Hetero-Molecular Interactions in Polar-Nonpolar Binary Mixture of Different Compositions and Studies on their Thermodynamic Properties
Article Information
Md Sydur Rahman1*, Muhammad Habibullah2
1Graduate Student, Department of Chemistry, Mississippi State University, USA
2Professor, Department of Chemistry, University of Chittagong, Bangladesh
*Corresponding Author: Md Sydur Rahman, Graduate Student, Department of Chemistry, Mississippi State University, USA
Received: 09 June 2022; Accepted: 17 June 2022; Published: 27 June 2022
Citation: Md Sydur Rahman, Muhammad Habibullah. Hetero-Molecular Interactions in Polar-Nonpolar Binary Mixture of Different Compositions and Studies on their Thermodynamic Properties. Journal of Analytical Techniques and Research 4 (2022): 102-121.
Share at FacebookAbstract
The thermodynamic properties of binary liquid mixture of 2-Bu-OH and Cumene (isopropylbenzene) have been determined at different temperatures (298.15-323.15) by 5K intervals over the whole composition range. The data have been utilized to estimate the excess thermodynamic properties (excess enthalpy, excess entropy, excess free energy). The excess values have been found to be useful in estimating the type and strength of the interactions between the polar-nonpolar binary mixture.
Keywords
Binary mixtures, bThermodynamic properties, Polar-nonpolar interactions, 2-Butanol, Cumene, Isopropylbenzene
Binary mixtures articles; Thermodynamic properties articles; Polar-nonpolar interactions articles; 2-Butanol articles; Cumene articles; Isopropylbenzene articles
Binary mixtures articles Binary mixtures Research articles Binary mixtures review articles Binary mixtures PubMed articles Binary mixtures PubMed Central articles Binary mixtures 2023 articles Binary mixtures 2024 articles Binary mixtures Scopus articles Binary mixtures impact factor journals Binary mixtures Scopus journals Binary mixtures PubMed journals Binary mixtures medical journals Binary mixtures free journals Binary mixtures best journals Binary mixtures top journals Binary mixtures free medical journals Binary mixtures famous journals Binary mixtures Google Scholar indexed journals bThermodynamic properties articles bThermodynamic properties Research articles bThermodynamic properties review articles bThermodynamic properties PubMed articles bThermodynamic properties PubMed Central articles bThermodynamic properties 2023 articles bThermodynamic properties 2024 articles bThermodynamic properties Scopus articles bThermodynamic properties impact factor journals bThermodynamic properties Scopus journals bThermodynamic properties PubMed journals bThermodynamic properties medical journals bThermodynamic properties free journals bThermodynamic properties best journals bThermodynamic properties top journals bThermodynamic properties free medical journals bThermodynamic properties famous journals bThermodynamic properties Google Scholar indexed journals Polar-nonpolar interactions articles Polar-nonpolar interactions Research articles Polar-nonpolar interactions review articles Polar-nonpolar interactions PubMed articles Polar-nonpolar interactions PubMed Central articles Polar-nonpolar interactions 2023 articles Polar-nonpolar interactions 2024 articles Polar-nonpolar interactions Scopus articles Polar-nonpolar interactions impact factor journals Polar-nonpolar interactions Scopus journals Polar-nonpolar interactions PubMed journals Polar-nonpolar interactions medical journals Polar-nonpolar interactions free journals Polar-nonpolar interactions best journals Polar-nonpolar interactions top journals Polar-nonpolar interactions free medical journals Polar-nonpolar interactions famous journals Polar-nonpolar interactions Google Scholar indexed journals 2-Butanol articles 2-Butanol Research articles 2-Butanol review articles 2-Butanol PubMed articles 2-Butanol PubMed Central articles 2-Butanol 2023 articles 2-Butanol 2024 articles 2-Butanol Scopus articles 2-Butanol impact factor journals 2-Butanol Scopus journals 2-Butanol PubMed journals 2-Butanol medical journals 2-Butanol free journals 2-Butanol best journals 2-Butanol top journals 2-Butanol free medical journals 2-Butanol famous journals 2-Butanol Google Scholar indexed journals Cumene articles Cumene Research articles Cumene review articles Cumene PubMed articles Cumene PubMed Central articles Cumene 2023 articles Cumene 2024 articles Cumene Scopus articles Cumene impact factor journals Cumene Scopus journals Cumene PubMed journals Cumene medical journals Cumene free journals Cumene best journals Cumene top journals Cumene free medical journals Cumene famous journals Cumene Google Scholar indexed journals Isopropylbenzene articles Isopropylbenzene Research articles Isopropylbenzene review articles Isopropylbenzene PubMed articles Isopropylbenzene PubMed Central articles Isopropylbenzene 2023 articles Isopropylbenzene 2024 articles Isopropylbenzene Scopus articles Isopropylbenzene impact factor journals Isopropylbenzene Scopus journals Isopropylbenzene PubMed journals Isopropylbenzene medical journals Isopropylbenzene free journals Isopropylbenzene best journals Isopropylbenzene top journals Isopropylbenzene free medical journals Isopropylbenzene famous journals Isopropylbenzene Google Scholar indexed journals physico-chemical properties articles physico-chemical properties Research articles physico-chemical properties review articles physico-chemical properties PubMed articles physico-chemical properties PubMed Central articles physico-chemical properties 2023 articles physico-chemical properties 2024 articles physico-chemical properties Scopus articles physico-chemical properties impact factor journals physico-chemical properties Scopus journals physico-chemical properties PubMed journals physico-chemical properties medical journals physico-chemical properties free journals physico-chemical properties best journals physico-chemical properties top journals physico-chemical properties free medical journals physico-chemical properties famous journals physico-chemical properties Google Scholar indexed journals molecular interactions articles molecular interactions Research articles molecular interactions review articles molecular interactions PubMed articles molecular interactions PubMed Central articles molecular interactions 2023 articles molecular interactions 2024 articles molecular interactions Scopus articles molecular interactions impact factor journals molecular interactions Scopus journals molecular interactions PubMed journals molecular interactions medical journals molecular interactions free journals molecular interactions best journals molecular interactions top journals molecular interactions free medical journals molecular interactions famous journals molecular interactions Google Scholar indexed journals experimental techniques articles experimental techniques Research articles experimental techniques review articles experimental techniques PubMed articles experimental techniques PubMed Central articles experimental techniques 2023 articles experimental techniques 2024 articles experimental techniques Scopus articles experimental techniques impact factor journals experimental techniques Scopus journals experimental techniques PubMed journals experimental techniques medical journals experimental techniques free journals experimental techniques best journals experimental techniques top journals experimental techniques free medical journals experimental techniques famous journals experimental techniques Google Scholar indexed journals theoretical techniques articles theoretical techniques Research articles theoretical techniques review articles theoretical techniques PubMed articles theoretical techniques PubMed Central articles theoretical techniques 2023 articles theoretical techniques 2024 articles theoretical techniques Scopus articles theoretical techniques impact factor journals theoretical techniques Scopus journals theoretical techniques PubMed journals theoretical techniques medical journals theoretical techniques free journals theoretical techniques best journals theoretical techniques top journals theoretical techniques free medical journals theoretical techniques famous journals theoretical techniques Google Scholar indexed journals
Article Details
1. Introduction
From the past few decades liquids have been studied extensively by both theoretical and experimental techniques using various physico-chemical properties. The practical nature of liquid mixtures rather than single component liquid systems, has gained much importance in assessing the nature of molecular interactions and investigating the physico-chemical behaviors [1,2]. In chemical process industries, materials are normally handled in fluid form and as a consequence, the physical, chemical, and transport properties of fluids draw attentions. Thermodynamic investigation of liquid mixtures consisting of polar and non-polar components is of considerable importance in understanding intermolecular interaction between the component molecules and in processing product formulation in several industrial and technological purposes. 2-Butanol (or 2-Bu-OH), is an organic polar compound normally found as an equal mixture of the two stereoisomers [3], a racemic mixture of (R)-(−)-2-Bu-OH and (S)-(+)-2-Bu-OH [4]. This secondary alcohol is a flammable, colorless liquid that is soluble in water and completely miscible with polar organic solvents such as ethers and other alcohols [5,6]. Cumene is also a colorless, flammable hydrocarbon but nonpolar liquid. It is an aromatic hydrocarbon, based on benzene having isopropyl substituent on the aromatic ring. It is part of the aromatic family and has a distinctive odor. The common uses of cumene are as solvent, dissolvent, fragrance intermediate [7]. Cumene is primarily used as a feedstock in the manufacture of phenol [8]. In view of this importance, it is of interest to study the thermodynamic properties in order to understand the interaction behavior in their binary mixtures of various proportions with the variation of temperature. In this paper, density and viscosity for the binary mixtures of 2-Bu-OH with Cumene at (298.15, 303.15, 308.15, 313.15, 318.15, 323.15) K are provided. From the experimental values of density and viscosity, thermodynamic properties and their excess thermodynamic properties have been estimated. The result of excess properties viz: excess enthalpy of activation (DH#E), excess entropy of activation (DS#E) and excess free energy of activation (DG#E) were fitted to the Redlich-Kister equation [9] and plotted by graphical presentation.
2. Experimental Details
All the chemicals used in this study were purchased from Sigma Aldrich Chemicals Company. According to the manufacturer, the purities of these compounds were >99%. The water used in all experimental work was double distilled water. The binary mixtures of 2-Bu-OH and Cumene were prepared by using an analytical electrical balance with a precision of ± o.1 µg and later were converted to different composition of the mixture using dilution method. Special care was taken to prevent evaporation and the introduction of moisture into the experimental samples. To measure the viscosity Stabinger viscometer (svm-3000-stabinger-viscometer) was used. The temperature was previously set up by 298.15-323.15 K. The measurement was accomplished by the descending of the temperature in the viscometer. Density of all binary mixtures including pure solvents was measured using an oscillation densimeter (Anton Paar DSA 5000). In both machinery processes the temperature was automatically controlled with an uncertainty of ±0.01K.
3. Theoretical Consideration
3.1 Calculation of different thermodynamic parameters for viscous flow
Liquid in a tube is considered as a combination of layers and that it flows as a rate process. To treat the viscosity as a rate process it is assumed that the motion of liquid layers involve the passage of a molecule from one equilibrium position to another in the same layer. In order to do so, it is necessary that a suitable ‘hole’ or site should be available and the production of such a ‘hole’ requires the expenditure of energy, since work must be done in pushing back other molecules. The jump of the moving molecules from one equilibrium position to the next may thus be regarded as equivalent to the passage of the system over a potential barrier. Eyring and his co-workers using absolute reaction rate theory and partition functions, correlated viscosity [10], as follows:
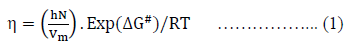
Where, DG# is the free energy of activation per mole for viscous flow, h is the Planck’s constant (= 6.6262 x 10-34 J.sec), N is the Avogadro number (= 6.023 x 1023 mol-1), R is the molar gas constant (= 8.3145 JK-1mol-1) T is the absolute temperature in Kelvin scale and h is the observed viscosity in mPa.s. According to the definition of DG# eq. (1) reduces to-
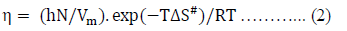
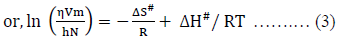
Where, DH# is the enthalpy of activation per mole and DS# is the entropy of activation per mole for viscous flow.
The plot of 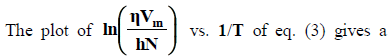 vs. 1/T of eq. (3) gives a straight line with slope, DH#/ R and intercept, -DS#/R assuming that DH# and DS# be almost independent of temperature. Therefore, DH# and DS# can easily be calculated from the slope and intercept of eq. (3) as,
vs. 1/T of eq. (3) gives a straight line with slope, DH#/ R and intercept, -DS#/R assuming that DH# and DS# be almost independent of temperature. Therefore, DH# and DS# can easily be calculated from the slope and intercept of eq. (3) as,
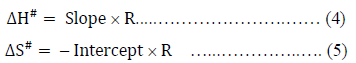
The free energy of activation, DG#, for viscous flow has been calculated by using the simple thermodynamic relation,

Calculation of different excess thermodynamic parameters for viscous flow:
The excess free energy of activation (DG#E), excess enthalpy of activation (DH#E) and excess entropy of activation (DS#E) for viscous flow were calculated by using the following relations,
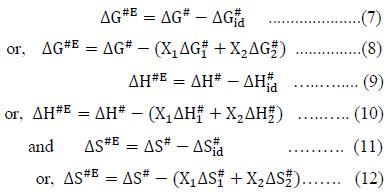
Where, the subscripts 1 and 2 represent the pure components of the mixture.
The experimentally obtained values of excess properties for a system can be fitted by the least square method using Redlich-Kister Eq. (13) of the form:
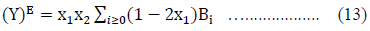
4. Results and Discussion
4.1 Density and Viscosity of the binary mixture
The density and viscosity values of binary mixture as a function of the mole fraction of 2-Bu-OH(x1) respectively at temperatures (298.15 to 323.15) K are listed in tables 1 and 2. The corresponding plotting of these values are displayed to observe the trend of changing these properties by figures 1 and 2. The changes of the properties are evaluated for the changes of each proportion of the mixture component along with the variation of temperatures.
|
Mole fraction of X |
Experimental Density r ×10-3 / kgm-3 |
||||||
|
2-Bu-OH |
Cumene |
298.15 |
303.15 |
308.15 |
313.15 |
318.15 |
323.15 |
|
x1 |
x2 |
||||||
|
0 |
1 |
0.8577 |
0.8535 |
0.8492 |
0.8449 |
0.8406 |
0.8363 |
|
0.05 |
0.95 |
0.8552 |
0.8509 |
0.8465 |
0.8422 |
0.8378 |
0.8334 |
|
0.1 |
0.9 |
0.8526 |
0.8483 |
0.8439 |
0.8395 |
0.835 |
0.8306 |
|
0.15 |
0.85 |
0.8503 |
0.8459 |
0.8415 |
0.8371 |
0.8326 |
0.8281 |
|
0.2001 |
0.7999 |
0.8479 |
0.8435 |
0.839 |
0.8345 |
0.83 |
0.8255 |
|
0.2503 |
0.7497 |
0.8454 |
0.841 |
0.8365 |
0.832 |
0.8275 |
0.823 |
|
0.3001 |
0.6999 |
0.843 |
0.8386 |
0.8341 |
0.8296 |
0.825 |
0.8205 |
|
0.3502 |
0.6498 |
0.8407 |
0.8362 |
0.8317 |
0.8272 |
0.8226 |
0.818 |
|
0.3992 |
0.6008 |
0.8385 |
0.834 |
0.8295 |
0.825 |
0.8204 |
0.8158 |
|
0.4502 |
0.5498 |
0.8361 |
0.8317 |
0.8271 |
0.8226 |
0.818 |
0.8134 |
|
0.5002 |
0.4998 |
0.8333 |
0.8288 |
0.8243 |
0.8198 |
0.8152 |
0.8105 |
|
0.5503 |
0.4497 |
0.8306 |
0.8261 |
0.8216 |
0.8171 |
0.8124 |
0.8078 |
|
0.5999 |
0.4001 |
0.8279 |
0.8234 |
0.8189 |
0.8143 |
0.8097 |
0.805 |
|
0.6502 |
0.3498 |
0.8251 |
0.8206 |
0.8161 |
0.8115 |
0.8068 |
0.8021 |
|
0.7001 |
0.2999 |
0.8223 |
0.8178 |
0.8133 |
0.8087 |
0.8041 |
0.7994 |
|
0.7503 |
0.2497 |
0.8193 |
0.8149 |
0.8104 |
0.8058 |
0.8011 |
0.7964 |
|
0.8001 |
0.1999 |
0.8163 |
0.8118 |
0.8073 |
0.8028 |
0.7981 |
0.7934 |
|
0.85 |
0.15 |
0.8131 |
0.8088 |
0.8043 |
0.7997 |
0.7951 |
0.7904 |
|
0.9001 |
0.1 |
0.8096 |
0.8053 |
0.8009 |
0.7964 |
0.7918 |
0.7871 |
|
0.9499 |
0.0501 |
0.8055 |
0.8013 |
0.7969 |
0.7925 |
0.788 |
0.7833 |
|
1 |
0 |
0.8027 |
0.7985 |
0.7942 |
0.7898 |
0.7853 |
0.7807 |
Table 1: Density (r) of 2-Bu-OH + Cumene system for different molar ratios at 298.15 K to 323.15 K by 5 K intervals
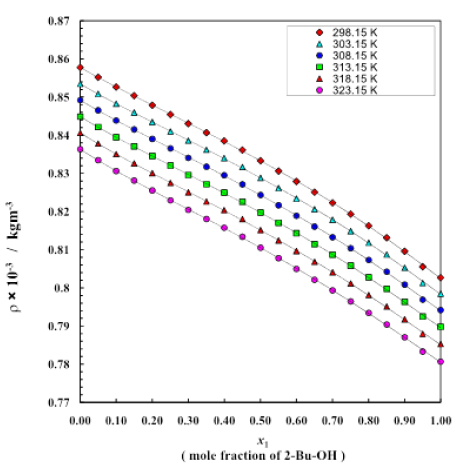
Figure 1: Density change for different mole fractions at different temperatures
|
Mole fraction of X |
Experimental Viscosity (η) mPa.s |
||||||
|
2-Bu-OH |
Cumene |
298.15 |
303.15 |
308.15 |
313.15 |
318.15 |
323.15 |
|
x1 |
x2 |
||||||
|
0 |
1 |
0.7267 |
0.68 |
0.6376 |
0.599 |
0.5637 |
0.5316 |
|
0.05 |
0.95 |
0.7189 |
0.6713 |
0.6282 |
0.5892 |
0.5536 |
0.5212 |
|
0.1 |
0.9 |
0.7244 |
0.6749 |
0.6303 |
0.5899 |
0.5531 |
0.5192 |
|
0.15 |
0.85 |
0.7356 |
0.6839 |
0.6372 |
0.5949 |
0.5564 |
0.5223 |
|
0.2001 |
0.7999 |
0.7495 |
0.6953 |
0.6465 |
0.6026 |
0.5626 |
0.5265 |
|
0.2503 |
0.7497 |
0.7668 |
0.7098 |
0.6588 |
0.6125 |
0.5706 |
0.5328 |
|
0.3001 |
0.6999 |
0.7867 |
0.7267 |
0.6729 |
0.6244 |
0.5805 |
0.5419 |
|
0.3502 |
0.6499 |
0.8107 |
0.7469 |
0.6899 |
0.6386 |
0.5924 |
0.552 |
|
0.3992 |
0.6008 |
0.8396 |
0.7704 |
0.7085 |
0.6542 |
0.6063 |
0.5612 |
|
0.4502 |
0.5498 |
0.8795 |
0.8028 |
0.7357 |
0.6763 |
0.6238 |
0.5785 |
|
0.5002 |
0.4998 |
0.9207 |
0.8376 |
0.7649 |
0.7007 |
0.6439 |
0.5948 |
|
0.5503 |
0.4497 |
0.997 |
0.8965 |
0.8097 |
0.7348 |
0.6711 |
0.6182 |
|
0.5999 |
0.4001 |
1.0685 |
0.9534 |
0.855 |
0.7714 |
0.7009 |
0.6429 |
|
0.6502 |
0.3498 |
1.1355 |
1.012 |
0.9064 |
0.816 |
0.7389 |
0.6742 |
|
0.7001 |
0.2999 |
1.2462 |
1.1024 |
0.9796 |
0.8751 |
0.7868 |
0.7138 |
|
0.7503 |
0.2497 |
1.3628 |
1.1994 |
1.0617 |
0.945 |
0.8457 |
0.7631 |
|
0.8001 |
0.1999 |
1.5256 |
1.3327 |
1.1714 |
1.0357 |
0.9212 |
0.8244 |
|
0.85 |
0.15 |
1.7423 |
1.5062 |
1.3112 |
1.1487 |
1.0122 |
0.9 |
|
0.9001 |
0.1 |
2.0491 |
1.7512 |
1.5072 |
1.3059 |
1.1387 |
1.0024 |
|
0.9499 |
0.0501 |
2.4528 |
2.0719 |
1.7634 |
1.5121 |
1.306 |
1.1392 |
|
1 |
0 |
3.0807 |
2.5601 |
2.1443 |
1.8103 |
1.5401 |
1.3207 |
Table 2: Viscosity of 2-Bu-OH + Cumene system for different molar ratios at 298.15 K to 323.15 K by 5 K intervals
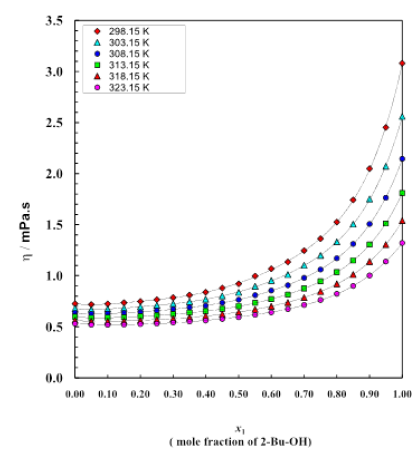
Figure 2: Viscosity curve for different mole fractions at different temperatures
4.2 Thermodynamic properties measurement
The three types of very important thermodynamic properties viz. enthalpy, entropy, and free energy were very intensively studied for the binary mixture of polar + nonpolar interaction. There excess values were also calculated and fitted in Redlich-kister fitting equation (eq.13). The overall data are provided by tables 3-10. The trend of changing due to miscibility is displayed by figures 3-8. Table 3 is for enthalpy study and its corresponding plots are by figures 3,4. Table 4 is for entropy study and it’s corresponding plots are by figures 5,6. Data for the free energy study of this binary mixture were provided by tables 5-10 and corresponding plotting are shown by figures 7,8.
|
Mole fraction of |
Enthalpy (Expt.) Δ H# kJmol-1 |
Enthalpy (Theor.) Δ H¹id kJmol-1 |
Excess Enthalpy Δ H#E kJmol-1 |
Fitting Δ H#E* kJmol-1 |
|
|
2-Bu-OH |
Cumene |
||||
|
x1 |
x2 |
||||
|
0 |
1 |
9.21 |
9.21 |
0 |
0 |
|
0.05 |
0.95 |
9.4792 |
10.0624 |
-0.5833 |
-0.6385 |
|
0.1 |
0.9 |
9.823 |
10.9158 |
-1.0928 |
-1.0867 |
|
0.15 |
0.85 |
10.1411 |
11.769 |
-1.6279 |
-1.5668 |
|
0.2001 |
0.7999 |
10.4602 |
12.623 |
-2.1628 |
-2.1274 |
|
0.2503 |
0.7497 |
10.8045 |
13.4785 |
-2.674 |
-2.726 |
|
0.3001 |
0.6999 |
11.0943 |
14.3285 |
-3.2342 |
-3.2848 |
|
0.3502 |
0.6498 |
11.4636 |
15.1822 |
-3.7186 |
-3.7445 |
|
0.3992 |
0.6008 |
12.0004 |
16.0194 |
-4.019 |
-4.0609 |
|
0.4502 |
0.5498 |
12.5615 |
16.8889 |
-4.3274 |
-4.2463 |
|
0.5002 |
0.4998 |
13.1307 |
17.7421 |
-4.6113 |
-4.3137 |
|
0.5503 |
0.4497 |
14.4789 |
18.5959 |
-4.117 |
-4.3121 |
|
0.5999 |
0.4001 |
15.4402 |
19.4413 |
-4.001 |
-4.2859 |
|
0.6502 |
0.3498 |
15.8413 |
20.3005 |
-4.4592 |
-4.2614 |
|
0.7001 |
0.2999 |
17.0098 |
21.1514 |
-4.1416 |
-4.2341 |
|
0.7503 |
0.2497 |
17.7091 |
22.0067 |
-4.2977 |
-4.1578 |
|
0.8001 |
0.1999 |
18.8262 |
22.856 |
-4.0297 |
-3.9493 |
|
0.85 |
0.15 |
20.2898 |
23.7076 |
-3.4177 |
-3.4981 |
|
0.9001 |
0.1 |
22.043 |
24.5613 |
-2.5183 |
-2.6984 |
|
0.9499 |
0.0501 |
23.714 |
25.4111 |
-1.6971 |
-1.5093 |
|
1 |
0 |
26.266 |
26.266 |
0 |
0 |
Table 3: Enthalpy (DH#), Excess Enthalpy (DH#E) and Fitting value (DH#E*) of activation for viscous flow for different molar ratios
|
D0 |
D1 |
D2 |
D3 |
D4 |
D5 |
SD |
|
-17.2544 |
-1.0101 |
-3.41 |
-33.4203 |
-3.8964 |
27.3415 |
0.17052 |
Table 3(a): Redlich-Kister equation constants
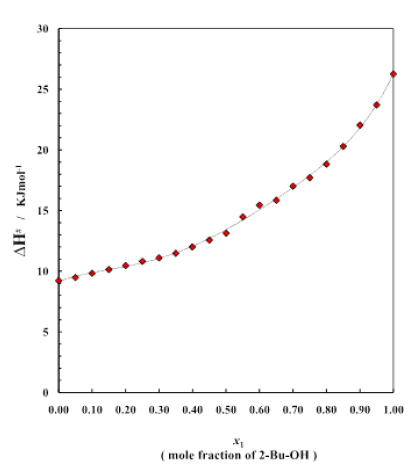
Figure 3: Enthalpy curve for different mole fractions
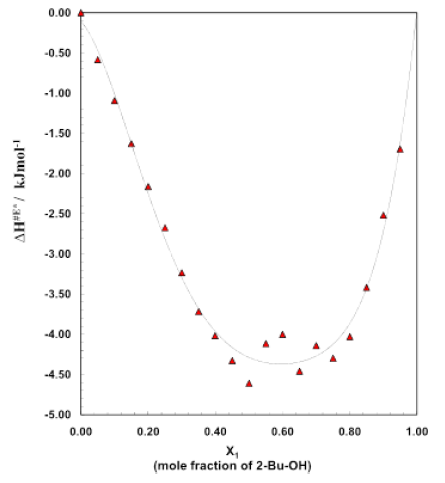
Figure 4: Excess enthalpy curve for different mole fractions
Figure 3 shows the plot of enthalpy of activation ΔH# for the transport process vs. mole fraction of alcohol for the system over the whole range of composition (0 to 1). It is noticed that for the binary system ΔH# value (table 3) continues to rise on addition of alcohol and eventually reach the value of pure 2-Bu-OH because H-bonded liquids, like 2-Bu-OH always require higher enthalpy for activation than that of the other low polar or nonpolar liquids. The excess enthalpies of the binary mixtures of aromatic hydrocarbons with 2-Bu-OH are represented by the figure 4. The excess enthalpy of activation for viscous flow (ΔH#E) show the negative values extended over a considerable region of concentrations.
|
Mole fraction of |
Entropy (Expt.) Δ S# Jmol-1 K-1 |
Entropy (Theor.) Δ S#id Jmol-1 K-1 |
Excess Entropy Δ S#E Jmol-1 K-1 |
Fitting Value |
|
|
2-Bu-OH |
Cumene |
||||
|
x1 |
x2 |
||||
|
0 |
1 |
-53.478 |
-53.47797 |
0 |
0 |
|
0.05 |
0.95 |
-52.3478 |
-51.04396 |
-1.3038 |
-1.3038 |
|
0.1 |
0.9 |
-51.1202 |
-48.60734 |
-2.5129 |
-2.5129 |
|
0.15 |
0.85 |
-50.0343 |
-46.17127 |
-3.863 |
-3.863 |
|
0.2001 |
0.7999 |
-48.9736 |
-43.73286 |
-5.2408 |
-5.2408 |
|
0.2503 |
0.7497 |
-47.8593 |
-41.29028 |
-6.569 |
-6.569 |
|
0.3001 |
0.6999 |
-46.9438 |
-38.86318 |
-8.0807 |
-8.0807 |
|
0.3502 |
0.6498 |
-45.7949 |
-36.42566 |
-9.3693 |
-9.3693 |
|
0.3992 |
0.6008 |
-44.1219 |
-34.03524 |
-10.0867 |
-10.0867 |
|
0.4502 |
0.5498 |
-42.4454 |
-31.55254 |
-10.8928 |
-10.8928 |
|
0.5002 |
0.4998 |
-40.7529 |
-29.11651 |
-11.6364 |
-11.6364 |
|
0.5503 |
0.4497 |
-36.6974 |
-26.67861 |
-10.0188 |
-10.0188 |
|
0.5999 |
0.4001 |
-33.8644 |
-24.26473 |
-9.5997 |
-9.5997 |
|
0.6502 |
0.3498 |
-32.8549 |
-21.81155 |
-11.0433 |
-11.0433 |
|
0.7001 |
0.2999 |
-29.5224 |
-19.38178 |
-10.1406 |
-10.1406 |
|
0.7503 |
0.2497 |
-27.7299 |
-16.93968 |
-10.7902 |
-10.7902 |
|
0.8001 |
0.1999 |
-24.7277 |
-14.51492 |
-10.2128 |
-10.2128 |
|
0.85 |
0.15 |
-20.717 |
-12.08335 |
-8.6336 |
-8.6336 |
|
0.9001 |
0.1 |
-15.9815 |
-9.64578 |
-6.3358 |
-6.3358 |
|
0.9499 |
0.0501 |
-11.6634 |
-7.21941 |
-4.444 |
-4.444 |
|
1 |
0 |
-4.7782 |
-4.77824 |
0 |
0 |
Table 4: Entropy (DS#), Excess Entropy (DS#E) and Fitting value (DS#E*) of Activation for viscous flow for different molar ratios
|
E0 |
E1 |
E2 |
E3 |
E4 |
E5 |
SD |
|
-42.8451 |
1.3533 |
-8.362 |
-103.6268 |
-9.7473 |
83.7869 |
0.53364 |
Table 4(a): Redlich-Kister equation constants
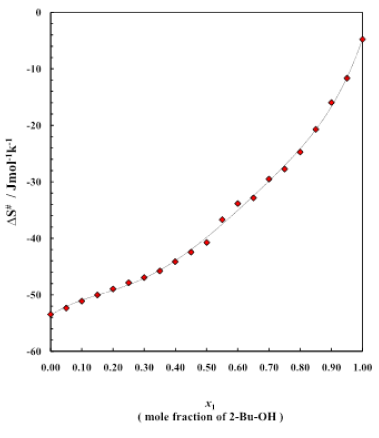
Figure 5: Entropy curve for different mole fractions
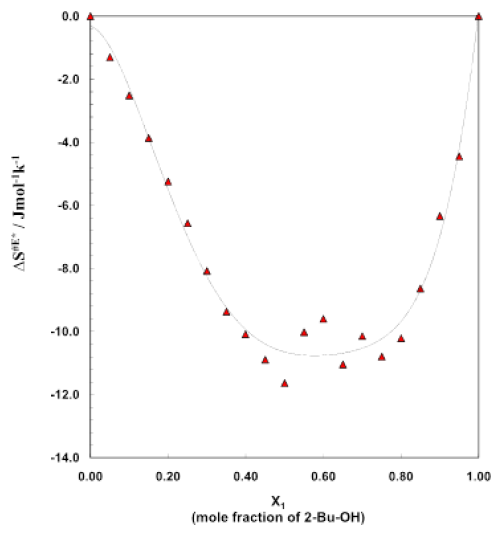
Figure 6: Excess entropy curve for different mole fractions
Figure 5 is for the plots of entropy of activation ΔS# for the viscous flow vs. mole fraction of alcohol for this binary system. The corresponding data are listed in table 4. The typical nature of the small and negative (ΔS#) value of alcohol indicates that during the viscous flow of initial H-bonded order breaks down to form the activated complex of little ordered structure. On the other hand, negative (ΔS#) value for the aromatic hydrocarbons revealed that during the flow process the activated complex formed, are much more ordered than of the initial state. Figure 5 also reveals that with increases of concentration of alcohol, the negative value decreases for the binary system of 2-Bu-OH + Cumene respectively. It indicates that during the flow process at the certain composition of mixtures, the molecular order of the activated and inactivated state for each of the mixtures is same. The excess entropy ΔS#E of activation for the system are shown (Figure 6) as a function of mole fraction of 2-Bu-OH. The plot of ΔS#E vs. mole fraction of 2-Bu-OH (Figure 6) for the binary mixture show an almost similar trend at those of the excess enthalpy (ΔH#E). The curve has been found to be concave in nature. The negative excess entropy signifies that the species formed in the activated state are more ordered than what is to be expected to the additive law.
|
Mole fraction of |
Free Energy (Expt.) Δ G# kJmol-1 |
Free Energy (Theor.) Δ G#id kJmol-1 |
Excess Free Energy Δ G#E kJmol-1 |
Fitting Value Δ G#E* kJmol-1 |
|
|
2-Bu-OH |
Cumene |
||||
|
x1 |
x2 |
||||
|
0 |
1 |
25.1544 |
25.1544 |
0 |
0 |
|
0.05 |
0.95 |
25.0867 |
25.2812 |
-0.1945 |
-0.1973 |
|
0.1 |
0.9 |
25.0645 |
25.4081 |
-0.3436 |
-0.3441 |
|
0.15 |
0.85 |
25.0588 |
25.535 |
-0.4761 |
-0.473 |
|
0.2001 |
0.7999 |
25.0616 |
25.6619 |
-0.6003 |
-0.5969 |
|
0.2503 |
0.7497 |
25.0737 |
25.7892 |
-0.7154 |
-0.7174 |
|
0.3001 |
0.6999 |
25.0906 |
25.9156 |
-0.825 |
-0.8296 |
|
0.3502 |
0.6499 |
25.1174 |
26.0425 |
-0.9251 |
-0.9294 |
|
0.3992 |
0.6008 |
25.1554 |
26.167 |
-1.0116 |
-1.0108 |
|
0.4502 |
0.5498 |
25.2166 |
26.2963 |
-1.0797 |
-1.0758 |
|
0.5002 |
0.4998 |
25.2812 |
26.4231 |
-1.1419 |
-1.1202 |
|
0.5503 |
0.4497 |
25.4203 |
26.5501 |
-1.1298 |
-1.1469 |
|
0.5999 |
0.4001 |
25.5369 |
26.6758 |
-1.1389 |
-1.1569 |
|
0.6502 |
0.3498 |
25.637 |
26.8036 |
-1.1666 |
-1.1504 |
|
0.7001 |
0.2999 |
25.8119 |
26.9301 |
-1.1182 |
-1.1245 |
|
0.7503 |
0.2497 |
25.9768 |
27.0573 |
-1.0806 |
-1.0721 |
|
0.8001 |
0.1999 |
26.1988 |
27.1836 |
-0.9848 |
-0.9836 |
|
0.85 |
0.15 |
26.4666 |
27.3102 |
-0.8436 |
-0.8448 |
|
0.9001 |
0.1 |
26.8079 |
27.4372 |
-0.6293 |
-0.6408 |
|
0.9499 |
0.0501 |
27.1914 |
27.5635 |
-0.3721 |
-0.3614 |
|
1 |
0 |
27.6907 |
27.6907 |
0 |
0 |
Table 5: Free energy (DG#), Excess Free energy (DG#E) and Fitting value (DG#E*) of Activation for viscous flow at 298.15 K
|
F0 |
F1 |
F2 |
F3 |
F4 |
F5 |
SD |
|
-4.4802 |
-1.4136 |
-0.9169 |
-2.5236 |
-0.9902 |
2.3599 |
0.0118 |
Table 5(a): Redlich-Kister equation constants
|
Mole fraction of |
Free Energy (Expt.) Δ G# kJmol-1 |
Free Energy (Theor.) Δ G#id kJmol-1 |
Excess Free Energy Δ G#E kJmol-1 |
Fitting Value Δ G#E* kJmol-1 |
|
|
2-Bu-OH |
Cumene |
||||
|
x1 |
x2 |
||||
|
0 |
1 |
25.4218 |
25.4218 |
0 |
0 |
|
0.05 |
0.95 |
25.3484 |
25.5364 |
-0.188 |
-0.1962 |
|
0.1 |
0.9 |
25.3201 |
25.6511 |
-0.3311 |
-0.3669 |
|
0.15 |
0.85 |
25.309 |
25.7658 |
-0.4568 |
-0.5164 |
|
0.2001 |
0.7999 |
25.3065 |
25.8806 |
-0.5741 |
-0.6457 |
|
0.2503 |
0.7497 |
25.313 |
25.9956 |
-0.6826 |
-0.756 |
|
0.3001 |
0.6999 |
25.3253 |
26.1099 |
-0.7846 |
-0.8479 |
|
0.3502 |
0.6499 |
25.3464 |
26.2246 |
-0.8783 |
-0.924 |
|
0.3992 |
0.6008 |
25.376 |
26.3372 |
-0.9612 |
-0.9841 |
|
0.4502 |
0.5498 |
25.4288 |
26.454 |
-1.0252 |
-1.0328 |
|
0.5002 |
0.4998 |
25.485 |
26.5687 |
-1.0837 |
-1.0666 |
|
0.5503 |
0.4497 |
25.6038 |
26.6835 |
-1.0797 |
-1.0854 |
|
0.5999 |
0.4001 |
25.7062 |
26.7971 |
-1.0909 |
-1.0885 |
|
0.6502 |
0.3498 |
25.8013 |
26.9126 |
-1.1114 |
-1.0762 |
|
0.7001 |
0.2999 |
25.9595 |
27.027 |
-1.0675 |
-1.0393 |
|
0.7503 |
0.2497 |
26.1154 |
27.142 |
-1.0266 |
-0.9777 |
|
0.8001 |
0.1999 |
26.3224 |
27.2562 |
-0.9337 |
-0.8769 |
|
0.85 |
0.15 |
26.5702 |
27.3706 |
-0.8004 |
-0.7279 |
|
0.9001 |
0.1 |
26.8878 |
27.4854 |
-0.5976 |
-0.5164 |
|
0.9499 |
0.0501 |
27.2497 |
27.5996 |
-0.3499 |
-0.2575 |
|
1 |
0 |
27.7145 |
27.7145 |
0 |
0 |
Table 6: Free energy (DG#), Excess Free energy (DG#E) and Fitting value (DG#E*) of Activation for viscous flow at 303.15 K
|
F0 |
F1 |
F2 |
F3 |
F4 |
F5 |
SD |
|
-4.2659 |
-1.4204 |
-0.8751 |
-2.0055 |
-0.9414 |
1.941 |
0.0092 |
Table 6(a): Redlich-Kister equation constants
|
Mole fraction of |
Free Energy (Expt.) Δ G# kJmol-1 |
Free Energy (Theor.) Δ G#id kJmol-1 |
Excess Free Energy Δ G#E kJmol-1 |
Fitting Value Δ G#E* kJmol-1 |
|
|
2-Bu-OH |
Cumene |
||||
|
x1 |
x2 |
||||
|
0 |
1 |
25.6892 |
25.6892 |
0 |
0 |
|
0.05 |
0.95 |
25.6102 |
25.7916 |
-0.1815 |
-0.1825 |
|
0.1 |
0.9 |
25.5757 |
25.8942 |
-0.3185 |
-0.3192 |
|
0.15 |
0.85 |
25.5592 |
25.9967 |
-0.4375 |
-0.4363 |
|
0.2001 |
0.7999 |
25.5514 |
26.0993 |
-0.5479 |
-0.5455 |
|
0.2503 |
0.7497 |
25.5523 |
26.2021 |
-0.6497 |
-0.65 |
|
0.3001 |
0.6999 |
25.56 |
26.3042 |
-0.7442 |
-0.7472 |
|
0.3502 |
0.6499 |
25.5753 |
26.4068 |
-0.8314 |
-0.835 |
|
0.3992 |
0.6008 |
25.5966 |
26.5073 |
-0.9108 |
-0.9085 |
|
0.4502 |
0.5498 |
25.641 |
26.6118 |
-0.9708 |
-0.9694 |
|
0.5002 |
0.4998 |
25.6888 |
26.7143 |
-1.0255 |
-1.0131 |
|
0.5503 |
0.4497 |
25.7873 |
26.8169 |
-1.0297 |
-1.0407 |
|
0.5999 |
0.4001 |
25.8756 |
26.9185 |
-1.0429 |
-1.052 |
|
0.6502 |
0.3498 |
25.9655 |
27.0217 |
-1.0562 |
-1.0461 |
|
0.7001 |
0.2999 |
26.1071 |
27.1239 |
-1.0168 |
-1.0202 |
|
0.7503 |
0.2497 |
26.254 |
27.2267 |
-0.9727 |
-0.9686 |
|
0.8001 |
0.1999 |
26.4461 |
27.3287 |
-0.8827 |
-0.8841 |
|
0.85 |
0.15 |
26.6738 |
27.431 |
-0.7573 |
-0.7558 |
|
0.9001 |
0.1 |
26.9677 |
27.5336 |
-0.5659 |
-0.5718 |
|
0.9499 |
0.0501 |
27.308 |
27.6357 |
-0.3277 |
-0.3229 |
|
1 |
0 |
27.7384 |
27.7384 |
0 |
0 |
Table 7: Free energy (DG#), Excess Free energy (DG#E) and Fitting value (DG#E*) of Activation for viscous flow at 308.15 K
|
F0 |
F1 |
F2 |
F3 |
F4 |
F5 |
SD |
|
-4.0517 |
-1.4272 |
-0.8333 |
-1.4873 |
-0.8927 |
1.522 |
0.0067 |
Table 7(a): Redlich-Kister equation constants
|
Mole fraction of |
Free Energy (Expt.) Δ G# kJmol-1 |
Free Energy (Theor.) Δ G#id kJmol-1 |
Excess Free Energy Δ G#E kJmol-1 |
Fitting Value Δ G#E* kJmol-1 |
|
|
2-Bu-OH |
Cumene |
||||
|
x1 |
x2 |
||||
|
0 |
1 |
25.9566 |
25.9566 |
0 |
0 |
|
0.05 |
0.95 |
25.8719 |
26.0469 |
-0.175 |
-0.1751 |
|
0.1 |
0.9 |
25.8313 |
26.1372 |
-0.3059 |
-0.3067 |
|
0.15 |
0.85 |
25.8093 |
26.2275 |
-0.4182 |
-0.4179 |
|
0.2001 |
0.7999 |
25.7962 |
26.3179 |
-0.5217 |
-0.5199 |
|
0.2503 |
0.7497 |
25.7916 |
26.4085 |
-0.6169 |
-0.6163 |
|
0.3001 |
0.6999 |
25.7948 |
26.4985 |
-0.7037 |
-0.7061 |
|
0.3502 |
0.6499 |
25.8043 |
26.5889 |
-0.7846 |
-0.7878 |
|
0.3992 |
0.6008 |
25.8172 |
26.6775 |
-0.8603 |
-0.8573 |
|
0.4502 |
0.5498 |
25.8533 |
26.7696 |
-0.9163 |
-0.9163 |
|
0.5002 |
0.4998 |
25.8925 |
26.8599 |
-0.9674 |
-0.9595 |
|
0.5503 |
0.4497 |
25.9707 |
26.9503 |
-0.9796 |
-0.9876 |
|
0.5999 |
0.4001 |
26.0449 |
27.0398 |
-0.9949 |
-0.9995 |
|
0.6502 |
0.3498 |
26.1298 |
27.1308 |
-1.001 |
-0.9939 |
|
0.7001 |
0.2999 |
26.2547 |
27.2208 |
-0.9661 |
-0.968 |
|
0.7503 |
0.2497 |
26.3927 |
27.3114 |
-0.9187 |
-0.9168 |
|
0.8001 |
0.1999 |
26.5697 |
27.4013 |
-0.8316 |
-0.8344 |
|
0.85 |
0.15 |
26.7774 |
27.4915 |
-0.7141 |
-0.7113 |
|
0.9001 |
0.1 |
27.0476 |
27.5818 |
-0.5342 |
-0.5372 |
|
0.9499 |
0.0501 |
27.3664 |
27.6718 |
-0.3055 |
-0.3036 |
|
1 |
0 |
27.7623 |
27.7623 |
0 |
0 |
Table 8: Free energy (DG#), Excess Free energy (DG#E) and Fitting value (DG#E*) of Activation for viscous flow at 313.15 K
|
F0 |
F1 |
F2 |
F3 |
F4 |
F5 |
SD |
|
-3.8375 |
-1.4339 |
-0.7915 |
-0.9693 |
-0.8439 |
1.1032 |
0.0045 |
Table 8(a): Redlich-Kister equation constants
|
Mole fraction of |
Free Energy (Expt.) Δ G# kJmol-1 |
Free Energy (Theor.) Δ G#id kJmol-1 |
Excess Free Energy Δ G#E kJmol-1 |
Fitting Value Δ G#E* kJmol-1 |
|
|
2-Bu-OH |
Cumene |
||||
|
x1 |
x2 |
||||
|
0 |
1 |
26.224 |
26.224 |
0 |
0 |
|
0.05 |
0.95 |
26.1336 |
26.3021 |
-0.1684 |
-0.1677 |
|
0.1 |
0.9 |
26.0869 |
26.3802 |
-0.2934 |
-0.2943 |
|
0.15 |
0.85 |
26.0595 |
26.4584 |
-0.3989 |
-0.3996 |
|
0.2001 |
0.7999 |
26.0411 |
26.5366 |
-0.4955 |
-0.4942 |
|
0.2503 |
0.7497 |
26.0309 |
26.615 |
-0.584 |
-0.5826 |
|
0.3001 |
0.6999 |
26.0295 |
26.6928 |
-0.6633 |
-0.6649 |
|
0.3502 |
0.6499 |
26.0333 |
26.771 |
-0.7377 |
-0.7406 |
|
0.3992 |
0.6008 |
26.0378 |
26.8477 |
-0.8099 |
-0.8062 |
|
0.4502 |
0.5498 |
26.0655 |
26.9273 |
-0.8618 |
-0.8631 |
|
0.5002 |
0.4998 |
26.0963 |
27.0055 |
-0.9092 |
-0.906 |
|
0.5503 |
0.4497 |
26.1542 |
27.0837 |
-0.9295 |
-0.9345 |
|
0.5999 |
0.4001 |
26.2142 |
27.1611 |
-0.9469 |
-0.9471 |
|
0.6502 |
0.3498 |
26.2941 |
27.2398 |
-0.9457 |
-0.9418 |
|
0.7001 |
0.2999 |
26.4024 |
27.3178 |
-0.9154 |
-0.9159 |
|
0.7503 |
0.2497 |
26.5313 |
27.3961 |
-0.8648 |
-0.8651 |
|
0.8001 |
0.1999 |
26.6933 |
27.4739 |
-0.7805 |
-0.7847 |
|
0.85 |
0.15 |
26.8809 |
27.5519 |
-0.6709 |
-0.6668 |
|
0.9001 |
0.1 |
27.1275 |
27.6301 |
-0.5026 |
-0.5027 |
|
0.9499 |
0.0501 |
27.4247 |
27.7079 |
-0.2832 |
-0.2844 |
|
1 |
0 |
27.7862 |
27.7862 |
0 |
0 |
Table 9: Free energy (DG#), Excess Free energy (DG#E) and Fitting value (DG#E*) of Activation for viscous flow at 318.15 K
|
F0 |
F1 |
F2 |
F3 |
F4 |
F5 |
SD |
|
-3.6233 |
-1.4407 |
-0.7497 |
-0.4511 |
-0.7952 |
0.6842 |
0.003 |
Table 9(a): Redlich-Kister equation constants
|
Mole fraction of |
Free Energy (Expt.) Δ G# kJmol-1 |
Free Energy (Theor.) Δ G#id kJmol-1 |
Excess Free Energy Δ G#E kJmol-1 |
Fitting Value Δ G#E* kJmol-1 |
|
|
2-Bu-OH |
Cumene |
||||
|
x1 |
x2 |
||||
|
0 |
1 |
26.4914 |
26.4914 |
0 |
0 |
|
0.05 |
0.95 |
26.3954 |
26.5573 |
-0.1619 |
-0.1603 |
|
0.1 |
0.9 |
26.3425 |
26.6233 |
-0.2808 |
-0.2818 |
|
0.15 |
0.85 |
26.3097 |
26.6892 |
-0.3796 |
-0.3812 |
|
0.2001 |
0.7999 |
26.286 |
26.7553 |
-0.4693 |
-0.4685 |
|
0.2503 |
0.7497 |
26.2702 |
26.8214 |
-0.5512 |
-0.5489 |
|
0.3001 |
0.6999 |
26.2642 |
26.8871 |
-0.6229 |
-0.6237 |
|
0.3502 |
0.6499 |
26.2623 |
26.9531 |
-0.6909 |
-0.6934 |
|
0.3992 |
0.6008 |
26.2584 |
27.0179 |
-0.7595 |
-0.755 |
|
0.4502 |
0.5498 |
26.2777 |
27.0851 |
-0.8074 |
-0.8099 |
|
0.5002 |
0.4998 |
26.3001 |
27.1511 |
-0.851 |
-0.8524 |
|
0.5503 |
0.4497 |
26.3377 |
27.2171 |
-0.8794 |
-0.8814 |
|
0.5999 |
0.4001 |
26.3835 |
27.2824 |
-0.8989 |
-0.8946 |
|
0.6502 |
0.3498 |
26.4584 |
27.3489 |
-0.8905 |
-0.8896 |
|
0.7001 |
0.2999 |
26.55 |
27.4147 |
-0.8647 |
-0.8637 |
|
0.7503 |
0.2497 |
26.67 |
27.4808 |
-0.8108 |
-0.8133 |
|
0.8001 |
0.1999 |
26.817 |
27.5465 |
-0.7295 |
-0.7349 |
|
0.85 |
0.15 |
26.9845 |
27.6123 |
-0.6278 |
-0.6223 |
|
0.9001 |
0.1 |
27.2074 |
27.6783 |
-0.4709 |
-0.4682 |
|
0.9499 |
0.0501 |
27.483 |
27.744 |
-0.261 |
-0.2651 |
|
1 |
0 |
27.8101 |
27.8101 |
0 |
0 |
Table 10: Free energy (DG#), Excess Free energy (DG#E) and Fitting value (DG#E*) of Activation for viscous flow at 323.15 K
|
F0 |
F1 |
F2 |
F3 |
F4 |
F5 |
SD |
|
-3.409 |
-1.4475 |
-0.7079 |
0.0671 |
-0.7465 |
0.2652 |
0.0035 |
Table 10(a): Redlich-Kister equation constants
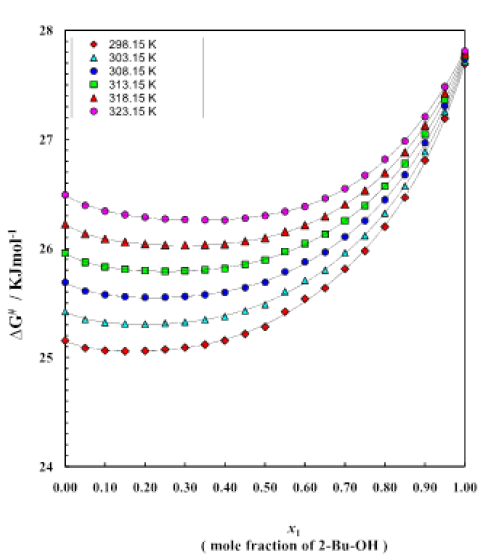
Figure 7:Free energy curve for different mole fractions at different temperatures
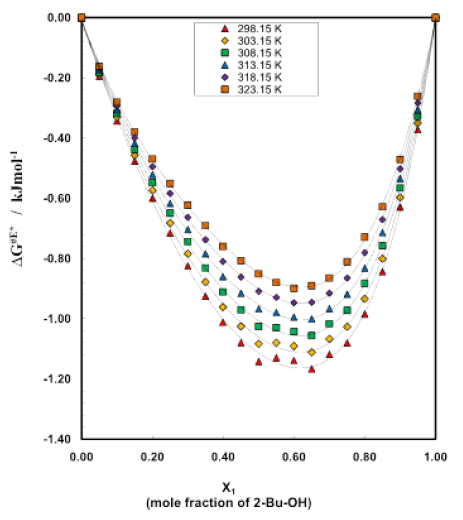
Figure 8: Excess free energy curve for different mole fractions at different temperatures
Tables 5-10 show the variation of free energy of activation ΔG# for the viscous flow of the system as a function of 2-Bu-OH under the whole range of composition at temperature 298.15 K to 323.15 K. And trend of changing this thermodynamic property are given by figure 7. The ΔG# values increase very slowly in the initial stage which is followed by a relatively greater rise with increasing concentration of 2-Bu-OH. The curves for ΔG# for the system are found to be smooth and similar. But a crossover of curves between temperature 298.15 K and 323.15 K is noticed in the system at higher mole fraction of 2-Bu-OH. It is also revealed from the figure 7 that the nonpolar (Cumene) rich region the ΔG# values increase slightly with the rise of temperature but at the alcohol rich region it is vice versa. Figure 8 shows the variation of excess free energy ΔG#E of activation at 298.15 K to 323.15 K over the whole composition range for this binary system (Table 5-10). In each case, the ΔG#E values are negative, but with the rise of temperature, the values are less negative, i.e., (δΔG#E/ΔT)p is positive. The general nature of the curves does not virtually change with the variation of temperature. The negative excess free energies indicate according to the Eyring 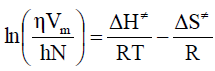 that the viscous flow of the solutions of the aromatic hydrocarbons in 2-Bu-OH is enhanced, causing the viscosity to decrease from the values expected ideally.
that the viscous flow of the solutions of the aromatic hydrocarbons in 2-Bu-OH is enhanced, causing the viscosity to decrease from the values expected ideally.
The excess values throughout the whole concentration range are negative with minima falling around 0.62 mole fraction of 2-Bu-OH. The free energy of activation is often regarded to be an energy barrier that a molecule must surmount in order to make a hole which is a necessary requirement for a molecule to flow through [11]. The negative excess free energy indicates the reduction of energy barrier height and hence increases of viscous flow.
5. Conclusion
The properties of binary liquid mixtures are of interest to experimental chemists and physicists and to the pharmaceutical as well as chemical industries [12,13]. An understanding of the thermodynamic properties of 2-Bu-OH and Cumene and their binary system have been measured at temperatures (298.15 to 323.15) K and in atmospheric pressure. Excess thermodynamic properties of binary mixture solvents were calculated and fitted with the Redlich–Kister equation. Besides knowing the very important thermodynamic behaviors of the liquid mixture at different proportions we also got some ideas about the polar-nonpolar interactions between these mixing components which are mainly for dipole–induced dipole interaction thus breaking of self-network built by H-bond among the polar molecules and rising dispersion force of nonpolar liquid. The observed excess values (excess enthalpy, excess entropy, excess free energy) in all the mixture indicate the significant interaction between the unlike molecules. Cumene is comparatively highly densed than 2-Bu-OH and the almost linear decreasing trend is observed with the addition of 2-Bu-OH as well as with the increasing of temperature (Figure 1). From table 2 and figure 2 it can be observed that viscosity of the binary mixture of 2-Bu-OH + Cumene increases in very disciplined way with the increasing proportion of 2-Bu-OH but decreases almost linearly with temperature rises. Here the nonpolar Cumene is low viscous liquid and when it interacts with the polar one it breaks the intermolecular network of the 2-Bu-OH which was built due to a lot of H-bonds and by polar-polar interactions between adjacent molecules. There was an induction of polarity occurred in Cumene and become induced dipole and attached to the polar 2-Bu-OH. Thus, the liquid mixture becomes more viscous than individual Cumene. From figure 2 the rising of viscosity was first observed slowly but when the polar proportion is higher it rises sharply upward.
Acknowledgement
This work was the partial fulfillment of academic degree of Master of Science of Md Sydur Rahman, financially supported by department of Chemistry of University of Chittagong and still the project is running in other significant areas of investigation. Teachers of this department and lab mates helped us in many ways during this research work to make it successful.
References
- Sudhamsa B, Babu MS, Narendra K. Study on thermodynamic properties of binary mixtures of diethyl carbonate with benzonitrile, benzaldehyde at different temperatures. Int Lett Chem, Phys, Astrono 38 (2014): 1-7.
- Hofmann A, Migeot M, Arens L, et al. Investigation of ternary mixtures containing 1-ethyl-3-methylimidazolium bis (trifluoromethanesulfonyl) azanide, ethylene carbonate and lithium bis (trifluoromethanesulfonyl)azanide. Int J Mol Sci 17 (2016): 670.
- Shi W, Nacev BA, Bhat S, et al. Impact of Absolute Stereochemistry on the Antiangiogenic and antifungal activities of itraconazole. ACS Med Chem Lett 1 (2010): 155-159.
- Rahman MS, Habibullah M. Estimation of acoustic impedance, it’s excess value and molar sound velocity of the binary mixture of 2-Butanol and m-Xylene for different compositions at different temperatures. Ind. J. of Chem Anal 3 (2020): 1-8.
- The national institute for occupational safety and health (NIOSH) (2011).
- Frank H, Thomas WM, Fookson A, et al. Preparation and physical properties of several aliphatic hydrocarbons and intermediates. J Res Nation Bur Stand 38 (1947): 1779.
- Salager JL. Surfactants types and uses. Universidad De Los Andes (2002).
- IARC monograph (2012).
- Chen F, Yang Z, Chen Z, et al. Density, Viscosity, Speed of sound, excess property and bulk modulus of binary mixtures of γ-butyrolactone with acetonitrile, dimethyl carbonate, and tetrahydrofuran at temperatures (293.15 to 333.15) K. J of Mol Liq 209 (2015): 683-692.
- Eyring H. Viscosity, plasticity, and diffusion as examples of absolute reaction rates. J Chem Phys 4 (1936): 283-291.
- Andrés J, Berski S, Domingo LR, et al. Describing the molecular mechanism of organic reactions by using aopological analysis of electronic localization function. Curr Org Chem 15 (2011): 1385.
- Md Sydur Rahman, Al-Nakib Chowdhury, Mahabubur Rahman, Md. Manwarul Islam. Drug Delivery: Bactericidal Effect of Manganese Oxide-Loaded Carbon Nanotubes Enhance Drug Efficiency. Journal of Biotechnology and Biomedicine 5 (2022): 137-147.
- Sydur Rahman Md. The Systematic Synthesis of Carbon Nanotubes from Aliphatic-Aromatic Compound Mixture Resolves Growth Uniformity and Production Complexity. Journal of Nanotechnology Research 2 (2020): 001-009.
