Impact of Clinical Pharmacist-Vancomycin Monitoring on Patient Safety Outcome
Article Information
Rana Al-Ruwaisan1*, Reem BaHmaid2, and Nora Al-Banyan3
1Clinical Pharmacist, King Fahad Medical City (KFMC), Riyadh, Kingdom of Saudi Arabia.
2 Clinical Pharmacist, King Fahad Medical City (KFMC), Riyadh, Kingdom of Saudi Arabia.
3 Clinical Pharmacist, King Fahad Medical City (KFMC), Riyadh, Kingdom of Saudi Arabia.
*Corresponding author: Rana Al-Ruwaisan, Clinical Pharmacist, King Fahad Medical City (KFMC), Riyadh, Kingdom of Saudi Arabia
Received: 21 September 2020; Accepted: 28 September 2020; Published: 05 October 2020
Citation: Rana Al-Ruwaisan, Reem BaHmaid, Nora Al-Banyan. Impact of Clinical Pharmacist- Vancomycin Monitoring on Patient Safety Outcome. Archives of Clinical and Biomedical Research 4 (2020): 441-452.
Share at FacebookAbstract
Introduction: Vancomycin is frequently used to treat gram-positive infections especially methicillin- resistant Staphylococcus aureus (MRSA). Vancomycin level in the blood should be kept in a specific range to give the optimal antimicrobial killing and avoid the development of resistant and nephrotoxicity with low or high serum levels, respectively. This is known as "Therapeutic Drug Monitoring (TDM)." We aimed to evaluate the safety consequence of clinical pharmacist- based vancomycin TDM versus physician-based vancomycin TDM.
Methods: Our study is a retrospective cohort study conducted at a single tertiary hospital, King Fahad Medical City (KFMC), Riyadh, Saudi Arabia. It included two groups one for physicians and one for clinical pharmacists. The patients were all adults more than 18 years old started on vancomycin intravenously for more than 24 hours for suspected or proven infection. The primary outcome was the development of nephrotoxicity. In addition to several secondary outcomes, that include appropriate vancomycin initial dosing, sampling time, interpretation of vancomycin level, and the development of other adverse reactions related to the use of vancomycin.
Results: A total of 100 patients were enrolled in the study with 53 patients in the physician group. There were no significant differences in baseline characteristics between the two groups. The defined endpoint was reported as 3.8% (n=2) in physician group and 12.8% (n=6) in Clinical pharmacist group with a P value of 0.143. Moreover, there was a significant difference in the defined secondary endpoints that include appropriate vancomycin initial dosing, sampling time, interpretation of vancomycin level, and other adverse reactions with a P value of less than 0.001.
Conclusion: There is a non-statistically significant higher rate of nephrotoxicity in
Keywords
Vancomycin; Clinical Pharmacist; Physician; Therapeutic Drug Monitoring; Nephrotoxicity
Article Details
Introduction
Vancomycin is one of the glycopeptides antibiotics that considered the gold standard treatment of severe gram-positive infections involving methicillin resistance Staphylococcus aureus (MRSA) [1]. Some adverse events that are well known to be associated with the use of vancomycin are infusion-related reactions especially "red man" or "red neck” syndrome associated with rapid infusion, nephrotoxicity, and ototoxicity [2].
The importance of vancomycin monitoring is rising from the development of resistant strains and therapeutic failure. These are associated with low serum vancomycin concentrations. The susceptibility and resistance breakpoints for the Minimum Inhibitory Concentration (MIC) of vancomycin against S. aureus are ≤2 mg/L for “susceptible,” 4–8 mg/L for “intermediate,” and ≥16 mg/L for “resistant” according to the Clinical and Laboratory Standards Institute (CLSI) [3]. There is a direct correlation between low serum vancomycin level and the development of Vancomycin-Resistant S. aureus (VISA) [4]. It is recommended to maintain vancomycin serum level >10 mg/L (7 µmol/L) to achieve successful therapy and avoid prolongation of treatment, therefore, prevent the development of resistant strains [4,5].
To interpret the results correctly, we should assure that the sample of blood used to measure the serum vancomycin level is collected at an exact time after steady state before the fourth dose. Around 25% of levels taken too early were misinterpreted by clinician and vancomycin dosage changes were done either by holding, decreasing, increasing, or discontinuing the dose [6]. Therapeutic drug monitoring (TDM) services by pharmacists have been shown to be of great benefit. Therapeutic drug monitoring is defined as the tailoring of the patient dosage regimen to maintain drug plasma level within therapeutic range by measuring clinical laboratory chemical parameters with an appropriate interpretation of the results [7]. The cost-effectiveness of vancomycin serum concentration monitoring was studied in one prospective randomized study in patients with hematologic malignancies. The study included 70 immunocompromised febrile patients with hematologic malignancy randomized to control group (n=33) and vancomycin therapeutic drug monitoring group (n=37). Their outcomes were length of hospital stay (TDM group: 24.5±15.5; non-TDM group: 24.1±15), improved clinical response (TDM group: 24; non-TDM group: 19), number of days of fever (TDM group: 5.6±4.7; non-TDM group: 7.1±5.3), time to reach apyrexia (TDM group: 4.1±3.9; non-TDM group: 5.4±4.9), duration of vancomycin therapy (TDM group: 18.2±8.9; non-TDM group: 20.3±9.1), modifications in antibiotic therapy, and the incidence of nephrotoxicity. Among these outcomes, only incidence of nephrotoxicity shows a statistically significant decrease in TDM group compared to non-TDM group (42.4% and 13.5%; p<0.05, respectively). Their cost analysis was based on the cost of serum vancomycin assays (drug assay kits, calibration, quality control) and the time spent by clinical pharmacist and nurses in performing TDM activities. It showed that it costs $435 per case to prevent mild or moderate nephrotoxicity [8].
Another prospective, cohort study that involved 116 patients, 61 patients were in TDM group and 55 patients in the non-TDM group, was conducted to evaluate the impact of vancomycin therapeutic drug monitoring. The decrease in renal function was significantly higher in the non-TDM group compared to TDM group (24%, 7%; P<0.05 respectively), so the decrease in incidence of nephrotoxicity was 17%. Vancomycin serum concentrations measured in all TDM group while in non-TDM only 71% had vancomycin serum concentrations measured. The difference in dose adjustments based on vancomycin levels was statistically significant with 1.1±0.8 changes per patient in TDM group compared to 0.7±1.1 per patient in the non-TDM group. There was no statistically significant difference concerning duration of therapy with a mean of 11.1±5.8 days in TDM group and 13.4±13.6 days in the non- TDM group. Patient care was not compromised by vancomycin TDM service [9].
Another prospective study was conducted in a tertiary care academic hospital to evaluate short-term impact of vancomycin dosing and therapeutic drug monitoring guidelines. Their primary objectives are the appropriateness of initial vancomycin dosing and appropriateness of sampling time. Three different educational interventions were used to implement the guideline. The first intervention was one- page guideline that was developed based on the IDSA/ASHP vancomycin guidelines. The second intervention was in-services education that was provided to nurses and phlebotomists regarding appropriate sampling time of trough level. The last intervention was dosing cards containing initial vancomycin dosing and TDM was distributed to medical staff and pharmacists. The number of patients included is 279 in pre-implementation phase and 200 in post-implementation phase. There was a significantly better initial dosing in post phase (78%) compared to pre-phase (51%), (p<0.0001). Sampling time was improved significantly from 36% in pre-phase to 55% in post phase (p<0.03). Education and training about vancomycin dosing and TDM are associated with a short-term benefit [10].
One systematic review and meta-analysis was addressed to evaluate the evidence for the necessity of vancomycin therapeutic drug monitoring in treating Gram-positive infections. Their primary outcome was clinical efficacy, and their secondary outcomes were nephrotoxicity, duration of vancomycin therapy, length of hospital stay and mortality. One randomized controlled trial and five cohort studies were analyzed. High rates of clinical efficacy was observed in TDM group (OR = 2.62, 95%CI 1.34–5.11 P = 0.005) and this group shows also a decreased rates of nephrotoxicity (OR = 0.25, 95%CI 0.13–0.48 P= 0.0001). On the other hand, the differences in remaining secondary outcomes were not significant, including vancomycin duration of therapy and length of stay [11].
The aim of the study
We aimed to study the difference between clinical pharmacist-based vancomycin TDM versus physician-based vancomycin TDM regarding safety outcome of vancomycin therapy.
Method/Procedure
The study is an observational retrospective cohort study that was conducted at King Fahad Medical City (KFMC), Riyadh, KSA, starting in September 2014. KFMC is a tertiary health care facility that contains four hospitals (Main, Women Specialized, Children, and Rehab hospitals) and four centers of excellence, which serve around 1095 beds. Either a clinical pharmacist or physician performs vancomycin TDM at KFMC. We conducted this observational study to determine the difference in patient safety outcome between TDM services led by clinical pharmacists versus physicians.
The primary outcome of the study was that nephrotoxicity which is defined as a rise in serum creatinine concentration of >0.5 mg/dL (44 µmol/L) or ≥50% increase from baseline on two consecutive days [5]. Secondary outcomes include appropriate initial dosage regimen, sampling time, interpretation of trough vancomycin levels as per KFMC approved vancomycin guideline, and other adverse reactions such as red- man syndrome, and anaphylactic reactions.
Procedure
In this study we screened all the patients started on vancomycin to see if they are followed by a clinical pharmacist or not, then we separated the patients into clinical pharmacist-based TDM group or physician-based TDM group (control group). Then, we collected the data from patients’ files and medical records after completion of vancomycin course. In case of clinical pharmacist consultation in physician group for vancomycin monitoring, the consulted case was shifted to clinical pharmacist TDM group.
Management Protocol
Patient’s evaluation and therapeutic drug monitoring for vancomycin were performed according to KFMC vancomycin guideline. In the guideline, Vancomycin dose of 15–20 mg/kg (as actual body weight) given every 8–12 hr in normal renal function is recommended. A loading dose of 25–30 mg/kg (based on actual body weight) is recommended in severely ill patients with complicated infection.
The doses in patients with renal impairment to be adjusted according to creatinine clearance see table (1). Patients on intermittent hemodialysis to be given a dose of 1000-1500 mg after 25–30 mg/kg loading dose, then 5-10 mg/kg after each dialysis session, based on pre-dialysis concentration, table (2). Doses for patients on continuous renal replacement therapy (CRRT) are according to the method of renal replacement, table (2).
|
Table 1: Dose Adjustment According to CrCl |
||
|
CrCl* |
Dose* |
Recommendation |
|
>65 ml/min |
Uncomplicated Infections: 10-15 mg/kg q12h |
Trough levels: 10-15 mg/L (7-10 µmol/L) |
|
Serious Infections: Consider loading dose of 25 mg/kg IV once, despite renal function followed by: 15-20 mg/kg q8-12h (45-60 mg/kg/day divided q12h or q8h) |
For patients with serious infections due to MRSA : o CNS infections o Endocarditis o VAP o Bacteremia o Osteomyelitis Trough levels: 15-20 mg/L (10-14mcmol/L) -ID Consult is recommended |
|
|
40-65 ml/min |
10-15 mg/kg q12-24h |
|
|
20-40 ml/min |
5-10 mg/kg q24h |
|
|
10-20 ml/min |
5-10 mg/kg q24-48h |
|
|
<10 ml/min |
10 - 15 mg/kg IV loading dose once; then 500 mg every 48 to 96 hours based on trough level |
|
|
*CrCl: Creatinine Clearance based on Cockcroft and Gault equation. |
||
|
Table 2: Dose Adjustment in Renal Impairment with Dialysis |
|
|
Intermittent hemodialysis (IHD)** |
|
|
Intermittent hemodialysis (IHD) |
Following loading dose, give 1000-1500 mg then 5-10 mg/kg after each dialysis session ** based on pre dialysis concentration |
|
Re-dosing based on pre-HD concentrations: |
If Vancomycin serum trough concentration is: < 10 mg/L (7 µmol/L): Administer 1000 mg after HD 10-25 mg/L (7-17 µmol/L): Administer 500-750 mg after HD > 25 mg/L (17 µmol/L): Hold vancomycin, & do random vancomycin level |
|
Redosing based on post-HD concentrations |
< 10-15 mg/L (7-10 µmol/L): Administer 500-1000 mg |
|
Continuous renal replacement therapy (CRRT)*** |
|
|
CVVH |
Loading dose of 15-25 mg/kg, followed by either 1000 mg every 48 hours or 10-15 mg/kg every 24-48 hours |
|
CVVHD |
Loading dose of 15-25 mg/kg, followed by either 1000 mg every 24 hours or 10-15 mg/kg every 24 hours |
|
CVVHDF |
Loading dose of 15-25 mg/kg, followed by either 1000 mg every 24 hours or 7.5-10 mg/kg every 12 hours. |
|
Note: **Dosing dependent on the assumption of 3 times/week, complete IHD sessions. ***For CRRT: vancomycin maintenance dose based on random vancomycin trough concentration, target level concentrations <10-15 mg/L (7-10 μmol/L) or 15-20 mg/L (10-14 μmol/L). CVVH: Continuous Veno-Venous Hemofiltration; CVVHD: Continuous Veno-Venous Hemodiallysis; CVVHDF: Continuous Veno-Venous Hemodiafiltration. |
|
Therapeutic drug monitoring and details about vancomycin serum levels are listed in table (3, 4). The minimum trough concentration should be maintained >10mg/L (>7 µmol/L) to avoid development of resistance, with a range from 10- 15mg/L (7-10 µmol/L) if MIC<1 mg/L. In case of complicated infections (bacteremia, endocarditis, osteomyelitis, meningitis, and hospital-acquired pneumonia caused by Staphylococcus aureus), or MIC 1-2 mg/L the recommended trough concentration is 15-20 mg/L (10-14 µmol/L), see tables 4 and 5.
|
Table 3: Therapeutic Drug Monitoring |
|
|
Monitoring |
Recommendation |
|
Trough serum concentration monitoring |
· The most accurate and practical method for monitoring efficacy. · Recommended for patients: o Requiring therapy > 4 days o With severe or life-threatening infections o Receiving concomitant nephrotoxic drugs (e.g., cyclosporine, amphotericin B, aminoglycosides) o Aggressive dosing. o Unstable renal function. o Morbidly obese |
|
Sample Time |
Trough levels should be obtained within 30 minutes before 4th dose of a new regimen or dosage change. |
|
Short duration of therapy (≤ three days) |
Vancomycin troughs are not recommended in those patients |
|
Oral vancomycin therapy Difficile –associated diarrhea |
Vancomycin troughs are not recommended in those patients |
|
Patients with stable renal function and clinical status |
Once weekly monitoring is reasonable. |
|
Hemodynamically unstable |
Draw trough concentrations more frequently or in some instances daily. |
|
Hemodialysis |
Trough levels are recommended for routine monitoring (for intermittent hemodialysis, a pre-dialysis level should be drawn). |
|
Random concentrations ONLY if: |
- Severe renal dysfunction or on dialysis. -Obtain a level after 3-4 days of therapy. More frequent sampling is usually not necessary. - Re-dose when serum level ≤15 mg/L.(≤10 µmol/L) |
|
Peak monitoring |
Data do not support using peak serum vancomycin concentrations to monitor for nephrotoxicity. Both trough and peak concentrations ONLY if: Severe infections requiring "high" concentrations to penetrate selected sites (e.g., endocarditis, osteomyelitis) OR not responding to therapy. - Obtain peak levels at least 1 hour after the end of the infusion. -Peak levels of 20-40 mg/L (14- 27 µmol/L) have been considered “therapeutic.” |
|
Monitoring serum vancomycin levels to prevent ototoxicity |
· Monitoring serum vancomycin levels to prevent ototoxicity is not recommended because this toxicity is rarely associated with monotherapy and does not correlate with serum vancomycin concentrations. · Monitoring may be more important when other ototoxic agents, such as aminoglycosides, are administered. |
|
Table 4: Therapeutic Trough Concentrations |
|
|
Type of Infection |
Target Trough Concentration |
|
Soft skin tissue infections , Abscess, cellulitis (MIC <1 mg/L) |
10-15 mg/L (7-10 µmol/L) |
|
Soft skin tissue infections , Abscess, cellulitis (MIC >1 mg/L) |
15 – 20 mg/L (10-14 µmol/L) |
|
Complicated infections (endocarditis, osteomyelitis, bacteremia, prosthetic joint infection, or Pneumonia) |
15 – 20 mg/L (10-14 µmol/L) |
|
Infection involving central nervous system ( meningitis) |
20-25 mg/L (14-17.5 µmol/L) |
Data Collection
The following patient data were collected: Medical Record Number (MRN), age, gender, weight, height, temperature, baseline serum creatinine levels, creatinine clearance and white blood cell count, an indication of vancomycin, and medication history for patients developed nephrotoxicity. Vancomycin initial dosing regimen, duration of therapy, time of vancomycin level sample, serum level, interpretation of vancomycin level, and dosage changes according to the level were collected. Serum creatinine during vancomycin was collected to observe any nephrotoxicity. Data collected included any reports of other adverse reactions such as red-man syndrome, anaphylactic reactions, and other adverse reactions.
Statistical Analysis
Considering a 24% prevalence of vancomycin-induced nephrotoxicity, 95% confidence interval and 5% margin of error, we calculated the sample size to be 105 patients (9). Categorical variables were presented as numbers and percentages. Continuous variables were expressed as Mean ± S.D. Independent sample t-test was used to find out the significant mean difference between initial vancomycin dose and primary outcome. Whereas Person's Chi-square or Fisher exact test was used according to whether the cell frequencies is smaller than 5 and consequently determine the significant association between the primary and secondary outcome of vancomycin effectiveness. P value < 0.05 will be considered as statistically significant. All data was entered and analyzed through statistical package SPSS version 20.
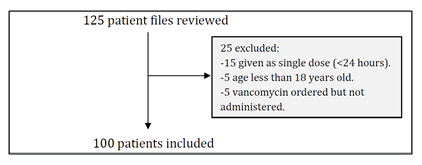
In this cohort study 125 patient files were reviewed, 25 of them excluded (Figure (1)) and 100 patients met the inclusion criteria. Fifty-three patients in physician group and 47 patients in clinical pharmacist group. Table (5) shows the baseline characteristics of the patients. The mean age of patients was 49.53 ± 21.50 in physician group and 54.85 ± 20.38 in clinical pharmacist group. In physician group, 49.1% (n=26) were female, while there were 44.7% (n=21) female patients in clinical pharmacist group. Before starting vancomycin the mean serum creatinine and creatinine clearance in physician group were 49.10 ± 26.01 and 36.33 ± 27.46 respectively. On the other hand, the baseline SCr and CrCl in clinical pharmacist group were 40.4 ± 23.13 and 35.80 ± 23.91 respectively. The mean duration of therapy in physician group was 7.91 ± 4.84 days and 10.47 ± 10.72 days in clinical pharmacist group. Vancomycin in physician group was used most frequently for patients having febrile neutropenia, while in clinical pharmacist group the use was mostly in patients with bacteremia.
|
Table 5: Baseline Characteristics |
||||
|
Characteristics |
Physician Group (n=53) |
Clinical Pharmacist Group (n=47) |
P. value |
|
|
Mean Age (Year) |
49.53 ± 21.50 |
54.85 ± 20.38 |
0.209 |
|
|
Gender Male Female |
26 (55.3%) 21 (44.7%) |
0.692 |
||
|
27 (50.9%) 26 (49.1%) |
||||
|
Mean SCr (umol/L) |
49.10 ± 26.01 |
40.4 ± 23.13 |
0.082 |
|
|
Mean CrCl (ml/min) |
36.33 ± 27.46 |
35.80 ± 23.91 |
0.92 |
|
|
Mean duration of therapy (Days) |
7.91 ± 4.84 |
10.47 ± 10.72 |
0.12 |
|
|
Indication of Vancomycin |
Febrile Neutropenia |
12 (28.6%) |
6 (17.1%) |
0.199 |
|
SSTI |
9 (21.4%) |
5 (14.3%) |
0.361 |
|
|
Urosepsis |
1 (2.4%) |
0.00% |
0.343 |
|
|
Bacteremia |
5 (11.9%) |
8 (22.9%) |
0.26 |
|
|
Infective Endocarditis |
1 (2.4%) |
2 (5.7%) |
0.488 |
|
|
Septic Shock |
2 (4.8%) |
5 (14.3%) |
0.179 |
|
|
Sepsis |
2 (4.8%) |
2 (5.7%) |
0.903 |
|
|
Empirically |
1 (2.4%) |
0.00% |
0.343 |
|
|
Pneumonia |
5 (11.9%) |
2 (5.7%) |
0.311 |
|
|
Aspiration Pneumonia |
1 (2.4%) |
3 (8.6%) |
0.252 |
|
|
Parotitis |
1 (2.4%) |
0.00% |
0.343 |
|
|
HAP |
1 (2.4%) |
1 (2.9%) |
0.931 |
|
|
Meningitis |
1 (2.4%) |
0.00% |
0.343 |
|
|
Valval Infection |
0.00% |
1 (2.9%) |
0.285 |
|
|
n: Number of patients; SCr: Serum Creatinine; CrCl: Creatinine Clearance; SSTI: Skin & Soft Tissue Infections; HAP: Hospital Acquired Pneumonia. |
||||
Nephrotoxicity was the primary outcome, it was reported in 3.8% (n=2) of patients in physician group and 12.8% (n=6) in clinical pharmacist group with a non- statistical significant P value of 0.143. There was no statistically significant difference in nephrotoxicity between patients with age more than 50 years old or less than 50 years old (P=0.464). The mean increase in serum creatinine was slightly higher in physician group 51± 12 umol/L, while it was 46± 28 umol/L in clinical pharmacist group. However, that difference was not statistically significant (P=0.239). The duration of vancomycin was statistically significant longer in clinical pharmacist group 9± 3 days compared with physician group 3± 0.1 days with a P value of 0.001. The concomitant use of other medications that can increase the serum creatinine level was associated more frequently in clinical pharmacist group than in physician group; see table (6). The use of Meropenem was statistically significant higher in clinical pharmacist group, and tend to be significantly higher with Furosemide.
|
Table 6: Concomitant Medications that Increase SCr |
|||
|
Concomitant Medication* |
Physician Group (n=2) |
Clinical Pharmacist Group (n=6) |
P. value |
|
Meropenem |
0.0% |
4 (8.5%) |
0.030 |
|
Colistin |
1 (2.4%) |
2 (5.7%) |
0.488 |
|
Amikacin |
1 (2.4%) |
0.0% |
0.343 |
|
Pipracilln/Tazobactam |
0.0% |
2 (5.7%) |
0.129 |
|
Acyclovir |
1 (2.4%) |
0.0% |
0.343 |
|
Ceftriaxone |
1 (2.4%) |
0.0% |
0.343 |
|
PPI |
1 (2.4%) |
4 (8.5%) |
0.129 |
|
Furosemide |
0.0% |
3 (6.3%) |
0.061 |
|
Voriconazole |
0.0% |
1 (2.1%) |
0.285 |
|
n: Number of patients; PPI: Proton Pump Inhibitors; SCr: Serum Creatinine *For patients who developed nephrotoxicity |
|||
The secondary outcomes were appropriate initial dosing regimen, appropriate sampling time, proper interpretation of vancomycin level and other adverse drug reactions, Table (7). Among the patients in clinical pharmacist group, 15 (31.9%) patients were not able to get their secondary outcome because their files were missing. The initial dosing regimen was appropriate in 84.9% (n=45) of patients in physician group and 87.5% (n=28) (P<0.001) in clinical pharmacist group. In physician group, the appropriate sampling time was 37.7% (n=20) while it was 62.5% (n=20) (P<0.001) in clinical pharmacist group. Regarding the interpretation of trough vancomycin levels, it was appropriate in 11.3% (n=6) of physician group patients and 48.9% (n=23) (P<0.001) in clinical pharmacist group. No reports of other adverse drug reactions of vancomycin in both groups.
|
Table 7: Secondary Outcomes |
|||
|
Outcome |
Physician Group (n=53) |
Clinical Pharmacist Group (n=32) |
P. value |
|
The appropriate initial dosing regimen Appropriate Inappropriate |
45 (84.9%) 8 (15.1%) |
28 (87.5%) 4 (12.5%) |
*< 0.004 |
|
Appropriate sampling time Appropriate Inappropriate |
20 (37.7%) 33 (62.3%) |
20 (62.5%) 12 (37.5%) |
*< 0.021 |
|
Appropriate interpretation of vancomycin level Appropriate Inappropriate |
6 (11.3%) 47 (88.7%) |
23 (48.9%) 9 (19.1%) |
*< 0.001 |
|
Other adverse reactions No |
53 (100%) |
32 (100%) |
*< 0.001 |
|
N/A: Not Available *From the above table, there is statistically significant (P < 0.004) associations was observed between the clinical pharmacist and physicians with respect dosage pattern. However, 8 (15.1%) Physicians were mentioned inappropriate dosage of vancomycin and 4 (8.5%) clinical pharmacist had an inappropriate dosage of vancomycin. *Similarly, there is statistically significant (P < 0.021) associations was observed between clinical pharmacist and physicians with respect vancomycin sampling procedure. On the other hand, 33 (62.3%) physicians had an inappropriate sampling of vancomycin and 12 (25.5%) clinical pharmacist had an inappropriate sampling of vancomycin. |
|||
Discussion
In this retrospective study, we included 100 patients received vancomycin for more than 24 hours. The vancomycin monitoring was performed by the physician in 53% of the patients, and 47% were followed by a clinical pharmacist. There was no significant difference between the patients in both groups in regards to age, gender, SCr, CrCl, duration of therapy and indications for the use of vancomycin. However, most of the cases in physician group received vancomycin for febrile neutropenia while in clinical pharmacist group it was used mostly for bacteremia. For this reason, the duration was longer in clinical pharmacist group.
The primary safety outcome in this study was the development of nephrotoxicity. The clinical pharmacist group had a non-statistically significant higher rate of nephrotoxicity compared to physician group 12.8% vs. 3.8% (P=0.143). This increase in nephrotoxicity can be explained by the nature of clinical pharmacist group as septic shock and aspiration pneumonia was higher. In addition to the use of other medications that can rise the serum creatinine in clinical pharmacist group including meropenem, colistin, piperacillin/tazobactam, and furosemide. All patients who develop nephrotoxicity in physician group were more than 50 years old while 50% of clinical pharmacist patients where in this age group. Among the patients who developed nephrotoxicity in both groups, the increase in serum creatinine was non- statistically significant higher in physician group patients compared with clinical pharmacist patients. The statistically significant difference was the duration of vancomycin therapy, which was almost six days longer in clinical pharmacist group that will increase the likelihood of serum creatinine increase.
The data for secondary outcomes were collected for all patients in the physician group. However, due to the missing files of patients in clinical pharmacist group, we were not able to get the data for 15 (31.9%) patients. Appropriateness of initial dosing regimen there was statistically significant (P < 0.001) associations observed between clinical pharmacist and physicians. However, 8 (15.1%) Physicians were mentioned inappropriate dosage of vancomycin and 4 (8.5%) clinical pharmacist had an inappropriate dosage of vancomycin. Similarly, concerning appropriate sampling time and interpretation of vancomycin level. Inappropriate sampling time was reported in 33 (62.3%) in physician and 12 (25.5%) in clinical pharmacist group. Additionally, inappropriate interpretation of vancomycin level was substantially higher in physician group compared to clinical pharmacist group (47 (88.7%) vs. 9 (19.1%) respectively). No other adverse reactions reported in both groups.
As an investigator we expected to have fewer nephrotoxicity in pharmacist group compared to physician group. Our assumption was based on previously published studies, the study has many limitations. The small number of patients can affect the significance of the study. The missing data from the clinical pharmacist group make the secondary outcome challenging to interpret. We recommend further validated studies to be conducted that overcome these limitations.
Conclusion
This retrospective study found that there is no statistically significant difference in the rate of vancomycin-induced nephrotoxicity between patients monitored by clinical pharmacists compared with those monitored by physicians. Given that the clinical pharmacist patients were having a more serious illness and received vancomycin for longer duration in addition to a concomitant administration of medications that cause an increase in serum creatinine. Other secondary outcomes were significantly favoring the clinical pharmacist group. No other adverse reactions reported in both groups.
References
- Moellering RC Jr. Vancomycin: a 50-year reassessment. Clin Infect Dis 42 (2006) :S3–4.
- Levine DP. Vancomycin: a history. Clin Infect Dis 42 (2006): S5–12.
- Tenover FC, Moellering RC Jr. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus Clin Infect Dis 44 (2007): 1208– 15.
- Sakoulas G, Gold HS, Cohen RA, et Effects of prolonged vancomycin administration on methicillin resistant Staphylococcus aureus (MRSA) in a patient with recurrent bacteraemia. J Antimicrob Chemother 57 (2006): 699– 704.
- Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC Jr, Craig WA, Billeter M, Dalovisio JR, Levine DP. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 29 (2009): 1275-9.
- Morrison AP, Melanson SE, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early?: frequency and impact on clinical actions. Am J Clin Pathol 137 (2012): 472-8.
- Ju-Seop Kang and Min-Ho Lee. Overview of Therapeutic Drug Monitoring. Korean J Intern Med 24 (2009): 1–10.
- Fernández de Gatta MD, Calvo MV, Hernández JM, Caballero D, San Miguel JF, Domínguez-Gil A. Cost-effectiveness analysis of serum vancomycin concentration monitoring in patients with hematologic malignancies. Clin Pharmacol Ther 60 (1996): 332-40.
- Welty TE, Copa AK. Impact of vancomycin therapeutic drug monitoring on patient care. Ann Pharmacother 28 (1994): 1335-9.
- Swartling M, Gupta R, Dudas V, Guglielmo BJ. Short term impact of guidelines on vancomycin dosing and therapeutic drug Int J Clin Pharm 34 (2012): 282-5.
- Ye ZK1, Tang HL, Zhai SD. Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta-analysis. PLoS One 8 (2013): e77169.