Guiding the Treatment of Hidradenitis Suppurativa: A Role for Therapeutic Drug Monitoring
Article Information
Charlotte Vanhoutte*, Tom Hillary, An Van Laethem
Department of Dermatology, University Hospital Leuven, Leuven, Belgium
*Corresponding Author: Charlotte Vanhoutte, Department of Dermatology, University Hospital Leuven, Leuven, Belgium.
Received: 19 November 2021; Accepted: 22 December 2021; Published: 17 January 2022
Citation: Charlotte Vanhoutte, Tom Hillary, An Van Laethem. Guiding the Treatment of Hidradenitis Suppurativa: A Role for Therapeutic Drug Monitoring. Archives of Clinical and Medical Case Reports 6 (2022): 11-16.
Share at FacebookAbstract
Background: Although the precise pathophysiology of Hidradenitis Suppurativa (HS) is yet to be elucidated, involvement of various cytokines has been proposed. Accordingly, the use of biologics has been introduced in the armamentarium and adalimumab is currently the only registered biologic for this indiciation. However, case series for IL-12/23 and IL 23-inhibitors are emerging. In case of therapeutic failure or loss of response to a biological, therapy is often blindly adapted, whereas Therapeutic Drug Monitoring (TDM) might assist in a personalized approach to optimize treatment. TDM has shown to be effective in other inflammatory diseases with overlapping pathogenesis.
Methods: We present a case of HS with waning response to first adalimumab and then guselkumab for both of which TDM was performed. Research of the literature was conducted.
Results: Our patient showed a waning clinical response that was paralleled by a drop in the Trough Levels (TL), first of adalimumab, then of guselkumab. Literature search showed only scarce data on TDM in HS patients.
Conclusion: Future research is warranted to determine optimal serum TL for therapeutic efficacy of the biologicals in the treatment of HS.
Keywords
Adalimumab; Guselkumab; Hidradenitis suppurativa; Therapeutic drug monitoring
Adalimumab articles; Guselkumab articles; Hidradenitis suppurativa articles; Therapeutic drug monitoring articles
Adalimumab articles Adalimumab Research articles Adalimumab review articles Adalimumab PubMed articles Adalimumab PubMed Central articles Adalimumab 2023 articles Adalimumab 2024 articles Adalimumab Scopus articles Adalimumab impact factor journals Adalimumab Scopus journals Adalimumab PubMed journals Adalimumab medical journals Adalimumab free journals Adalimumab best journals Adalimumab top journals Adalimumab free medical journals Adalimumab famous journals Adalimumab Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Guselkumab articles Guselkumab Research articles Guselkumab review articles Guselkumab PubMed articles Guselkumab PubMed Central articles Guselkumab 2023 articles Guselkumab 2024 articles Guselkumab Scopus articles Guselkumab impact factor journals Guselkumab Scopus journals Guselkumab PubMed journals Guselkumab medical journals Guselkumab free journals Guselkumab best journals Guselkumab top journals Guselkumab free medical journals Guselkumab famous journals Guselkumab Google Scholar indexed journals Hidradenitis suppurativa articles Hidradenitis suppurativa Research articles Hidradenitis suppurativa review articles Hidradenitis suppurativa PubMed articles Hidradenitis suppurativa PubMed Central articles Hidradenitis suppurativa 2023 articles Hidradenitis suppurativa 2024 articles Hidradenitis suppurativa Scopus articles Hidradenitis suppurativa impact factor journals Hidradenitis suppurativa Scopus journals Hidradenitis suppurativa PubMed journals Hidradenitis suppurativa medical journals Hidradenitis suppurativa free journals Hidradenitis suppurativa best journals Hidradenitis suppurativa top journals Hidradenitis suppurativa free medical journals Hidradenitis suppurativa famous journals Hidradenitis suppurativa Google Scholar indexed journals Ultra Sound articles Ultra Sound Research articles Ultra Sound review articles Ultra Sound PubMed articles Ultra Sound PubMed Central articles Ultra Sound 2023 articles Ultra Sound 2024 articles Ultra Sound Scopus articles Ultra Sound impact factor journals Ultra Sound Scopus journals Ultra Sound PubMed journals Ultra Sound medical journals Ultra Sound free journals Ultra Sound best journals Ultra Sound top journals Ultra Sound free medical journals Ultra Sound famous journals Ultra Sound Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Therapeutic drug monitoring articles Therapeutic drug monitoring Research articles Therapeutic drug monitoring review articles Therapeutic drug monitoring PubMed articles Therapeutic drug monitoring PubMed Central articles Therapeutic drug monitoring 2023 articles Therapeutic drug monitoring 2024 articles Therapeutic drug monitoring Scopus articles Therapeutic drug monitoring impact factor journals Therapeutic drug monitoring Scopus journals Therapeutic drug monitoring PubMed journals Therapeutic drug monitoring medical journals Therapeutic drug monitoring free journals Therapeutic drug monitoring best journals Therapeutic drug monitoring top journals Therapeutic drug monitoring free medical journals Therapeutic drug monitoring famous journals Therapeutic drug monitoring Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Torticollis articles Torticollis Research articles Torticollis review articles Torticollis PubMed articles Torticollis PubMed Central articles Torticollis 2023 articles Torticollis 2024 articles Torticollis Scopus articles Torticollis impact factor journals Torticollis Scopus journals Torticollis PubMed journals Torticollis medical journals Torticollis free journals Torticollis best journals Torticollis top journals Torticollis free medical journals Torticollis famous journals Torticollis Google Scholar indexed journals
Article Details
1. Introduction
HS is a chronic, inflammatory disease characterised by recurrent (sub) cutaneous inflammation in the form of painful Nodules (N) and Abscesses (A), which can give rise to pus draining fistula and scarring [1, 2]. Pathogenesis remains poorly understood. It is believed that follicular keratinisation leads to occlusion, dilatation and subsequent rupture of the pilosebaceous unit, followed by a profound perifollicular lympho-histiocytic inflammation [1, 3]. The role of Tumor Necrosis Factor (TNF)-α as a primary driver of the inflammatory process was suggested by the finding that TNF-α concentration is higher in the serum and skin of HS patients4. Furthermore, Schlapbach et al demonstrated a predominant expression of IL-23 and a distinct infiltration of IL-17 producing T helper cells in HS lesional skin, supporting an important role for the IL23/Th17-axis in HS skin lesions (3). Consistent with these findings, the introduction of biologics (TNF-α-, IL12/23-, IL17-, and IL23-blockers) to treat HS opened new therapeutic avenues with promising results [4-11]. TDM (Figure 1a,b) is the clinical practice of measuring drug trough levels (lowest level, measured before the next scheduled administration) and/or Anti-Drug Antibody (ADA) concentrations to correlate these findings with clinical response.
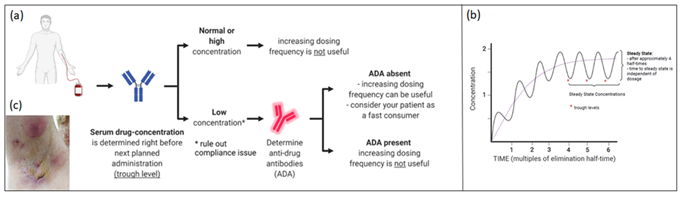
Figure 1: A. In Therapeutic Drug Monitoring (TDM) serum drug concentration is measured before next planned administration; B. Evolution of serum drug concentrations; C. Five months after start of adalimumab a clinical deterioration was seen, serum trough levels were undetectable.
The use of TDM in case of insufficient clinical response to biologics is well established in inflammatory diseases with similar cytokine-driven immunopathological mechanisms: rheumatoid arthritis, spondyloarthritis, psoriasis arthritis, inflammatory bowel disease and psoriasis [12]. Accordingly, we reviewed the literature on TDM in the treatment of HS. The use of TDM in this area is limited and no clear recommendations on therapeutic serum levels have been published except for adalimumab [13]. We present a patient with HS for whom TL were measured to adjust his treatment with first adalimumab and subsequently guselkumab. We hope this case can be a stepping stone to promote future investigations.
2. Case report
We present a 46 year old male patient with debilitating HS for more than 10 years. Previous topical and surgical treatment, supportive therapy (metformin, zinc suppletion) as well as long course antibiotic treatments (rifampicin (600 mg daily) combined with clindamycin (300 mg 2x/day); lymecycline 300 mg 2x/day) failed to obtain satisfying disease control. Upon clinical examination we observed 10 abscesses and nodules (AN count 10) and grade 2 on the Hurley-scale. Adalimumab (Humira®) was initiated according to the label, which was well tolerated. 10 weeks later, AN-count was reduced to 4 and minocycline 100 mg daily was added to further improve disease control. However, five months after the start of adalimumab, increased disease activity was observed (AN-count reaching 7 with increased number of draining fistula compared to the baseline number and DLQI 26) (Figure 1c). Serum TL was undetectable (<0,5 µg/ml) and ADA were absent (<3 ng/ml). To rule out compliance issues, adalimumab was administered in the dermatology department and serum was collected already after three days (way earlier than when lowest concentration is to be expected). Surprisingly, adalimumab levels were undetectable, again in the absence of ADA. We categorized the patient as a fast consumer and deemed it unlikely to obtain clinical improvement by increasing the dosing frequency. One month later guselkumab (Tremfya®) was initiated (off-label) with subcutaneous injections of 100 mg at start and at week 4, followed by 100 mg every 8 weeks (according to psoriasis label).
3. Discussion
This case of a patient with moderate (PGA of 3-4; IHS4 > 11) HS demonstrates the medical need for this disease. Adalimumab is the only approved biologic for the treatment of HS and guselkumab is currently under investigation for this indication [4, 5, 8]. In our patient, the initial good clinical response to adalimumab faded over time and TDM demonstrated undetectable serum levels of adalimumab on repeated measurements. Immunogenicity is a frequent cause for secondary loss of response (LOR). First, formation of ADA results in neutralizing the biologic activity of the drug through blocking the binding site. Secondly, decreased bioavailability due to the formation of immune-complexes has been described. In their large cohort of HS patients, Nader et al. [13] report 6,5% of patients to have anti-adalimumab antibodies (AAA). In general, the mean serum adalimumab concentrations were lower in AAA+ patients compared with that observed in the AAA− patients. The development of AAA appeared to correlate with lower adalimumab efficacy [13]. Little is known, however, on the LOR observed in patients that show undetectable serum levels and therefore don’t reach a therapeutic steady state concentration, without the formation of AAA, as presented in our patient [14-16]. In IBD compliance issues, TNF-mediated flares that “consume” the anti-TNF drug and non–immune drug clearance are proposed causes for this loss of anti- TNF activity [17].
Pharmacokinetics of adalimumab in the treatment of HS were investigated by Nader et al. [13]. In the 40 mg weekly dosing regimen, starting at week 4 after the loading phase of 160 mg at week 0 and 80mg at week 2, the mean serum steady state concentration of adalimumab (7-9 μg/mL) was reached at week 2 and maintained through week 12. A clear exposure-response relationship to adalimumab was demonstrated, with higher HiSCR response rates observed at week 12 at higher steady-state adalimumab concentrations. To our knowledge, the optimal target therapeutic trough range of adalimumab is not known for HS. Optimal TL of biologics appear to vary amongst diseases and thus cannot be compared. In psoriasis, for example, Liau et al18 found ADA to adalimumab in 6,5-45% of psoriasis patients and the target therapeutic trough range varied between 3,51 and 9,7 μg/l. The American Gastroenterological Association guidelines on TDM of adalimumab in Crohn’s disease (CD) suggest an optimal TL of >7,5 µg/L, whereas other algorithms recommend aiming for a window of 5,0-12 µg/ml. Immunogenicity rates to adalimumab in CD vary around 15% [18].
Analysing the guselkumab TL in our patient (Figure 2), we cannot find a clear correlation between drug levels and clinical response. Of interest is the progressive decrease in trough concentration while receiving a stable treatment. No remarkable changes in weight or life-style (eg. smoking) were reported. We note this evolution would probably result in undetectable TL, similar to the findings when the patient was treated with adalimumab. Therefore, we consider this patient to be a “fast consumer” of biologics. Although this entity is also recognised in other inflammatory diseases such as psoriasis and IBD, to date, the pathways associated with this process remain unknown.
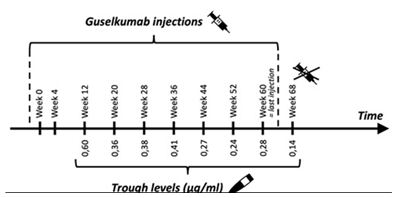
Figure 2: Number of injections and trough levels of guselkumab in our patient.
Little is known about the exposure –response relationship in terms of therapeutic threshold or range, nor the immunogenicity potential of the newer biologics such as guselkumab. To date no optimal TL are known for the use of guselkumab in HS and also in other inflammatory diseases data are scarce.
TDM can be a strategy to optimize the biologic effectiveness and individualize therapeutic decision making. In IBD multiple studies demonstrated that adequate drug TL of TNF-inhibitors are associated with a better long term outcome, better quality of life and less surgeries or hospitalisations. High TL on the other hand might correlate with more side effects (eg. psoriasiform eczema, arthralgia), further supporting a role for TDM. If routine TDM is not feasible, Vermeire et al. [12]. recommended that it should be performed in both primary and secondary non-responders. The rationale for this is that improvement is unlikely in the presence of high antibody titers and to prevent prolonged use of costly, inadequate or unsuitable biologic therapy.
4. Conclusion
We present a case of HS with a waning response to first adalimumab and then guselkumab for both of which TDM was performed. A paucity of data with regards to ideal drug TL in HS, makes TDM a limited tool.
Furthermore, future research to increase insight into the pathways responsible for increased biologic clearance is warranted.
Acknowledgment
We would like to thank Tine Vanhoutvin (Dermatology Department UZ Leuven) as well as the Laboratory for Therapeutic and Diagnostic Antibodies, Department of Pharmaceutical and Pharmacological Sciences (KU Leuven, Belgium). Figures created with BioRender.com.
Declaration of Interest Statement
The authors report no conflict of interest.
References
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa. J Eur Acad Dermatol Venerol 29 (2015): 619-644.
- Tzellos T, Zouboulis CC. Review of Comorbidities of Hidradenitis Suppurativa: Implications for Daily Clinical Practice. Dermatol Ther (Heidelb) 10 (2020): 63-71.
- Schlapbach C, Hänni T, Yawalkar N, et al.. Expression of IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol 65 (2011): 790-798.
- Savage KT, Flood KS, Porter ML, et al. TNF-α inhibitors in the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis (2019): 10.
- Casseres RG, Kahn JS, Min BS, et al. Guselkumab in the treatment of hidradenitis suppurativa: A retrospective chart review. J Am Acad Dermatol 81 (2019): 265-267.
- Gulliver WP, Jemec GBE, Baker KA. Experience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativa. J Eur Acad Dermatol Venerol 26 (2012): 911-914.
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and search for potential biomarkers in serum. Br J Dermatol 174 (2016): 839-846.
- Kovacs M, Podda M. Guselkumab in the treatment of severe hidradenitis suppurativa. J Eur Acad Dermatol Venerol 33 (2018): 140-142.
- Kimball AB, Okun MM, Williams DA, et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med 375 (2016): 422-434.
- Frew J, Navrazhina K, Grand D, et al. The effect of subcutaneous brodalumab on clinical disease activity in hidradenitis suppurativa: An open-label cohort study. JAAD 83 (2020): 1341-1348.
- Megna M. Ixekizumab: An efficacious treatment for both psoriasis and hidradenitis suppurativa. Letter in Dermatologic Therapy (2020).
- Vermeire S, Gils A. Value of drug level testing and antibody assays in optimising biological therapy. Frontline Gastroenterology 4 (2013): 41-43.
- Nader A, Beck D, Noertersheuer P, et al. N. Population Pharmacokinetics and Immunogenicity of Adalimumab in Adult Patients with Moderate-to-Severe Hidradenitis Supprativa. Clinical Pharmacokinetics 56 (2017): 1091-1102.
- Sotiriou E, Goussi C, Lallas A, et al. A prospective open-label clinical trial of efficacy of the every week administration in the treatment of hidradenitis suppurativa. J Drugs Dermatol 11 (2012): 15-20.
- Zouboulis CC, Hansen H, Caposiena CC, et al. Adalimumab Dose Intensification in Recalcitrant Hidradenitis Suppurativa / Acne Inversa. Dermatology 236 (2020): 25-30.
- Strik AS, Bots SJA, D’Haens G, et al. Optimization of anti-TNF therapy in patients with Inflammatory Bowel Disease, Expert Review of Clinical Pharmacology 9 (2016): 429-439.
- Liau MM, Oon, HH. Therapeutic drug monitoring of biologicals in psoriasis. Biologics 13 (2019): 127-132.
- Plevris N, Lyons M, Jenkinson P, et al. Higher Adalimumab Drug Levels During Maintenancy Therapy for Crohn’s Disease Are Associated With Biologic Remission. Inflammatory Bowel Diseases 25 (2019): 1036-1043.
