Gestational Age-Related Variation of Central Thickness of Placenta of Healthy Pregnant Mother of Bangladesh
Article Information
Dr. Sanjib Kumar Bose*1, Dr. Rawshon Ara Naznin2, Dr. Afsana Khanam3, Dr. Rafuja Afrin Shanto4, Dr. Kimty Tasmia5, Dr. Tamanna Jannat6, Dr. Labiba Jabeen7, Dr. Sharmin Akter Sumi8
1Assistant Professor, Department of Anatomy, Gonoshasthaya Samaj Vittik Medical College, Dhaka, Bangladesh
2Associate professor, Department of Anatomy, TMSS Medical College, Bogura, Bangladesh
3Associate Professor, Department of Anatomy, TMSS Medical College, Bogura, Bangladesh
4Assistant Professor, Department of Anatomy, Dhaka Central International Medical College, Dhaka, Bangladesh
5Assistant Professor, Department of Anatomy, Gonoshasthaya Samaj Vittik Medical College, Dhaka, Bangladesh
6Associate professor, Department of Pharmacology & Therapeutics, Bikrampur Bhuiyan Medical College, Munshigonj, Bangladesh
7Associate Professor, Department of Anatomy, Asgar Ali Medical College, Dhaka, Bangladesh
8Assistant Professor, Department of Anatomy, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
*Corresponding author: Sanjib Kumar Bose, Assistant Professor, Department of Anatomy, Gonoshasthaya Samaj Vittik Medical College, Dhaka, Bangladesh.
Received: 02 August 2024; Accepted: 08 August 2024; Published: 20 August 2024
Citation: Sanjib Kumar Bose, Rawshon Ara Naznin, Afsana Khanam, Rafuja Afrin Shanto, Kimty Tasmia, Tamanna Jannat, Labiba Jabeen, Sharmin Akter Sumi. Gestational Age-Related Variation of Central Thickness of Placenta of Healthy Pregnant Mother of Bangladesh. Fortune Journal of Health Sciences. 7 (2024): 481-486.
Share at FacebookAbstract
Introduction: The placenta is the organ that facilitates nutrient and gas exchange between the maternal and fetal components. The fetal component of the placenta is derived from the trophoblast and extraembryonic mesoderm (the chorionic plate). The maternal component is derived from the uterine endometrium.
Aim of the study: The aim of this study was to evaluate the variation in central placental thickness across different gestational ages in healthy pregnant women in Bangladesh.
Methods: This cross-sectional descriptive study was conducted in the Department of Anatomy, Mymensingh Medical College, Mymensingh, from January 2018 to December 2018. This study was performed on 80 human placentas to find out the variation in central thickness of placenta of healthy Bangladeshi mother in relation to different gestational age.
Result: The mean central thickness was maximum in group C (3.07 cm) and was minimum in group A (2.14 cm). It was also observed that the mean thickness at center of the placenta increased with gestational age. The mean difference of the placental thickness at the center between groups A and B and A and C was statistically highly significant (p < 0.001) but difference between B and C was statistically moderately significant (p < 0.01).
Conclusion: The present study showed that the central thickness of placenta was variable; however, maximum number of cases had normal thickness.
Keywords
Placenta, Central Thickness, Gestational age, Healthy pregnant mother
Article Details
1. Introduction
The placenta is the organ that facilitates nutrient and gas exchange between the maternal and fetal components. The fetal component of the placenta is derived from the trophoblast and extraembryonic mesoderm (the chorionic plate). The maternal component is derived from the uterine endometrium. At full term, the placenta is discoid with a diameter of 15 to 25 cm, is approximately 3 cm thick, and weighs about 500 to 600 gm. At birth, it is torn from the uterine wall and, approximately 30 minutes after birth of the child, is expelled from the uterine cavity as the after birth. When the placenta is viewed from the maternal side, 15 to 20 slightly bulging areas, the cotyledons, covered by a thin layer of decidua basalis, are clearly recognizable. The fetal surface of the placenta is covered entirely by the chorionic plate [1]. At full term the placenta is disc-like and presents after separation from uterine wall fetal and maternal surfaces, and peripheral margin. The peripheral margin is continuous with the fetal membrane which consists from outside inwards of fused decidua parietalis and capsularis, chorionic leave and amnion. A full-term placenta occupies about 30% of the uterine wall. The placenta consists of chorionic plate on the fetal side, basal plate on the maternal side, stem villi extending between the plates, and intervillous space between the stem villi filled with the maternal blood.
The chorionic plate covered by the amniotic membrane, is composed of (fetus to mother) the primary mesoderm containing branches of umbilical vessels (fetal), cytotrophoblast and syncytiotrophoblast. The basal plate consists from outside inwards (mother to fetus) of stratum spongiosum of decidua basalis containing maternal blood vessels, outer layer of syncytiotrophoblast, outer shell of cytotrophoblast and inner layer of syncytiotrophoblast [2]. In human, maternal blood is separated from fetal blood by chorionic tissue; hence human placenta is termed hemochorial [3]. The fetal surface is covered by the smooth glistening amnion with the umbilical cord attached at or near to its center. The maternal surface has a rough and spongy appearance. A shaggy layer may be visible on the maternal surface, remnant of decidua basalis. The maternal surface is divided into several cotyledons (15-20) by septae arising from the maternal tissues. Each cotyledon may be supplied by its own spiral artery [4]. The placenta consists of vascular tissue in which oxygen and nutrients can pass from the mother’s blood into that of the fetus and waste products can pass in the reverse direction [5]. There are some diseases and abnormalities of placenta. These are succenturiate placenta, ring shaped placenta, membranous placenta, fenestrated placenta, extrachorial placenta, placental polyp, placental infarcts [6]. As placenta is the mirror of maternal and fetal status, it reflects the changes associated with anaemia, hypertension, preeclamptic toxemia, eclampsia, diabetes and various type of placental disorder. Pregnancy related clinical disorder is most common medical complications of human pregnancy responsible for both maternal and perinatal morbidity. Like other developing countries Bangladesh still has alarming maternal and fetal mortality rate [7]. Bangladesh is one of those countries that have very high maternal and fetal mortality rates, obviously there are various socio-cultural, economic, health reason, medical problem and placental disorder behind there high rates [8]. The purpose of the present study of measuring placental thickness at the center of placenta was to assess the relationship of placental thickness with gestational age and also to assess the thickness pattern of placenta with advancing gestational age.
2. Materials & Methods
This cross sectional descriptive study was performed on 80 human placentas to find out the variation in central thickness of placenta of healthy Bangladeshi mother in relation to different gestational age. This study was conducted in the Department of Anatomy, Mymensingh Medical College, Mymensingh, from January to December 2018. Specimens containing placenta were collected just after delivery on different dates from April 2018 to September 2018 from the Department of Obstetrics and Gynaecology of Mymensingh Medical College Hospital, Mymensingh. All the specimens were collected from healthy pregnancy of gestational age at 28 weeks and above. All patient’s information regarding the exclusion criteria (below 28 weeks of gestation, ante partum haemorrhage, multiple pregnancies, pre eclamptic toxaemia, eclampsia, Rh-incompatibility, retained placenta, diabetes mellitus, pregnancy induced hypertension) were collected from the hospital records of MMCH. At first, after dissecting of umbilical cord from the placenta, the placenta was dried with tissue paper properly. The placental thickness was measured at three areas- central, middle and peripheral. The thickness of placenta was measured by piercing a needle through the whole thickness of placenta in such a way that other end came out and a marking was given on the needle at the point of exit. Then the marking was measured on the scale which gave the thickness of the placenta in centimeter (cm). The center of the maternal surface of the placenta was considered as the first point (Figure I) where central thickness was measured. Each placenta was allotted an identification number tagged with a piece of waxed cloth. The gestational age of the mother was collected from MMCH records and noted in a record book against respective identification number. The collected specimens were divided into 3 groups e.g. A, B, and C according to the gestational age, on the basis of maturation of baby such as group A pre-term 28-36 weeks, group B term 37-40 weeks, group C late term above 40 weeks (Table I) for convenience of differentiating the variation of central thickness of placenta at different gestational age. The central thickness of placenta was recorded in the predesigned data sheet, analyzed by SPSS program & compared with the findings of other national and international studies and standard textbooks.
Table I: Gestational Age Grouping of Samples for Morphological Study (n=80)
|
Group |
Gestational Age in week |
Number of specimen |
|
A |
28 - 36 |
20 |
|
B |
37 - 40 |
42 |
|
C |
Above 40 weeks |
18 |
|
Total |
80 |
3. Results
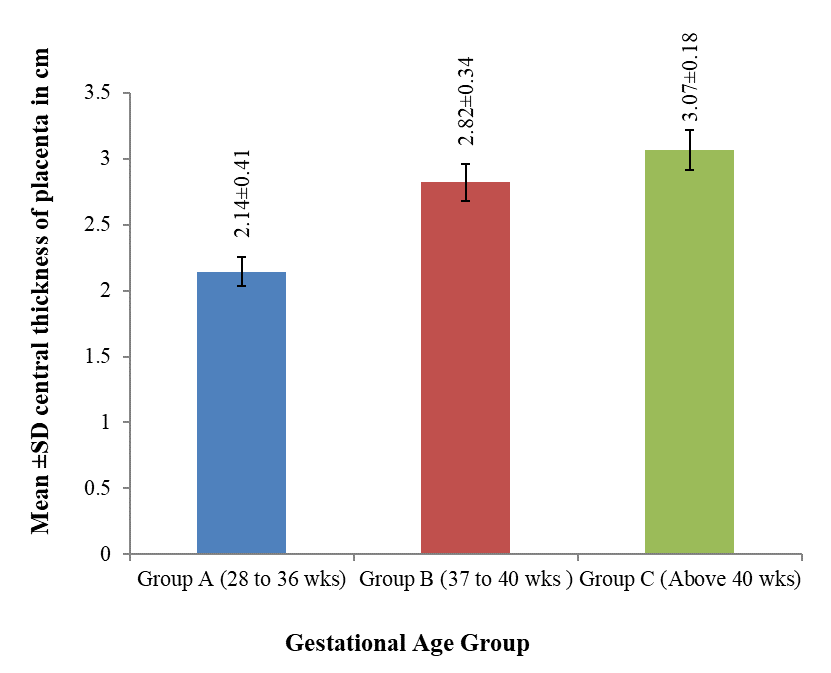
The maximum thickness at the center of the placenta was 3 cm in Group A, 3.5 cm in group B, 3.4 cm in Group C. The minimum thickness at the center of the placenta was 1.6 cm in Group A, 2 cm in Group B and 2.5 cm in Group C. The mean (± SD) thickness at the center was 2.14±0.41 cm in group A, 2.82±0.34 cm in group B and 3.07±0.18 cm in group C. The mean central thickness was maximum in group C (3.07 cm) and was minimum in group A (2.14 cm). It was also observed that the mean thickness at center of the placenta increased with gestational age. The mean difference of the placental thickness at the center between groups A and B and A and C was statistically highly significant (p < 0.001) but difference between B and C was statistically moderately significant (p < 0.01). Above findings are shown in the table II, III and figure 2.
Table II: Central Thickness of Placenta in Different Gestational Age Groups
|
Gestational Age Group |
Number of Specimen |
Central Thickness (cm) |
|
(n = 80) |
Mean±SD |
|
|
(Minimum - Maximum) |
||
|
A |
18 |
2.14±0.41 |
|
(28 to 36 weeks) |
(1.6 - 3.0) |
|
|
B |
42 |
2.82±0.34 |
|
(37 to 40 weeks) |
(2.0 - 3.5) |
|
|
C |
20 |
3.07±0.18 |
|
(Above 40 week) |
(2.5 - 3.4) |
Table III: Comparison of thickness at the center of placenta among the gestational age groups
|
Comparison between gestational age groups |
Mean Difference |
Standard Error of Difference |
t |
p |
Level of significance |
|
A & B |
-0.67698 |
0.11031 |
-6.137 |
0 |
Highly significant |
|
B & C |
-0.24357 |
0.06615 |
-3.682 |
0.001 |
Moderately significant |
|
A & C |
0.92056 |
0.10578 |
8.702 |
0 |
Highly significant |
4. Discussion
The maximum thickness at the center of the placenta was 3 cm in group A, 3.5 cm in group B, 3.4 cm in group C. The minimum thickness at the center of the placenta was 1.6 cm in group A, 2 cm in group B and 2.5 cm in group C. The mean (± SD) thickness at the center was 2.14±0.41 cm in group A, 2.82±0.34 cm in group B and 3.07±0.18 cm in group C. The mean central thickness was maximum in group C (3.07 cm) and was minimum in group A (2.14 cm). It was also observed that the mean thickness at center of the placenta increased with age. The mean difference of the placental thickness at the center between groups A and B, A and C was statistically highly significant (p < 0.001) but difference between B and C was statistically moderately significant (p < 0.01).
D’sa and Sangeetha (2018) elaborated that the mean placental thickness was 1.82±0.25 cm in normal birth weight group and 1.75±0.30 cm in the low-birth-weight group. It was found to be statistically significant [10]. Korantema & DuBois (2018) revealed that the mean placental thickness was 1.96 cm (1.2 cm -2.5 cm) which was studied on 40 human placentas in Ghana [11]. Das (2015) stated that the thickness and diameter of placentae were reduced in 62.5% of toxemia cases [12]. Gunasegaran (2017) described that the average thickness of placenta at term was about 3 cm [13]. Nagamani et al. (2015) performed a study on 500 observations and reported that the mean placental thickness was 3.10 cm (76% had normal thickness) [14]. Sadler (2015) elaborated that the average weight of placenta at term was about 3 cm at its center [1]. Hatti, Imran & ashwini (2013) calculated that the mean thickness of the term placenta was 2.1 cm [15]. In the study of Balihallimath et al. (2013), the mean placental thickness was 2.1 cm, the 5th and 95th percentiles of placental thickness varied from 1.5 to 3.0 cm [16]. Moore (2013) mentioned that the thickness of term placenta was 2-3 cm [17]. Decherney (2018) stated that the thickness of term placenta was 2-4 cm [4]. Datta (2012) observed that the thickness of full-term placenta was 3 cm at the center [2]. Gunapriya et al. (2011) reported that the mean thickness of the term placenta was 2.1 cm [18]. Dutta (2011) stated that the average thickness of the placenta at term was about 3 cm at its center and thins off towards the edge [19]. Raghunath, Vijayalakshmi & Shenoy (2011) stated that the average thickness of placenta was 2.1 cm [20]. Begum (2010) made a study on 60 human placentas and revealed that the mean±SD placental thickness was 1.54±0.33 cm in between 28 to 32 weeks, 1.89±0.39 cm in between 33 to 37 weeks and 2.80±0.21 cm in between 38 to above weeks of gestation [21]. Kishwara (2009) mentioned that the mean±SD thickness of the placenta was 1.59±0.39 cm in group A (n = 30, normal pregnant women) and 1.51±0.37 cm in group B (n = 30, pregnancy complicated by pre-eclampsia). Statistically the difference between groups A and B was not significant [22]. Salafia & Popek (2008) showed that thickness of term placenta was 2.3 cm (range 1.1-4.1 cm) [23]. Huppertz (2008) studied in the anatomy of the normal placenta and found that central thickness was 2 - 5 cm [24]. Saha et al (2006) studied in 35-40 weeks of normotensive, normoglycemic and hypertensive mother and they found that thickness of placenta of control was 0.9 - 1.9 cm and pregnancy induced hypertension was 1.2 - 2.06 cm and mean±SD value in case of control was 1.36±0.33 and pregnancy induced hypertension was 1.56±0.31 cm [25]. Sultana (2005) conducted a study on 45 placentas (20 control group and 25 eclamptic mothers) and revealed the range of thickness of placenta was 1.08 to 3.10 cm with a mean±SD) thickness was 2.037±0.452 cm in eclampsia. Whereas in control group the thickness of placenta ranged from 1.50 to 3.00 cm with a mean±SD thickness of placenta was 2.290 ± 0.422 cm [26]. Dawn (2004) stated that the thickness of the placenta at the center was 2.5 cm [27]. According to a study by Habib (2002), the warning limit of a placental thickness was 2 cm at 36 weeks gestation which was calculated to predict low birth weight infants [28]. Yetter et al (1998) stated that the thickness of full-term placenta was about 2.0 to 2.5 cm. They also described that the thickness less than 2 cm was possibly placental insufficiency with intrauterine growth restriction and in case of maternal diabetes mellitus the placenta became thick and more than 4 cm [29]. Cunningham et al. (1985) were reviewed the sonograms of 200 randomly selected singleton pregnancies. Placental thickness was measured and correlated with menstrual age. The placenta was demonstrated to increase in thickness with advancing menstrual age. At no stage of pregnancy was the normal placenta greater than 4 cm in thickness [6]. According to the Boyd and Hamilton (1970), the average thickness of placenta at term was 23 mm [30]. Aladjem et al (1967) studied morphologic aspects of the placenta in gestational diabetes seen by phase contrast microscopy and found that the thickness of placenta ranged from 1.8 to 3.2 cm and average was 2.5 cm [31].
Finding of the present study in group B and C was more or less similar to the findings of above mention authors. In group A, the finding of the present study was higher than the findings of Begum (2010) but less than those of rest of the authors. It may be due to different in age grouping or ranges, different authors have different inclusion criteria, racial factors and effect of fixative etc.
Limitations of the study
The study was conducted in a single hospital with a small sample size. So, the results may not represent the whole community.
Conclusion
The present study showed that the central thickness of placenta was variable; however, maximum number of cases had normal thickness. The mean central thickness was maximum in group C (3.07 cm) and was minimum in group A (2.14 cm). It was also observed that the mean thickness at center of the placenta increased with gestational age.
References
- Sadler TW. Langman’s medical embryology. 13th ed. Philadelphia: Wolters Kluwer (2015): 109-13.
- Datta AK. The placenta. In: Essentials of human embryology. 6th ed. Kolkata: Current Books International (2014): 58-9.
- Singh V. Extraembryonic membranes, placenta, and multiple pregnancy. In: Textbook of clinical embryology. 2nd ed. India: Elsevier Health Sciences (2017): 77-8.
- Decherney AH, Nathan L, Laufer N, Roman AS. Current diagnosis & treatment obstetrics & gynecology. 12th ed. New York: McGraw Hill Education 108 (2018): 171-8.
- Stevenson A. Oxford medical dictionary in English. 3rd ed. Oxford: OUP Oxford (2010): 28.
- Cunningham FG, Gant NF, Leveno KJ, Gilstrap LC, Hauth JC, Wenstrom KD. Williams Obstetrics. 21st ed. New York: McGraw Hill Medical Publishing (2001): 661-4.
- Durnwald CP, Mercer BM. Ultrasonographic estimation of placental thickness with advancing gestational age. Am J Obstet Gynecol 191 (2004): 1709-1714.
- Rahman MH. Gross morphological and arterial features of human placenta in overt diabetes with and without hypertension [thesis]. Dhaka: Bangabandhu Sheikh Mujib Medical University (2003).
- Sharma U, Banerjee K, Jyoti. Ultrasound evaluation of placental thickness during pregnancy as a predictor for low-birth-weight babies in a rural population of India. Int J Reprod Contracept Obstet Gynecol 7 (2018): 2686-2692.
- D’Sa DS, Sangeetha V. Morphometric study of placenta in relation to birth weight of full-term newborns. Int J Anat Res 6 (2018): 4924-7.
- Korantema T, DuBois A. Mode of umbilical cord insertion and neonatal weight and some placental factors. Int J Anat Res 6 (2018): 5471-6.
- Das SR, Kar P, Sahoo S, Panda SK, Chowdhury S, Nayak SK, et al. Morphological study of placenta in pregnancy with hypertension in western Odisha. Int J Pharm Sci Rev Res 33 (2015): 74-8.
- Gunasegaran JP. Textbook of histology. 3rd ed. New Delhi: Reed Elsevier India Pvt. Ltd (2017): 334-6.
- Nagamani G, Seshu KK, Vandana RG. Placental size and perinatal outcomes. J Evid Based Med Healthc 2 (2015): 1651-6.
- Hatti MA, Imran SS, Ashwini H. Effect of birth order on placental morphology and its ratio to birth weight. Int J Biomed Res 4 (2013): 2765-71.
- Balihallimath RL, Shirol VS, Gan AM, Tyagi NK, Bandankar MR. Placental morphometry determines the birth weight. J Clin Diagn Res 7 (2013): 2428-31.
- Moore KL, Persaud TVN, Torchia MG. The developing human: clinically oriented embryology. 9th ed. Philadelphia: Saunders Elsevier (2013): 230-4.
- Gunapriya R, Vijayalakshmi, Varsha S. A study on the morphology and the morphometry of the human placenta and its clinical relevance in a population in Tamil Nadu. J Clin Diagn Res 5 (2011): 282-6.
- Dutta DC. DC Dutta’s textbook of obstetrics including perinatology and contraception. 8th ed. New Delhi: Jaypee Brothers Medical Publishers Pvt. Ltd (2015): 28-45.
- Ragunath G, Vijayalakshmi, Shenoy V. A study of morphology and morphometry of the human placenta and its clinical relevance in a population in Tamil Nadu. J Clin Diagn Res 5 (2011): 282-6.
- Begum T. Gross and histomorphological study of human placenta and umbilical cord in different gestational age group in Bangladesh [thesis]. Mymensingh: Mymensingh Medical College (2010).
- Kishwara S, Ara S, Rayhan KA, Begum M. Morphological changes of placenta in preeclampsia. Bangladesh J Anat 7 (2009): 49-54.
- Salafia CM, Popek EJ. Placental development and early pregnancy pathology. Global Library of Women’s Medicine (2008).
- Huppertz B. The anatomy of the normal placenta. J Clin Pathol 61 (2008): 1296-302.
- Saha RR, Ferdousi R, Shamim KM, Ganguly D. A study of gross morphology of the placenta in pregnancy-induced hypertension. Bangladesh J Anat 4 (2006): 45-50.
- Sultana S. A comparative study of gross and histomorphological changes of human placenta and umbilical cord in normal and eclamptic pregnancy. Mymensingh: Mymensingh Medical College (2005).
- Dawn CS. Textbook of obstetrics and neonatology. 16th ed. Kolkata: Pratap Medical Publishers (2004): 46-50.
- Habib FA. Prediction of low-birth-weight infants from ultrasound measurement of placental diameter and placental thickness. Ann Saudi Med 22 (2002): 312-4.
- Yetter JF. Examination of the placenta. Am Fam Physician 57 (1998): 1045-54.
- Boyd JD, Hamilton WJ. The human placenta. 1st ed. England: Heffer (1970): 143, 264.
- Aladjem S, Perrin E, Fenaroff A. Placental score and neonatal outcome. Obstet Gynecol 39 (1970): 591-602.