Impact and Potential Risk of Acute Myocardial Infarction on Consultation Type During the COVID-19 Pandemic: A Single-Center Experience
Article Information
Masato Furui1,2*, Kenji Kawajiri3, Takeshi Yoshida2, Bunpachi Kakii2, Norikazu Oshiro2, Mai Asanuma2, Hiroaki Nishioka4, and Hideichi Wada1
1Cardiovascular Surgery Department, Fukuoka University Hospital, 7-45-1 Nanakuma, Fukuoka, Fukuoka, Japan
2Cardiovascular Surgery Department, Matsubara Tokushukai Hospital, 7-13-26 Amamihigashi, Matsubara, Osaka, Japan
3Cardiology Department, Matsubara Tokushukai Hospital, 7-13-26 Amamihigashi, Matsubara, Osaka, Japan
4 Surgery Department, Matsubara Tokushukai Hospital, 7-13-26 Amamihigashi, Matsubara, Osaka, Japan
*Corresponding author: Masato Furui. Cardiovascular Surgery Department, Fukuoka University Hospital, 7-45-1 Nanakuma, Fukuoka, Fukuoka, Japan.
Received: 22 May 2023; Accepted: 30 May 2023; Published: 19 June 2023
Citation: Masato Furui, Kenji Kawajiri, Takeshi Yoshida, Bunpachi Kakii, Norikazu Oshiro, Mai Asanuma, Hiroaki Nishioka, Hideichi Wada. Impact and Potential Risk of Acute Myocardial Infarction on Consultation Type During the COVID-19 Pandemic: A Single-Center Experience. Cardiology and Cardiovascular Medicine. 7 (2023): 169-177.
Share at FacebookAbstract
Backgraound: Studies considering consultation types, such as walk-in, direct arrival by emergency medical service, or referral are rare in the coronavirus disease 2019 (COVID-19) era. The aim of this study was to compare the time course and outcome of acute myocardial infarction (AMI) and to examine the relation of the consultation types (walk-in and referral) with the time course during COVID-19 era.
Methods: In total, 503 patients who underwent emergency percutaneous coronary intervention between January 2011 and December 2021 at our institution were reviewed retrospectively. The AMI time course, mechanical complications, and mortality before and after the COVID-19 emergency declaration were compared.
Results: Overall, 426 patients with ST-segment elevation myocardial infarction (STEMI) and 77 patients with non-STEMI were identified. In STEMI patients, the onset-to-door time was prolonged (181 vs. 156 min, P=0.001) and mechanical complications worsened (7.8% vs. 2.6%, P=0.025) after the emergency declaration, compared with the findings before the pandemic. Multivariable analysis revealed that post-declaration, age, walk-ins, and referrals became independent risk factors for mechanical complications in STEMI patients.
Conclusions: Arrival by referral or walk-in which can cause treatment delay was identified as an independent risk factor of mechanical complications in addition to age and the time period post-declaration. Longitudinal research is needed to corroborate these potential risks during COVID-19 era.
Keywords
Coronavirus infection; Mechanical complication; Percutaneous coronary intervention; Referral; Risk polarization; Treatment delay; Walk-in
Coronavirus infection articles; Mechanical complication articles; Percutaneous coronary intervention articles; Referral articles; Risk polarization articles; Treatment delay articles; Walk-in articles
Article Details
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has been in existence since 2020. The infection situation is not much different in Japan to that worldwide, and the nation has already experienced the eighth wave of COVID-19. Common behavioral patterns have been adjusted to reduce infection risk, and patients are being asked to avoid population-dense areas for the same reason. Accordingly, a reduction in hospitalizations for acute myocardial infarction (AMI) has been reported [1-6]. Although the impact of the COVID-19 pandemic on door-to-balloon time and mechanical complications of AMI have been previously examined, there are only a few such reports in Japan [7-9]. Some studies on percutaneous coronary intervention (PCI) impact in Tokyo, Japan, have been reported; however, whether these are relevant to other regions is unknown due to the rural-urban emergency care disparity [10,11]. Even Osaka, the second largest prefecture in Japan, especially South Osaka which includes a rural area, has differing accessibility to PCI-capable institutions than that in Tokyo [12,13]. After the pandemic, studies considering consultation types, such as walk-ins, direct arrival by emergency medical service (EMS), or referral transport from other non-PCI-capable facilities, are rare [13]. Therefore, this study compared the time course and outcomes of AMI, including mechanical complications and in-hospital mortality, before and after the COVID-19 pandemic declaration at a regional core hospital in South Osaka, Japan. Moreover, this study examined whether differences in the form of hospital visits is a predictor of the outcomes.
2. Materials and Methods
2.1 Study design and population
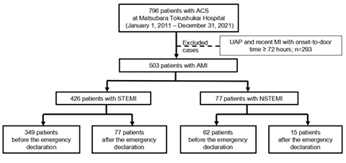
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the Matsubara Tokushukai Hospital (approval number: 22-01). The informed consent requirement from patients was waived due to the retrospective nature of this study. The corresponding author (Masato Furui) has full access to all data in this study and takes responsibility for data integrity and analysis. This study was a retrospective observational cohort study conducted on patients who underwent emergency PCI for AMI at the Matsubara Tokushukai Hospital in Osaka, Japan, between January 2011 and December 2021. The medical records of 796 consecutive patients with acute coronary syndrome (ACS) were reviewed, after excluding COVID-19 patients because our hospital was not designated for COVID-19. The data of 503 AMI patients were extracted after excluding those with recent myocardial infarction (MI) with an onset-to-door time >14 days and unstable angina pectoris. The participants were classified into ST-segment elevation myocardial infarction (STEMI) and non-STEMI (NSTEMI) groups. The Japanese government declared a state of emergency on April 7, 2020, following the World Health Organization’s initial declaration of COVID-19 as a pandemic on March 11, 2020 [14,15]. With this emergency declaration as a border line, the characteristics, time course, and outcomes were compared before and after the declaration to evaluate the impact of the COVID-19 pandemic on both the STEMI and NSTEMI groups (Figure 1).
Abbreviations:
ACS, acute coronary syndrome; AMI, acute myocardial infarction; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; UAP, unstable angina pectoris.
Definitions
AMI was defined based on the fourth universal definition of MI, and ACS was defined according to the Japanese Circulation Society guidelines [16,17]. Infarction was classified into STEMI or NSTEMI, depending on the presence or absence of ST-segment elevation. Onset-to-door time and door-to-balloon time were defined as the time from symptom onset to hospital arrival and from hospital arrival to balloon dilation or thrombus aspiration, respectively [18]. In the present study, renal dysfunction was defined as estimated glomerular filtration rate (eGFR) < 60 (mL/min/1.73 m2) [19]. Left ventricular ejection fraction (LVEF) was assessed using echocardiography. Primary PCI was defined as urgent balloon angioplasty (with or without stenting) without employing fibrinolytic therapy to open the infarct-related artery. The primary outcomes were onset-to-balloon time and in-hospital mortality. The secondary outcome was the incidence of mechanical complications. Therefore, we compared onset-to-door time, mechanical complications, and hospital mortality between patients before and after the COVID-19 emergency declaration in Japan. Mechanical complications, including cardiac rupture, ventricular septal perforation, and papillary muscle rupture, were assessed using echocardiography [20-22].
2.1 Statistical analysis
Categorical variables are expressed as numbers and proportions, whereas continuous variables are presented as means ± standard deviations. The Student’s t-test, chi-squared test, and Mann-Whitney U test were used for other statistical analyses. Multivariate logistic regression analysis was performed for risk factors associated with hospital mortality and mechanical complications in STEMI patients. This analysis considered age, walk-ins, referrals, out-of-hospital cardiac arrest (OHCA), LVEF, intra-aortic balloon pump (IABP) use, mechanical complications, and the time period post-declaration, including known factors [7,22,23]. The odds ratios (OR) and 95% confidence intervals (CI) were calculated. The number of patients who underwent venoarterial extracorporeal membrane oxygenation (VA-ECMO) was few; therefore, it was not entered because we could not effectively analyze it. Moreover, NSTEMI analysis was not valid due to the small number. All statistical analyses were performed using JMP® version 9.0 (SAS Institute Inc., Cary, NC), and differences were considered statistically significant at a P-value <0.05.
3. Results
We extracted the data of 796 patients who required emergency PCI for ACS from medical records. Of these, 503 AMI patients were identified as participants in this study (Figure 1), of whom 426 and 77 patients were categorized into the STEMI and NSTEMI groups, respectively. Table 1 summarizes their basic characteristics, including the patients’ background and pre-PCI examination findings before and after the COVID-19 declaration in each group. In the STEMI group, the number of patients with Killip classes III-IV was significantly higher after the declaration than it was before the declaration (12.0% [42/349] vs. 21.3% [16/77], P=0.010). Although the prevalence of OHCA was not significantly different, it tended to be higher after the declaration (2.3% [8/349] vs. 6.5% [5/77], P=0.052). There were no significant differences in comorbidities, clinical presentation, and laboratory data before and after the declaration, except for Killip III-IV classification in the STEMI group, as previously described.
Table 1: Basic characteristics of AMI patients before and after the COVID-19 declaration
|
Demographics n (%) or mean±SD |
STEMI (n=426) |
NSTEMI (n=77) |
|||||
|
Before declaration (n=349) |
After declaration (n=77) |
P- value |
Before declaration (n=62) |
After declaration (n=15) |
P- value |
||
|
Age (year) |
67.7±12.6 |
68.7±11.9 |
0.514 |
67.3±12.7 |
71.6±11.4 |
0.152 |
|
|
Sex (male) |
268 (76.8%) |
55 (71.4%) |
0.139 |
46 (74.2%) |
7 (46.7%) |
0.842 |
|
|
Body mass index (kg/m2) |
24.0±4.0 |
23.7±4.0 |
0.642 |
23.7±3.9 |
22.3±3.5 |
0.703 |
|
|
Comorbidities |
|||||||
|
Hypertension |
189 (54.2%) |
45 (58.4%) |
0.494 |
35 (56.5%) |
8 (53.3%) |
0.075 |
|
|
Diabetes mellitus |
100 (28.7%) |
24 (31.2%) |
0.66 |
15 (24.2%) |
3 (20.0%) |
0.737 |
|
|
Dyslipidemia |
121 (34.7%) |
30 (39.5%) |
0.428 |
20 (32.3%) |
7 (46.7%) |
0.063 |
|
|
Renal dysfunction |
163 (46.7%) |
30 (39.0%) |
0.217 |
25 (40.3%) |
4 (26.7%) |
0.327 |
|
|
Dialysis |
4 (1.1%) |
2 (2.6%) |
0.328 |
1 (1.6%) |
0 (0%) |
0.621 |
|
|
Cerebral infarction |
22 (6.3%) |
5 (6.5%) |
0.951 |
5 (8.1%) |
3 (20.0%) |
0.102 |
|
|
COPD |
3 (0.9%) |
0 (0%) |
0.414 |
0 (0%) |
10 (66.7%) |
0.041* |
|
|
Prior PCI |
36 (10.3%) |
7 (9.1%) |
0.747 |
9 (14.5%) |
2 (13.3%) |
0.187 |
|
|
Clinical presentation |
|||||||
|
Killip classification† |
I-II |
307 (88.0%) |
59 (78.7%) |
0.010* |
58 (93.5%) |
10 (100%) |
0.312 |
|
III-IV |
42 (12.0%) |
16 (21.3%) |
4 (6.5%) |
0 (0%) |
|||
|
OHCA |
8 (2.3%) |
5 (6.5%) |
0.052 |
1 (1.6%) |
1 (6.7%) |
0.27 |
|
|
LVEF (%) |
47±12 |
47±13 |
0.618 |
48±14 |
50±15 |
0.374 |
|
|
Laboratory data |
|||||||
|
CPK‡ (U/L) |
453±771 |
398±622 |
0.562 |
529±881 |
255±234 |
0.256 |
|
|
Peak CPK (U/L) |
3198±2808 |
3428±3038 |
0.528 |
2073±2380 |
799±730 |
0.156 |
|
|
CK-MB‡ (U/L) |
49±84 |
39±52 |
0.377 |
69±122 |
30±34 |
0.262 |
|
|
Peak CK-MB (U/L) |
297±302 |
274±216 |
0.531 |
189±199 |
80±75 |
0.248 |
|
|
Troponin I‡ (pg/mL) |
9378±36961 |
5491±18821 |
0.406 |
7905±28590 |
5855±1041 |
0.702 |
|
MI, acute myocardial infarction; CK-MB, creatine kinase-myocardial band; COPD, chronic obstructive pulmonary disease; CPK, creatine phosphokinase; LVEF, left ventricular ejection fraction; NSTEMI, non- ST-segment elevation myocardial infarction; OHCA, out-of-hospital cardiac arrest; PCI, percutaneous coronary intervention; SD, standard deviation; STEMI, ST-segment elevation myocardial infarction.
*P<0.05.
†Killip classification after the declaration has a defect value of 2 in STEMI and 5 in NSTEMI.
‡Value at admission.
Table 2 shows consultation forms, such as walk-ins, arrival by EMS, and referrals; time course; and outcomes before and after the declaration in each group. In the STEMI group, there were fewer referred patients after the declaration than there were before the declaration. However, there was no significant difference in the door-to-balloon time before and after the declaration (109±129 vs. 96±54 min, P=0.420). Onset-to-door time (a primary outcome) in STEMI patients was significantly longer after the declaration than it was before the declaration (124±134 vs. 180±144 min, P=0.001). Hospital mortality, another primary outcome, occurred in 11.0% (47/426) of STEMI patients and 7.8% (6/77) of NSTEMI patients. Contrarily, mechanical complications, a secondary outcome, occurred in 3.5% (15/426) of STEMI patients and 2.6% (2/77) of NSTEMI patients. The mechanical complication rate was significantly higher after the declaration than it was before the declaration (2.6% [9/349] vs. 7.8% [6/77], P=0.025) in the STEMI group; however, there was no significant difference in hospital mortality (10.3% [36/349] vs. 14.3% [11/77], P=0.299). In the post-intervention course of all patients, ten patients with four cases of ventricular septal perforation, three of cardiac rupture and three of papillary muscle rupture needed urgent surgery for mechanical complications, although seven patients could not undergo surgery in time because of cardiac rupture in the general ward. Operative mortality for mechanical complications was 10.0% (1/10); only one cardiac rupture needed VA-ECMO after a sudden change in ward out of a total of ten surgical cases, died after surgery.
Table 2: Consultation type, time course, and outcomes in AMI patients before and after the declaration
|
Demographics n (%) or mean±SD |
STEMI (n=426) |
NSTEMI (n=77) |
||||
|
Before declaration (n=349) |
After declaration (n=77) |
P- value |
Before declaration (n=62) |
After declaration (n=15) |
P- value |
|
|
Consultation type |
||||||
|
Walk-in |
50 (14.3%) |
12 (16.0%) |
0.733 |
9 (14.5%) |
3 (20%) |
0.599 |
|
EMS |
296 (85.3%) |
64 (83.1%) |
0.71 |
52 (83.9%) |
12 (80.0%) |
0.72 |
|
In-hospital patients |
2 (0.6%) |
1 (1.3%) |
0.491 |
1 (1.6%) |
0 (0%) |
0.621 |
|
Referral |
94 (27.0%) |
11 (14.3%) |
0.019* |
19 (30.7%) |
2 (13.3%) |
0.177 |
|
Time course |
||||||
|
Onset-to-door (min) |
124±134 |
180±144 |
0.001* |
126±134 |
186±158 |
0.327 |
|
Door-to-balloon (min) |
109±129 |
96±54 |
0.42 |
120±60 |
117±54 |
0.609 |
|
Culprit |
||||||
|
LMT |
6 (1.7%) |
1 (1.3%) |
0.785 |
6 (9.7%) |
0 (0%) |
0.096 |
|
LAD |
160 (45.9%) |
34 (44.2%) |
19 (30.7%) |
6 (40%) |
||
|
pLAD |
67 (19.2%) |
15 (19.5%) |
7 (11.3%) |
3 (20.0%) |
||
|
mLAD |
86 (24.6%) |
17 (22.1%) |
12 (19.4%) |
3 (20.0%) |
||
|
dLAD |
7 (2.0%) |
2 (2.6%) |
0 (0%) |
0 (0%) |
||
|
LCX |
34 (9.7%) |
9 (11.7%) |
16 (25.8%) |
8 (53.3%) |
||
|
RCA |
148 (42.4%) |
32 (41.6%) |
19 (30.7%) |
1 (6.7%) |
||
|
Diagonal branch |
1 (0.3%) |
1 (1.3%) |
2 (3.2%) |
0 (0%) |
||
|
Culprit in proximal part of LCA; LMT + pLAD |
73 (20.9) |
16 (20.8) |
0.979 |
13 (21.0%) |
3 (20.0%) |
0.934 |
|
Mechanical Circulatory Support |
||||||
|
IABP |
22 (6.3%) |
7 (9.1%) |
0.383 |
7 (11.3%) |
0 (0%) |
0.172 |
|
VA-ECMO |
11 (3.2%) |
4 (5.2%) |
0.382 |
2 (3.2%) |
0 (0%) |
0.481 |
|
Outcomes |
||||||
|
Mechanical complications |
9 (2.6%) |
6 (7.8%) |
0.025* |
1 (1.6%) |
1 (6.7%) |
0.27 |
|
Cardiac rupture |
6 (1.7%) |
3 (4.0%) |
0.231 |
1 (1.6%) |
0 (0%) |
0.621 |
|
VSP |
2 (0.6%) |
2 (2.7%) |
0.1 |
0 (0%) |
0 (0%) |
-† |
|
PMR |
1 (0.3%) |
1 (1.3%) |
0.241 |
0 (0%) |
1 (6.7%) |
0.041* |
|
Operative mortality rate for mechanical complications |
1/5a (20.0%) |
0/4b (0%) |
0.343 |
-c |
0/1 (0%) |
-† |
|
30-day mortality |
34 (9.7%) |
10 (13.0%) |
0.397 |
5 (8.1%) |
1 (6.7%) |
0.856 |
|
In-hospital mortality |
36 (10.3%) |
11 (14.3%) |
0.299 |
5 (8.1%) |
1 (6.7%) |
0.856 |
AMI, acute myocardial infarction; EMS, emergency medical service; IABP, intra-aortic balloon pump; LAD, left anterior descending coronary artery; LCA, left coronary artery; LCX, left circumflex coronary artery; LMT, left main trunk; NSTEMI, non-ST-segment elevation myocardial infarction; dLAD, distal LAD; mLAD, mid LAD; pLAD, proximal LAD; PMR, papillary muscle rupture; RCA, right coronary artery; SD, standard deviation; STEMI, ST-segment elevation myocardial infarction; and VA-ECMO, venoarterial extracorporeal membrane oxygenation; VSP, ventricular septal perforation.
*P<0.05.
†The value is not valid.
aFour, btwo, and cone case with cardiac rupture could not undergo surgery.
Table 3 presents the results of the multivariate analysis for the primary outcomes. Age (1-year increase: OR 1.087, 95% CI 1.045-1.135, P≤0.0001), OHCA (OR 61.883, 95% CI 9.726-595.506, P<0.0001), LVEF (1% increase: OR 0.943, 95% CI 0.911-0.975, P=0.0004), and mechanical complications (OR 10.724, 95% CI 2.233-57.236, P=0.003) were established as independent predictors of hospital mortality. Table 4 presents the results of the multivariate analysis for the secondary outcome. Age (1-year increase: OR 1.115, 95% CI 1.037-1.221, P=0.0019), walk-in visits (OR 14.695, 95% CI 3.265-80.468, P=0.0005), referral visits (OR 4.854, 95% CI 1.050-25.003, P=0.043), and post-declaration (OR 5.006, 95% CI 1.131-22.443, P=0.035) were identified as independent predictors of mechanical complications.
Table 3: Multivariate logistic regression analysis of risk factors for STEMI
|
Dependent variable: hospital mortality |
|||
|
Odds ratio |
95% CI |
P-value |
|
|
Age (per year) |
1.087 |
1.045-1.135 |
<0.0001* |
|
Walk-in |
2 |
0.527-10.829 |
0.332 |
|
Referral |
0.744 |
0.289-1.999 |
0.55 |
|
OHCA |
61.883 |
9.726-595.506 |
<0.0004* |
|
LVEF (1% increase) |
0.943 |
0.911-0.975 |
0.0019* |
|
Mechanical complications |
10.724 |
2.233-57.236 |
0.0030* |
|
Post-declaration (vs. pre-declaration) |
1.03 |
0.293-3.129 |
0.9603 |
CI, confidence interval; LVEF: left ventricular ejection fraction; OHCA, out-of-hospital cardiac arrest; and STEMI, ST-segment elevation myocardial infarction.
*P<0.05.
Table 4: Multivariate logistic regression analysis of risk factors for STEMI
|
Dependent variable: mechanical complications |
|||
|
Odds ratio |
95% CI |
P-value |
|
|
Age (per year) |
1.115 |
1.037-1.221 |
0.0019* |
|
Walk-in |
14.695 |
3.265-80.468 |
0.0005* |
|
Referral |
4.854 |
1.050-25.003 |
0.0432* |
|
LVEF (1% increase) |
0.971 |
0.915-1.030 |
0.276 |
|
Post-declaration (vs. pre-declaration) |
5.006 |
1.131-22.443 |
0.035* |
CI, confidence interval; LVEF, left ventricular ejection fraction; STEMI, ST-segment elevation myocardial infarction.
*P<0.05.
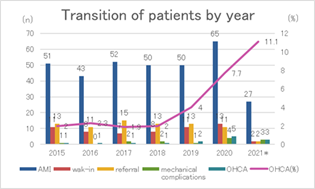
Figure 2 shows transition of patients with total AMI, walk-in, referral, mechanical complications, OHCA and OHCA rates (%) the last seven years. Total AMI counts in 2020 and 2021 (the post-COVID-19) varied, however, remained relatively stable except for OHCA and OHCA ratio.
* 2021only includes from April to December, other years include from April to next March.
Abbreviations: AMI, acute myocardial infarction; OHCA, out-of-hospital cardiac arrest
4. Discussion
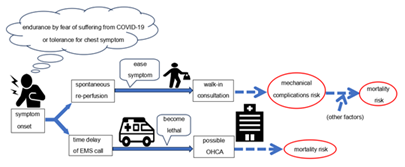
Several studies have reported the impact of the COVID-19 pandemic on AMI in Tokyo [3,7]. However, our hospital’s secondary medical service area in South Osaka is distinct from that in Tokyo and has an unevenly distributed population, similar to hospitals, especially PCI-capable facilities [12,24,25]. Moreover, arrival by walk-in or referral as risk factors for AMI have rarely been discussed following the COVID-19 pandemic. We established that onset-to-door time was prolonged and mechanical complications developed more frequently in STEMI patients after the declaration. These findings suggest that, in addition to post-declaration, walk-ins and referrals were risk factors for mechanical complications. To the best our knowledge, this is the first study that pointed out the risk factor of walk-in and referral visits, discussed after the COVID-19 pandemic. Regarding risk factors for hospital mortality, it was reported that patients with lower LVEF have higher mortality rates and develop heart failure easily [26,27]. Once mechanical complications occur, they can fatally affect patients’ hemodynamics. Although various operative procedures and strategies in surgery timing have been devised, operative mortality remains high [20,21,28]. Therefore, investigating the risk factors and preventing subsequent complications may be important in reducing associated mortality. Several risk factors for mechanical complications are known; previous studies have shown that established mechanical complication risk factors include age, female sex, anterior MI, de novo MI, and single-vessel disease [21,22]. Once mechanical complications occur, patients often experience cardiogenic shock, thus requiring IABP use, which may naturally become a risk factor. Considering previous findings, the identification of walk-ins and referrals as risk factors for mechanical complications are unique points in the present study. Walk-in patients often have mild symptoms, making this seem contradictory. However, in some cases with mild symptom presentation, walk-in patients are unrestricted in their activities or mobility until AMI diagnosis. That is, patients’ hearts can suffer from unnoticed load until AMI diagnosis, even after visiting the hospital. Failure to limit this activity may become a mechanical complication risk. Thus, the time course for walk-in patients is distinct from that for patients transported by ambulance. Actual onset-to-door time in walk-in patients was over 50 minutes longer than that in non-walk-in patients. Patients who notify the EMS are likely to immediately undergo consultation and check-ups by emergency doctors who begin monitoring as soon as the patient arrives at the hospital. Useful information by rescue crews generally aids in smooth AMI diagnosis. Conversely, walk-in patients have to wait longer for consultation or check-ups. Our hospital aggressively receives EMS patients from the surrounding area; however, only one or two doctors and a few emergency department nurses deal with EMS patients and out-of-hours walk-in patients, in that order. Although minimal triage is performed, walk-in patients may have waiting times because EMS patients are prioritized. Therefore, there may be a pronounced time lag from their arrival to diagnosis or PCI, compared with that in patients who notify EMS. Referred patients were reported to have a prolonged onset-to-door time because they came from a nearby clinic or were transferred from non-PCI-capable facilities [13,18]. Previous studies reported that the time interval from an AMI onset to ventricular septal perforation manifestation had a bimodal distribution [23]. Therefore, a longer ischemic time can become a risk factor for mechanical complications due to interactions with unknown factors. In any case, because a referral is indispensable due to the surrounding non-PCI-capable facilities, we suggest that an effective EMS system and crew education on carrying suspected patients to PCI-capable facilities is important [10,12,13]. In the future, telemedicine will also have an important role in AMI treatment [29]. In the STEMI group, OHCA rates tended to be higher after the declaration than they were before, although this failed to reach statistical significance. In the NSTEMI group in Table 2, the rate of lesions at left main was lower after the COVID-19 declaration. However, the lower rate may reflect milder AMI patients, after exclusion of AMI patients with severe left main who did not reach hospital. Accordingly, we cannot tell if this reduced number indicates real or pretending decrease. A previous study stated that 32% of patients with AMI at a left main lesion who were only medically treated medically died [30]. That is, AMI at left main may easily lead to death and hesitation to visiting hospital can be deadly. The OHCA may be just the tip of the iceberg. Given the potential for a higher OHCA rate and the risk factors for mechanical complications such as walk-ins and referrals shown in Table 4, AMI risk polarization may have emerged on the clinical scene during the COVID-19 pandemic. Although rare, spontaneous reperfusion can occur in 7-30% of STEMI patients, as stated in previous studies [31,32]. In addition, patients who follow stay-at-home orders and initially tolerate or adapt to their symptoms may likely experience spontaneous reperfusion. Walk-in patients with apparently mild symptoms or referral STEMI patients may be at risk of mechanical complications after the COVID-19 pandemic. The mechanical complications were identified in the present study as a mortality risk in STEMI. Therefore, care should be taken regarding walk-in or referral patients suspected of STEMI. Conversely, more severe symptoms can result in patients notifying EMS; however, their situation can become critical due to delays that may lead to OHCA during transport to PCI-capable facilities. The fear of contracting COVID-19 may have led to patient hesitation to visit hospitals, resulting in risk polarization (Figure 3). Although we can not just blame it on walk-in and referral, referral and walk-in which can lead treatment delay may contribute to increased OHCA rates as shown in Figure 2, in our medical area. We believe that the upward trend in OHCA rates means that more patients tend to endure until the last minute. Timely arrival to PCI-capable facilities without hesitation is necessary for patients at risk of AMI.
Abbreviations: EMS, emergency medical service; OHCA, out-of-hospital cardiac arrest.
The first limitation of this study is its retrospective observational design coupled with limited sample size and performance at a single center. Our hospital covers only one of the areas in South Osaka. Thus, our data may not accurately represent Osaka as a whole because of emergency care or PCI volume disparity [10-12]. Second, additional data, encompassing EMS calls, transfer-to-door time, or COVID-19 examination waiting time, may be desirable in future studies to support the initial findings outlined in this study. Finally, the number of patients who died at home or during transport to our hospital was unknown. They were excluded, and AMI patients who were COVID positive were not included in the study population. Therefore, OHCA and AMI prevalence rates may have been underestimated. Future research including these data will be required to further evaluate AMI-related mortality risk or mechanical complications during the COVID-19 pandemic.
5. Conclusion
The onset-to-door time was prolonged and mechanical complications developed more frequently in STEMI patients after the COVID-19 pandemic declaration than they were before. Age, low LVEF, OHCA, and mechanical complications were established as independent risk factors for hospital mortality in STEMI patients. Additionally, age, walk-ins, and referrals were identified as independent predictors of mechanical complications in those with STEMI. Arrival by walk-in or referral, chosen by patients with seemingly mild disease may also carry risks and may need to be handled with caution. Therefore, longitudinal studies are needed as the risks posed by the COVID-19 pandemic may be changing.
Author Contributions:
Conceptualization, M.F., K.K. and T.Y.; methodology, M.F.; validation, M.F. and H.W.; formal analysis, M.F. and H.N.; investigation, B.K., N.O. and M.A.; data curation, M.F.; writing—original draft preparation, M.F.; writing—review and editing, M.F. and H.W.; supervision, H.W. All authors have read and agreed to the published version of the manuscript.
Funding:
This research received no external funding.
Institutional Review Board Statement:
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the Matsubara Tokushukai Hospital (approval number: 22-01).
Informed Consent Statement:
The informed consent requirement from patients was waived due to the retrospective nature of this study.
Data Availability Statement:
The original contributions presented in this study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments:
We would like to thank Editage (www.editage.com) for English language editing.
Conflicts of Interest:
The authors declare no conflict of interest.
References
- Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol 76 (2020): 280-288.
- Toscano O, Cosentino N, Campodonico J, et al. Acute myocardial infarction during the COVID-19 pandemic: an update on clinical characteristics and outcomes. Front Cardiovasc Med 8 (2021): 648290.
- Arai R, Fukamachi D, Ebuchi Y, et al. Impact of the COVID-19 outbreak on hospitalizations and outcomes in patients with acute myocardial infarction in a Japanese single center. Heart Vessels 36 (2021): 1474-1483.
- Morishita T, Takada D, Shin JH, et al. Trends, treatment approaches, and in-hospital mortality for acute coronary syndrome in Japan during the coronavirus disease 2019 pandemic. J Atheroscler Thromb 29 (2022): 597-607.
- De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J 41 (2020): 2083-2088.
- Erol MK, Kayıkçıoglu M, Kılıçkap M, et al. Treatment delays and in-hospital outcomes in acute myocardial infarction during the COVID-19 pandemic: a nationwide study. Anatol J Cardiol 24 (2020): 334-342.
- Watanabe Y, Miyachi H, Mozawa K, et al. Impact of the COVID-19 pandemic on ST-elevation myocardial infarction from a single-center experience in Tokyo. Intern Med 60 (2021): 3693-3700.
- Kitahara S, Fujino M, Honda S, et al. COVID-19 pandemic is associated with mechanical complications in patients with ST-elevation myocardial infarction. Open Heart 8 (2021): 001497.
- Kobayashi S, Sakakura K, Jinnouchi H, et al. Comparison of door-to-balloon time and in-hospital outcomes in patients with ST-elevation myocardial infarction between before versus after COVID-19 pandemic. Cardiovasc Interv Ther 37 (2022): 641-650.
- Masuda J, Kishi M, Kumagai N, et al. Rural-urban disparity in emergency care for acute myocardial infarction in Japan. Circ J 82 (2018): 1666-1674.
- Matsuzawa Y, Konishi M, Nakai M, et al. In-hospital mortality in acute myocardial infarction according to population density and primary angioplasty procedures volume. Circ J 84 (2020): 1140-1146.
- Kinoshita N, Imai K, Kinjo K, et al. Longitudinal study of acute myocardial infarction in the southeast Osaka District from 1988 to 2002. Circ J 69 (2005): 1170-1175.
- Higuma T, Hanada H, Okumura K. Direct transfer, shorter onset-to-balloon time, and better clinical outcome in ST-segment elevation myocardial infarction. Circ J 79 (2015): 1897-1899.
- Mahase E. COVID-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ 368 (2020): 1036.
- Looi MK. COVID-19: Japan declares state of emergency as Tokyo cases soar. BMJ 369 (2020): 1447.
- Thygesen K, Alpert JS, Jaffe AS, et al. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 72 (2018): 2231-2264.
- Kimura K, Kimura T, Ishihara M, et al. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ J 83 (2019): 1085-1196.
- Tsukui T, Sakakura K, Taniguchi Y, et al. Association between the door-to-balloon time and mid-term clinical outcomes in patients with ST-segment elevation myocardial infarction. Intern Med 59 (2020): 1597-1603.
- Glassock RJ, Winearls C. Screening for CKD with eGFR: doubts and dangers. Clin J Am Soc Nephrol 3 (2008): 1563-1568.
- Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction—etiologies, management and outcome: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol 36 (2006): 1063-1070.
- Honda S, Asaumi Y, Yamane T, et al. Trends in the clinical and pathological characteristics of cardiac rupture in patients with acute myocardial infarction over 35 years. J Am Heart Assoc 3 (2014): 000984.
- Damluji AA, van Diepen S, Katz JN, et al. American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Surgery and Anesthesia; and Council on Cardiovascular and Stroke Nursing. Mechanical complications of acute myocardial infarction: a scientific statement from the American Heart Association. Circulation; Council on Cardiovascular Surgery and Anesthesia 144 (2021): 16-35.
- Novak M, Hlinomaz O, Groch L, et al . Ventricular septal rupture - a critical condition as a complication of acute myocardial infarction. J crit care Med (targu mures). J Crit Care Med (Targu Mures) 1 (2015): 162-166.
- Osaka Prefectural Government-Estimated Population. Statista Research Department (2022).
- Japan medical analysis platform by the Japan Medical Association (2022).
- Murphy SP, Ibrahim NE, Januzzi JL. Heart failure with reduced ejection fraction: a review. JAMA 324 (2020): 488-504.
- Packer M, Anker SD, Butler J, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced Trial. Circulation 143 (2021): 326-336.
- Furui M, Sakurai Y, Kakii B, et al. Benefits and risks of delayed surgery for ventricular septal rupture after acute myocardial infarction. Int Heart J 63 (2022): 433-440.
- Ghilencea LN, Chiru MR, Stolcova M, et al. Telemedicine: benefits for cardiovascular patients in the COVID-19 era. Front Cardiovasc Med 9 (2022): 868635.
- Atié J, Brugada P, Brugada J, et al. Clinical presentation and prognosis of left main coronary artery disease in the 1980s. Eur Heart J 12 (1991): 495-502.
- Uriel N, Moravsky G, Blatt A, et al. Acute myocardial infarction with spontaneous reperfusion: clinical characteristics and optimal timing for revascularization. Isr Med Assoc J 9 (2007): 243-246.
- Li X, Li B, Gao J, et al. Influence of angiographic spontaneous coronary reperfusion on long-term prognosis in patients with ST-segment elevation myocardial infarction. Oncotarget 8 (2017): 79767-79774.