Incidence, Trends and In-Hospital Course and Outcomes of Torsades de Pointes
Article Information
Malik Shehadeh1, Saurabh Sudesh2, Matias Pollevick2, Geoffrey A. Rubin2, Elaine Y. Wan2, Deepak Saluja2, Jose M. Dizon2, Angelo Biviano2, Hasan Garan2, Hirad Yarmohammadi2*
1Columbia University Division of Cardiology, Mount Sinai Heart Institute, Miami, FL, USA
2Columbia University Vagelos College of Physicians and Surgeons, Division of Cardiology, New York, NY, USA
*Corresponding author: Hirad Yarmohammadi MD, MPH, FAHA, FACC, FHRS. Columbia University Irving Medical Center 177 Fort Washington Avenue, Room 637 New York, NY 10032, USA.
Received: 28 October 2023; Accepted: 07 November 2023; Published: 12 January 2024.
Citation: Malik Shehadeh, Saurabh Sudesh, Matias Pollevick, Geoffrey A. Rubin, Elaine Y. Wan, Deepak Saluja, Jose M. Dizon, Angelo Biviano, Hasan Garan, Hirad Yarmohammadi. Incidence, Trends and In-Hospital Course and Outcomes of Torsades de Pointes. Cardiology and Cardiovascular Medicine. 8 (2024): 25-32.
Share at FacebookAbstract
Background: Torsades de Pointes (TdP) is a polymorphic ventricular tachycardia (VT) that occurs in the setting of QT prolongation. While it can terminate spontaneously, TdP can also degenerate into ventricular fibrillation and results in sudden cardiac death.
Objective: To investigate the incidence, trends, and in-hospital outcomes of TdP events. Methods: Our hospital database was queried from January 1, 2016- December 31, 2020 to identify patients with polymorphic VT. The mode of onset, telemetry strips and ECGs were reviewed to confirm the diagnosis of TdP. The in-hospital course, outcomes and predictors of in-hospital mortality were evaluated.
Results: Of 3,575 patients with VT episodes, 74 patients had confirmed TdP events (56.8% males, mean age 60.3 ± 17.1 years). The number of TdP cases remained stable throughout 2016-2020, ranging from 12-16 per year. The proportion of TdP/VT cases was significantly higher in 2020 during COVID-19 pandemic compared to prior years (6.8% vs 1.7%, p<.001), due mainly to a four-fold decrease in overall VT cases presented to the hospital that year. Forty-two patients (56.8%) needed defibrillation while the rest were hemodynamically stable. Fifteen patients (20.3%) died during admission. The proportion of TdP patients who died during admission was not significantly different across 2016-2020 (P=.76).
Conclusion: There was a relative and significant increase in the number of TdP as a proportion of VT cases in 2020 during COVID-19 pandemic year when it was compared to the previous years. However, in-hospital all-cause mortality rate was not significantly different during the 5-year study period.
Keywords
Torsades de Pointes; Polymorphic Ventricular Tachycardia; Ventricular Fibrillation; COVID-19.
Torsades de Pointes articles; Polymorphic Ventricular Tachycardia articles; Ventricular Fibrillation articles; COVID-19 articles.
Article Details
Abbreviations and Acronyms:
ECG: Electrocardiogram
ICD: Implantable cardioverter defibrillator
Ms: milliseconds
QTc: Corrected QT interval
TdP: Torsades de Pointes
VT: Ventricular tachycardia
1. Introduction
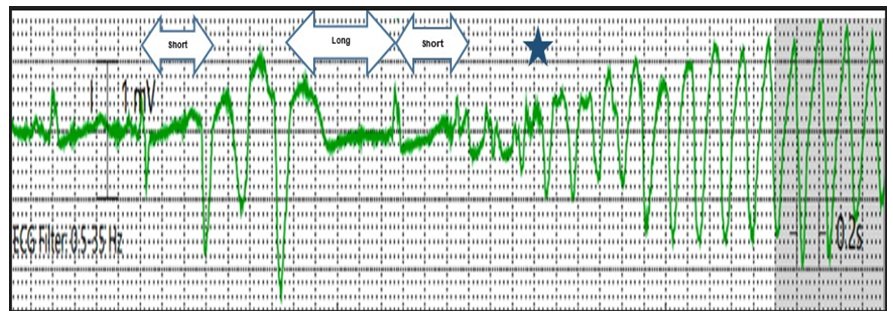
In 1966, the French cardiologist Dessertenne used the term Torsades de Pointes (TdP) to describe a polymorphic ventricular tachycardia characterized by a pattern of twisting pointes [1]. In the following years, TdP was defined as a form of polymorphic ventricular tachycardia (VT) that arises from early afterdepolarizations in the setting of QT prolongation [2, 3]. Early afterdepolarizations occur during phase 3 of the cardiac action potential and if they are of sufficient magnitude to reach the threshold, the chain of action potentials will generate TdP [4-6]. The risk of TdP is primarily assessed by the QT interval which is dynamic and depends largely on the previous cycle length (R-R interval); the longer the previous R-R interval, the longer the QT interval and the higher the risk of TdP [3, 7]; hence the treatment aims to eliminate pauses by increasing the heart rate. A corrected QT interval (QTc) of more than 500 milliseconds (ms) has been shown to correlate with a higher risk of TdP events, but there is no established threshold below which the proarrhythmic risk abates [3, 8, 9]. Most of the TdP events tend to occur in relatively short bursts with nearly half of the patients remaining hemodynamically stable and exhibiting no symptoms [2, 7, 10]. While TdP can be asymptomatic and terminate spontaneously, it can also degenerate into ventricular fibrillation and result in sudden cardiac death [2, 5, 11, 12]. The TdP event usually shows characteristic features. First, it usually starts with a short-long-short pattern of R-R cycles consisting of a short-coupled premature ventricular complex followed by a compensatory pause and then another premature ventricular complex that typically falls close to the peak of the T wave and provokes the TdP event. Second, once the arrhythmia is initiated, a switching of QRS morphology from predominately positive to predominately negative complexes, or vice versa, will be noted due to the polymorphic nature of TdP [13] (Figure 1).
Figure 1: Initiation of Torsades de Pointes (TdP) after a short-long-short cycle sequence by a premature ventricular complex (PVC) with a coupling interval measuring 560 milliseconds. The PVC falls near the peak of the T wave and provokes a TdP event. The (blue star) indicates the abrupt switching of QRS morphology due to polymorphic nature of TdP.
At the onset of COVID-19 pandemic, critically ill patients were being treated with QT-prolonging medications such as hydroxychloroquine and azithromycin. Therefore, there was a concern about potential increase in the incidence of TdP. However, initial reports found slight prolongation in QT interval with minimal to no increase in TdP events [14-18]. The aim of this study was to investigate the incidence, trends and in-hospital course and outcomes of TdP events before and during the COVID-19 pandemic in the epicenter of COVID-19 infection, a major New York City medical center.
2. Methods
This was a single-center, retrospective study performed at the New York Presbyterian Hospital/Columbia campus. The study was approved by Institutional Review Board. This study complied with the guidelines set forth in the Declaration of Helsinki. Our hospital database was queried from January 1, 2016 through December 31, 2020 using ICD-10 codes to identify all patients with diagnosis of VT. Patients with diagnosis of polymorphic VT were identified. The mode of onset, telemetry strips and electrocardiograms (ECGs) prior to and after the onset of the events were reviewed to confirm the diagnosis of TdP. For patients with cardiac implantable electronic devices who had no available rhythm strips, device interrogation was performed and the TdP event was confirmed by an electrophysiologist. Patients were included as a TdP case in the final retrospective analysis if the TdP event fell within the study time period. All ECGs for eligible patients were reviewed by an electrophysiologist to ensure accurate QTc measurements. All pre- and post-TdP ECGs were obtained within 7-days from reported arrhythmia event. The pre-TdP QTc and post-TdP QTc for each patient were measured from the ECGs that were temporally closest to the TdP event. Serial ECGs following the TdP event were reviewed until a QTc interval of less than 500 ms was observed. A prolonged QT was defined as a QTc ≥ 450 ms for males and ≥ 460 ms for females (these values represent the 97th to 99th percentile of the normal QT) [19]. For patients with a wide QRS (defined as QRS ≥ 120 ms during sinus rhythm), we used a QRS-adjusted QTc calculated by adding their measured JTc to an ideal QRS, where JTc = QTc—QRS [20]. Recovery of QTc interval to less than 500 ms was defined as slow if it was greater than 3 days. The in-hospital course including risk factors, hemodynamic stability during TdP event, invasive interventions such as temporary venous pacemaker insertion and implantable cardioverter defibrillator (ICD) implantation, and post-TdP QTc recovery were reviewed for all patients. The in-hospital outcomes and predictors of in-hospital mortality were evaluated.
Statistical Analysis:
Baseline demographic and relevant clinical data were collected for all patients. Continuous variables were reported as mean ± standard deviation, while categorical variables were reported as frequencies with percentages. Chi-squared test, Fisher’s exact test, one-way ANOVA, univariate and multivariable logistic regression analyses were conducted as appropriate. All data analyses were performed using Stata version 17.0 (Stata, College Station, TX, USA).
3. Results:
A total of 3,575 patients were diagnosed with VT between 2016 to 2020. Among them, 74 patients had confirmed diagnosis of TdP. The mean age was 60.3 ± 17.1 years, and 42 (56.8%) were males. At the time of reported TdP event, most patients were in the intensive care unit (70.3%) while the rest were on a telemetry floor (14.9%) or in the emergency department (12.2%). One patient (1.3%) was in the operating room at the time of TdP event, and another one (1.3%) was in the post-anesthesia care unit. Eight patients (10.8%) had an ICD for primary prevention, and four patients (5.4%) had permanent pacemaker prior to their TdP event. The baseline characteristics are listed in (Table 1).
Table 1: Baseline Characteristics
|
Age, (mean ± SD) years |
60.3 ± 17.1 |
|
Male, n (%) |
42 (56.8) |
|
BMI, (mean ± SD) |
27.1 ± 6.6 |
|
Comorbidities, n (%) |
|
|
Hypertension |
39 (52.7) |
|
Diabetes mellitus |
25 (33.8) |
|
Hyperlipidemia |
9 (12.2) |
|
Chronic kidney disease |
16 (21.6) |
|
Coronary artery disease |
19 (25.7) |
|
Atrial fibrillation/flutter |
30 (40.5) |
|
Heart Failure |
39 (52.7) |
|
Ischemic cardiomyopathy |
13 (17.6) |
|
Non-ischemic cardiomyopathy |
26 (35.1) |
|
Left Ventricular Ejection Fraction, n (%) |
|
|
≥ 50% |
36 (48.6) |
|
40-49% |
4 (5.4) |
|
30-39% |
11 (14.9) |
|
20-29% |
7 (9.5) |
|
< 20% |
16 (21.6) |
|
Cardiac implantable electronic devices, n (%) |
12 (16.2) |
|
ICD |
8 (10.8) |
|
Permanent pacemaker |
4 (5.4) |
|
Patient Location at time of TdP, n (%) |
|
|
Intensive care unit |
52 (70.3) |
|
Telemetry floor |
11 (14.9) |
|
Emergency department |
9 (12.2) |
|
Other (Operating room, post-anesthesia care unit) |
2 (2.7) |
|
QTc interval*, ms. (mean ± SD) |
|
|
Pre-TdP QTc |
530.9 ± 8.4 |
|
Post-TdP QTc |
547.8 ± 6.6 |
|
Hemodynamic stability at time of TdP, n (%) |
|
|
Stable |
32 (43.2) |
|
Unstable |
42 (56.8) |
BMI= Body mass index; ICD= Implantable cardioverter defibrillator; ms=milliseconds; QTc= Corrected QT interval; TdP= Torsades de Pointes.
*within 7 days of TdP event
None of the patients in our cohort was admitted with COVID-19 infection or received hydroxychloroquine during hospitalization. Four patients (5.4%) were found to have congenital long QT syndrome (diagnosed after the events). The rest of TdP events (94.6%) were attributed to acquired long QT syndrome. Most of the cases occurred in the setting of QT-prolonging medications (64.7%), brady-arrhythmias (44.6%) and electrolyte disturbances (29.7%). The underlying cause of prolonged QT interval was multifactorial in twenty-one patients (28.4%). The proposed underlying causes and risk factors for TdP events are listed in (Table 2).
Table 2: Torsades de Pointes Risk Factors/Causes
|
Electrolyte disturbances, n (%) |
22 (29.7) |
|
Hypokalemia |
13 (17.6) |
|
Hypocalcemia |
2 (2.7) |
|
Hypomagnesemia |
7 (9.4) |
|
QT prolonging medications, n (%) |
48 (64.7) |
|
Antiarrhythmics |
22 (29.7) |
|
Amiodarone |
20 (27.1) |
|
Procainamide |
1 (1.3) |
|
Dofetilide |
1 (1.3) |
|
Antibiotics |
2 (2.7) |
|
Azithromycin |
2 (2.7) |
|
Antifungals |
5 (6.7) |
|
Antiemetics |
2 (2.7) |
|
Antipsychotics |
4 (5.4) |
|
Opioids |
11 (14.8) |
|
Antidepressants |
2 (2.7) |
|
Bradyarrhythmia, n (%) |
33 (44.6) |
|
Post-atrial fibrillation/flutter cardioversion, n (%) |
20 (27.1) |
|
Congenital long QT, n (%) |
4 (5.4) |
|
LQTS-1 |
1 (1.3) |
|
LQTS-2 |
2 (2.7) |
|
LQTS-3 |
1 (1.3) |
LQTS= Long-QT syndrome
Twenty patients (27%) experienced their TdP event following restoration of sinus rhythm after atrial fibrillation/flutter cardioversion. The termination of atrial fibrillation/flutter occurred within 7 days of reported TdP event in all patients. In eleven out of twenty patients (55%), the restoration of sinus rhythm occurred within 2 days of TdP event. Among this cohort of patients with post-cardioversion TdP, 10 patients (50%) had an underlying heart failure with severely reduced ejection fraction, two patients (10%) had heart failure with moderately reduced ejection fraction and one patient (5%) had heart failure with preserved ejection fraction. The remaining seven patients (35%) had no prior history of heart failure. All patients had their electrolytes corrected prior to cardioversion. Thirteen patients (65%) were noted to be bradycardic post-cardioversion. At the time of TdP event, 10 patients (50%) were on amiodarone, one patient (5%) was on dofetilide, and the rest (45%) were not on any antiarrhythmics.
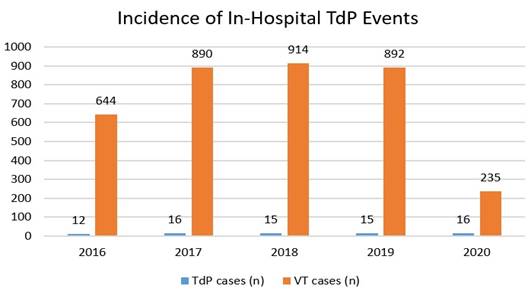
Mean pre-TdP QTc and post-TdP QTc within 7 days of reported arrhythmia event were 530.9 ± 8.4 ms and 547.8 ± 6.6ms, respectively. The mean pre-TdP QTc and mean post-TdP QTc did not significantly differ across 2016-2020 (P=.78, P=.14, respectively). Median time for the QTc interval to recover to less than 500 ms was 3 days. Time periods greater than 3 days were defined as slow recovery of QTc interval. In our cohort, 23 (47.9%) patients had slow recovery of their QTc interval, while the rest (52.1%) were found to have fast recovery (≤3 days for QTc interval to become <500 ms). History of coronary artery disease was borderline statistically significant (P=.05) for prediction of slow QTc recovery in the univariate analysis. However, after adjustment for age, gender, body mass index, left ventricular ejection fraction, hypertension, diabetes mellitus, chronic kidney disease, pre-TdP QTc, post-TdP QTc, cardioversion and the need for temporary venous pacing, no variables were predictive of slow QTc recovery on multivariable logistic regression analysis. Twenty patients (27%) exhibited T-wave inversion in at least one of the precordial leads after their TdP event (prior to getting temporary pacemaker). These patients tended to have pre-existing conduction disorders when it was compared to the remainder of the cohort. The absolute number of TdP cases remained stable throughout 2016-2020, ranging from 12-16 per year. The proportion of TdP/VT cases remained stable throughout 2016-2019. The proportion of TdP/VT cases was significantly higher in 2020 during COVID-19 pandemic compared to prior years (6.8% vs 1.7%, p<.001). This increase is attributed mainly to four-fold decrease in overall VT cases diagnosed in the hospital during that year (Figure 2).
During their hospital course, in all patients with TdP, all QT prolonging medications were discontinued, and electrolyte abnormalities were corrected. All patients received an intravenous magnesium infusion to stabilize the cardiac membrane irrespective of the serum magnesium level. Most of the cohort (56.8%) developed long runs (more than 10 seconds) of TdP, were hemodynamically unstable and required defibrillation. The rest of the patients (43.2%) were hemodynamically stable and had short bursts of TdP. Eight patients (10.8%) received an isoproterenol infusion. Nineteen patients (25.7%) continued to have intermittent runs of TdP, despite medical treatment, and required temporary transvenous pacing. Twenty-one patients (28.4%) had a pulseless TdP event during hospitalization attributed to irreversible causes and received an ICD prior to discharge. None of our patients experienced anti-tachycardia pacing therapy or ICD shock during hospitalization (post ICD implantation).
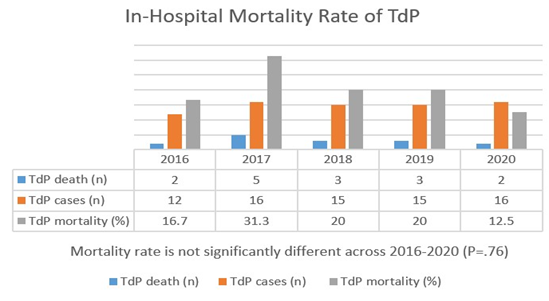
During the in-hospital course, fifteen patients in our cohort (20.3%) died. Of these 15 patients, 20% died of cardiopulmonary arrest, with cardiac arrest and various forms of shock being most frequent thereafter. The remaining patients (79.7%) survived their TdP event. Proportion of TdP patients who died during an admission was not significantly different across 2016-2020 (P=.76) (Figure 3). At univariate analysis, older age and male gender were borderline statistically significant (P=.05, P=.06, respectively) as predictors for in-hospital mortality after TdP. However, in multivariable regression analysis, no variable was significant.
Discussion
In this study, our main goal was to investigate the incidence, trends and outcomes of in-hospital monitored TdP events before and during the COVID-19 pandemic. The proportion of TdP/VT cases was estimated to be 1.7% per year in 2016-2019. The year 2020 during COVID-19 pandemic was an exceptional year and showed a statistically significant rise in the proportion of TdP/VT cases to 6.8% compared to prior years (p<.001). This observation was due to a four-fold decrease in overall VT cases presented to the hospital during that year which may be due to less patients seeking non-COVID related healthcare at the time, and transforming the healthcare system through telemedicine [21, 22]. The spread of the COVID-19 pandemic has led to decreased health care utilization by about a third globally. This correlates with Fire Department of New York data which found a significant rise in out-of-hospital cardiac arrests in 2020 compared to prior years [22-24]. Given the absolute number of TdP cases remained stable in 2020 despite a drastic drop in the total number of VT cases, we speculate that the incidence of TdP possibly increased in the year 2020 during COVID-19 pandemic. Congenital long QT syndrome constituted only 5.4% among all the cases in our cohort, while the rest were appeared to be due to acquired long QT syndrome. Although most of the TdP events occurred in the setting of QT-prolonging medications, twenty patients (27.1%) were on chronic amiodarone which, unlike other QT-prolonging medications, has been postulated to uniformly delay repolarization in all myocardial wall layers. This decreases transmural repolarization heterogeneity, which is believed to be a crucial substrate for the development of arrhythmia [25]. We also noted that all TdP events in atrial fibrillation/flutter patients occurred after restoration of sinus rhythm which might be in most patients due to the low heart rate that often accompanies cardioversion, and reverse-use dependence properties of class III antiarrhythmic medications which are frequently used in such patients [26, 27]. Darbar et al. and colleagues reported the occurrence of TdP after the termination of atrial fibrillation in 17 out of 78 torsades patients (22%) [28]. In our cohort, twenty patients (27.1%) experienced their TdP event within 7 days after atrial fibrillation/flutter termination. Among them, 10 patients (50%) were on amiodarone, 1 patient (5%) was on dofetilide, and the rest (45%) were not on any antiarrhythmics. None of our patients experienced TdP while they are in atrial fibrillation despite the frequent short-long-short cycles usually noted in such patients. This is in accord with previous reports indicating low risk of TdP during atrial fibrillation and elevated risk after cardioversion [29, 30]. Clinical anecdotes have confirmed that QT intervals are longer for any given RR interval after restoration of sinus rhythm, and the slope of the QT-RR relationship is unexpectedly flat in atrial fibrillation when compared to sinus rhythm [31]. It is well known from prior studies that there is a gradual increase in the risk of TdP as the QTc interval increases [9, 32]. Despite lacking threshold at which TdP is certain to occur, data from both congenital and acquired long QT studies showed an increased risk of TdP when the threshold of QTc > 500 ms is exceeded [33-36]. Therefore, we used a QTc > 500 ms within 7 days of reported TdP event as an inclusion criterion for our cohort, and we monitored the QTc interval post-TdP event till it recovered to less than 500 ms. TdP cause is usually multifactorial. The presence of QTc interval > 500 ms carries a high risk, but there are other contributing risk factors as well, such as genetic polymorphism, female gender, coronary artery disease, bradycardia, heart failure, renal and hepatic insufficiency [3, 8, 10, 11]. The mean pre- and post-TdP QTc in our cohort were 530.9 ± 8.4 ms and 547.8 ± 6.6 ms, respectively, and neither differed significantly across the study period. The temporal recovery of QTc interval to less than 500 ms has not been well studied in the literature, and therefore might be a physiologically distinct entity with disparate risk factors. Based on our analysis, the median time for QTc recovery to less than 500 ms was 3 days. We performed univariate and multivariable analyses to identify factors associated with slow QTc recovery (>3 days for QTc interval to become <500 ms) and found that a history of coronary artery disease was borderline statistically significant (P=.05) for prediction of slow QTc recovery. This finding correlates with prior studies which showed a relationship between coronary artery disease and long QTc interval [3, 37-39]. Macroscopic T-wave inversion is also a known risk marker for cardiac events [40]. However, the exact reason and the significance for the presence of T-wave inversion post-TdP is not clear.
The role of ICD following a survived pulseless TdP event is controversial, especially in those with acquired long QT syndrome [41, 42]. Gerold et al. (2012) and colleagues studied 43 patients with an ICD implanted for secondary prevention following a pulseless TdP event in the setting of acquired long QT syndrome [43]. The remarkable finding of their study was the occurrence of appropriate ICD shocks in 19 patients (44%) during follow up even in the absence of structural heart disease or obvious QT prolonging triggers. This is believed to be due to the presence of reduced repolarization reserve in such individuals which can be unmasked by unknown factors that lead to QT prolongation and subsequent TdP event that can degenerate into ventricular fibrillation and results in a sudden cardiac death. In our cohort, twenty-one patients (28.4%) had a pulseless TdP event during hospitalization attributed to irreversible causes and received an ICD prior to discharge. None of our patients experienced anti-tachycardia pacing therapy or ICD shock during hospitalization. The prognosis of congenital long QT is known to be good if promptly diagnosed and treated appropriately, whereas all-cause mortality, cardiovascular mortality and sudden cardiac death associated with acquired long QT are reported to be high [44, 45]. Huagaa et al. (2013) at Mayo Clinic Rochester, Minnesota used a QTc threshold of 500 ms to investigate the survival outcomes and reported 19% all-cause mortality in patients with QTc of 500 ms or greater compared with 5% in patients with QTc < 500 ms [46]. In our study, we investigated the survival outcomes in patients with TdP event and QTc > 500 ms within 7 days of reported arrhythmia. Fifteen patients in our cohort (20.3%) died during admission. The proportion of TdP patients who died during admission in our study was not significantly different across 2016-2020 (P=.76). At univariate analysis, older age and male gender were borderline statistically significant (P=.05, P=.06, respectively) as predictors for in-hospital mortality. This correlates with other studies which identified age and male gender as predictors for mortality in patients with QT prolongation [47].
4. Study limitations
The analysis is limited by the retrospective nature of the study and small number of patients. This study is also subject to detection bias due to frequently lost rhythm strips, selection bias and unmeasured confounders that affect the QTc interval and defy multivariable analysis. It is also possible that due to lack of specific coding, additional cases of TdP may have been missed. Moreover, the measurement of the QTc interval itself is subject to substantial variability due to multiple factors such as diurnal variation, autonomic factors, electrolytes, drugs, acquisition of the ECG recording and intra-observer variation in measurement [3]. Additionally, the QTc variability and QT-RR relationship significantly differs between patients with normal and prolonged QT intervals [48]. Furthermore, the outward ion channel properties and repolarization reserve affect the QTc variability differently in patients with normal structural heart as opposed to those with known heart failure or prior myocardial infarction [49]. Despite these limitations, we believe that our observations provide new insights into the incidence, outcomes and predictors of mortality and QTc recovery in patients with TdP events. Our findings can be strengthened by large multi-center trials with detailed follow-up data.
Conclusions:
In this study of hospitalized patients at a quaternary medical academic institution in a COVID-19 epicentre, we found that the proportion of TdP as a fraction of overall VT cases increased in 2020. The in-hospital all-cause mortality rate was not significantly different during the 5-year study period. Older age and male gender were borderline statistically significant for prediction of in-hospital mortality.
Conflict of Interest:
Authors declare that they have no conflicts of interest
References:
- Coeur FDAM, Ventricular tachycardia has two variable opposing foci. ci.nii.ac.jp (1966).
- El-Sherif N, Turitto G, Boutjdir M. Acquired long QT syndrome and torsade de pointes. PACE - Pacing Clin Electrophysiol 41 (2018): 414-421.
- Al-Khatib SM, Allen LaPointe NM, Kramer JM, et al. What Clinicians Should Know About the QT Interval. JAMA 289 (2003): 2120-2127.
- Shimizu W, Antzelevitch C. Cellular basis for long QT, transmural dispersion of repolarization, and torsade de pointes in the long QT syndrome. J Electrocardiol 32 (1999): 177-184.
- Ponce-Balbuena D, Deschênes I. Long QT syndrome - Bench to bedside. Hear Rhythm 2 (2021): 89-106.
- Priori SG, Napolitano C, Paganini V, et al. Molecular biology of the long QT syndrome:Impact on management. PACE - Pacing Clin Electrophysiol 20 (1997): 2052-2057.
- Khan IA. Long QT syndrome: diagnosis and management. Am Heart J 143 (2002): 7-14.
- Bednar MM, Harrigan EP, Anziano RJ, et al. The QT interval. Prog Cardiovasc Dis 43 (2001): 1-45.
- Moss AJ, Schwartz PJ, Crampton RS, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation 84 (1991): 1136-1144.
- Sauer AJ, Newton-Cheh C. Clinical and genetic determinants of torsade de pointes risk. Circulation 125 (2012): 1684-1694.
- Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol 51 (2008): 2291-2300.
- Schwartz PJ, Woosley RL, Woosley RL. Predicting the Unpredictable: Drug-Induced QT Prolongation and Torsades de Pointes. J Am Coll Cardiol 67 (2016): 1639-1650.
- Drew BJ, Ackerman MJ, Funk M, et al. Prevention of Torsade de Pointes in Hospital Settings. Circulation 121 (2010): 1047-1060.
- Chorin E, Wadhwani L, Magnani S, et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Hear Rhythm 17 (2020): 1425-1433.
- Saleh M, Gabriels J, Chang D, et al. Effect of Chloroquine, Hydroxychloroquine, and Azithromycin on the Corrected QT Interval in Patients with SARS-CoV-2 Infection. Circ Arrhythmia Electrophysiol 13 (2020): 496-504.
- Rubin GA, Desai AD, Chai Z, et al. Cardiac Corrected QT Interval Changes Among Patients Treated for COVID-19 Infection During the Early Phase of the Pandemic. JAMA Netw open 4 (2021): 216842.
- Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT Interval Prolongation Associated with Use of Hydroxychloroquine with or without Concomitant Azithromycin among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 5 (2020): 1036-1041.
- O’Connell TF, Bradley CJ, Abbas AE, et al. Hydroxychloroquine/Azithromycin Therapy and QT Prolongation in Hospitalized Patients With COVID-19. JACC Clin Electrophysiol 7 (2021): 16-25.
- Vink AS, Neumann B, Lieve KVV, et al. Determination and Interpretation of the QT Interval. Circulation 138 (2018): 2345-2358.
- Yankelson L, Hochstadt A, Sadeh B, et al. New formula for defining “normal” and “prolonged” QT in patients with bundle branch block. J Electrocardiol 51 (2018): 481-486.
- Mann DM, Chen J, Chunara R, et al. COVID-19 transforms health care through telemedicine: Evidence from the field. J Am Med Informatics Assoc 27 (2020): 1132-1135.
- Moynihan R, Sanders S, Michaleff ZA, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open 11 (2021): e045343.
- Mountantonakis SE, Saleh M, Coleman K, et al. Out-of-Hospital Cardiac Arrest and Acute Coronary Syndrome Hospitalizations During the COVID-19 Surge. J Am Coll Cardiol 76 (2020): 1271.
- Schriger DL. Learning from the Decrease in US Emergency Department Visits in Response to the Coronavirus Disease 2019 Pandemic. JAMA Intern Med 180 (2020): 1334-1335.
- Lazzara R. Amiodarone and torsade de pointes. Ann Intern Med 111 (1989): 549-551.
- Roden DM, Kannankeril P, Darbar D. On the relationship among QT interval, atrial fibrillation, and torsades de pointes. Europace 9 (2007): 1-3.
- Choy AMJ, Darbar D, Dell’Orto S, et al. Exaggerated QT prolongation after cardioversion of atrial fibrillation. J Am Coll Cardiol 34 (1999): 396-401.
- Darbar D, Kimbrough J, Jawaid A, et al. Persistent Atrial Fibrillation is Associated with Reduced Risk of Torsades de Pointes in Patients with Drug-Induced Long QT Syndrome. J Am Coll Cardiol 51 (2008): 836.
- Choy AMJ, Darbar D, Dell’Orto S, et al. Exaggerated QT prolongation after cardioversion of atrial fibrillation. J Am Coll Cardiol 34 (1999): 396-401.
- Nowinski K, Gadler F, Jensen-Urstad M, et al. Transient proarrhythmic state following atrioventricular junction radiofrequency ablation: pathophysiologic mechanisms and recommendations for management. Am J Med 113 (2002): 596-602.
- Darbar D, Hardin B, Harris P, et al. A Rate-Independent Method of Assessing QT-RR Slope Following Conversion of Atrial Fibrillation. J Cardiovasc Electrophysiol 18 (2007): 636-641.
- Zareba W, Moss AJ, Schwartz PJ, et al. Influence of the Genotype on the Clinical Course of the Long-QT Syndrome. N Engl J Med 339 (1998): 960-965.
- Priori SG, Schwartz PJ, Napolitano C, et al. Risk Stratification in the Long-QT Syndrome. N Engl J Med 348 (2003): 1866-1874.
- Sauer AJ, Moss AJ, McNitt S, et al. Long QT Syndrome in Adults. J Am Coll Cardiol 49 (2007): 329-337.
- L De Bruin CM, De Bruin ML, J Langendijk PN, et al. In-hospital cardiac arrest is associated with use of non-antiarrhythmic QTc-prolonging drugs. Wiley Online Libr 63 (2006): 216-223.
- Roden D, Woosley R, journal RPA heart. Incidence and clinical features of the quinidine-associated long QT syndrome: implications for patient care. Elsevier (1986).
- Rebeiz A, Cardiology SAC. A case of severe ischemia-induced QT prolongation. ncbi.nlm.nih.gov (2001).
- journal SAE heart. QT interval prolongation in acute myocardial infarction. academic.oup.com (1985).
- Dekker JM, Schouten EG, Klootwijk P, et al. Association between QT interval and coronary heart disease in middle-aged and elderly men. The Zutphen Study. Circulation 90 (1994): 779-785.
- Aro AL, Anttonen O, Tikkanen JT, et al. Prevalence and prognostic significance of T-wave inversions in right precordial leads of a 12-lead electrocardiogram in the middle-aged subjects. Circulation 125 (2012): 2572-2577.
- Zipes D, Camm A, … MBE heart. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—executive summary: a report of. academic.oup.com (2006).
- Schwartz PJ, Spazzolini C, Priori SG, et al. Who are the long-QT syndrome patients who receive an implantable cardioverter-defibrillator and what happens to them?: Data from the European Long-QT syndrome implantable cardioverter-defibrillator (LQTS ICD) registry. Circulation 122 (2010): 1272-1282.
- Mönnig G, Köbe J, Löher A, et al. Role of implantable cardioverter defibrillator therapy in patients with acquired long QT syndrome: A long-term follow-up. EP Eur 14 (2012): 396-401.
- Schwartz P, Crotti L, and RICA. Long-QT syndrome: from genetics to management. Am Hear Assoc (2012).
- Zhang Y, Post WS, Blasco-Colmenares E, et al. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology 22 (2011): 660.
- Haugaa KH, Bos JM, Tarrell RF, et al. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc 88 (2013): 315-325.
- Gibbs C, Thalamus J, Heldal K, et al. Predictors of mortality in high-risk patients with QT prolongation in a community hospital. EP Eur 20 (2018): f99-f107.
- Chan A, Isbister GK, Kirkpatrick CMJ, et al. Drug-induced QT prolongation and torsades de pointes: evaluation of a QT nomogram. QJM An Int J Med 100 (2007): 609-615.
- Nattel S, Maguy A, Le Bouter S, et al. Arrhythmogenic ion-channel remodeling in the heart: Heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 87 (2007): 425-456.