Separate Analysis of Cardiovascular Patients. J Clin Med 9 (2020): 659-668.
- Goyal M, Johnson J. Obstructive Sleep Apnea Diagnosis and Management. Mo Med 114 (2017): 120-124.
- Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5 (2008): 144-153.
- Sanders AE. et al. Sleep apnea symptoms and risk of temporomandibular disorder: OPPERA cohort. J Dent Res 92 (2013): 70-77.
- Wu JH. The Association between Temporomandibular Disorder and Sleep Apnea-A Nationwide Population-Based Cohort Study. Int J Environ Res Public Health 17 (2020): 8212-8234.
- Alakuijala A, Salmi T. Predicting Obstructive Sleep Apnea with Periodic Snoring Sound Recorded at Home. J Clin Sleep Med 12 (2016): 953-958.
- Markiewicz MR, Ohrbach R, McCall WD, et al. Oral behaviors checklist: reliability of performance in targeted waking-state behaviors. J Orofac Pain 20 (2006): 306-316.
- Weir CB, Jan A. in StatPearls (StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., (2022).
- Nuckton TJ, Glidden DV, Browner WS, et al. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep 29 (2006): 903-908.
- Shah JA, George A, Chauhan N, et al. Obstructive Sleep Apnea: Role of an Otorhinolaryngologist. Indian J Otolaryngol Head Neck Surg 68 (2016): 71-74.
- Wang J, Wang Y, Feng J, Chen, B. Y. & Cao, J. Complex sleep apnea syndrome. Patient Prefer Adherence 7 (2013): 633-641.
- Goyal M, Johnson J. Obstructive Sleep Apnea Diagnosis and Management. Mo Med 114 (2017): 120-124.
- Metz, C. E. Basic principles of ROC analysis. Semin Nucl Med 8 (1978): 283-298.
- Menon SM, Sampangiramaiah S, Mathew M. Cross Sectional Observational Study Performed to See for Relation of Mallampati Score and Extended Mallampati Score with Body Mass Index. J Clin Diagn Res 11 (2017): ug01-ug03.
- Rodrigues MM, Dibbern RS. Goulart CW. Nasal obstruction and high Mallampati score as risk factors for Obstructive Sleep Apnea. Braz J Otorhinolaryngol 76 (2010): 596-599.
- Eckert DJ, Jordan AS, Merchia P, et al. Central sleep apnea: Pathophysiology and treatment. Chest 131 (2007): 595-607.
- Orr, J. E., Malhotra, A. & Sands, S. A. Pathogenesis of central and complex sleep apnoea. Respirology 22 (2017): 43-52.
- Khan MT, Franco RA. Complex sleep apnea syndrome. Sleep Disord 12 (2014): 798487.
- Marrone O. Complex sleep apnea and obesity hypoventilation syndrome. Opposite ends of the spectrum of obstructive sleep apnea? Med Hypotheses 73 (2009): 488-492.
- Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med 4 (2008) 403-405.
- Wimms A, Woehrle H, Ketheeswaran S, et al. Obstructive Sleep Apnea in Women: Specific Issues and Interventions. Biomed Res Int 9 (2016): 1764837.
- Hoffstein V, Mateika JH, Mateika S. Snoring and sleep architecture. Am Rev Respir Dis 143 (1991): 92-96.
- Haack M, Simpson N, Sethna N, et al. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology 45 (2020): 205-216.
- Lee YH, Auh QS, An JS, et al. Poorer sleep quality in patients with chronic temporomandibular disorders compared to healthy controls. BMC Musculoskeletal Disorders 23 (2002): 246.
- Romero-Corral A, Caples SM, Lopez-Jimenez F, et al. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest 137 (2010): 711-719.
- Curtin LR. National Health and Nutrition Examination Survey: sample design, 2007-2010. Vital Health Stat 2 (2013) 1-23.
- Lee YH, Auh QS. Clinical factors affecting depression in patients with painful temporomandibular disorders during the COVID-19 pandemic. Scientific Reports 12 (2022): 14667.
- Lee YH, Lee KM, Auh QS, et al. Sex-related differences in symptoms of temporomandibular disorders and structural changes in the lateral pterygoid muscle after whiplash injury. J Oral Rehabil 46 (2019): 1107-1120.
