Fulminant Diabetes in the Context Of COVID-19 Pandemic: The Resurgence of Pancreatic Insulitis?
Article Information
Soghomonian A1, Vincentelli C1, Jacquier A3,4, Di Bisceglie M5, Chaumoitre K5, Ancel P2, Taieb D6, Sahakhian N1,6, Ronsin O1, Boullu S1, Darmon P1,2, Dutour A1,2, Gaborit B1,2*
1Department of Endocrinology, metabolic diseases and nutrition, pôle ENDO, APHM, Marseille, France
2Aix Marseille Univ, INSERM, INRAE, C2VN, Marseille, France
3Aix-Marseille Univ, CNRS, CRMBM, Marseille, France
4Radiology department, CHU La Timone, Marseille, France
5Radiology department, CHU Nord, Marseille, France
6Department of Nuclear Medicine, Aix-Marseille University, Endo-ERN Reference Center for Rare Genetic Tumor Syndromes, Assistance Publique-Hopitaux de Marseille, Marseille, France
*Corresponding Authors: Bénédicte Gaborit, Endocrinology, Metabolic Diseases and Nutrition Department, Pole ENDO, Hôpital Nord, Chemin des Bourrely, 13915 Marseille cedex 20, France
Received: 27 October 2020; Accepted: 04 November 2020; Published: 20 November 2020
Citation: Soghomonian A, Vincentelli C, Jacquier A, Di Bisceglie M, Chaumoitre K, Ancel P, Taieb D, Sahakhian N, Ronsin O, Boullu S, Darmon P, Dutour A, Gaborit B. Fulminant Diabetes in the Context Of COVID-19 Pandemic: The Resurgence of Pancreatic Insulitis?. Archives of Clinical and Biomedical Research 4 (2020): 704-708.
Share at FacebookKeywords
COVID-19; Fulminant Diabetes
COVID-19 articles
Article Details
1. Introduction
Viral infection and its association with diabetes is a familiar concept. However, beyond the well-recognized impairment in glycemic control associated with infectious disease, there is scarce data on new-onset diabetes during Covid-19 outbreak [1].
2. Case Presentation
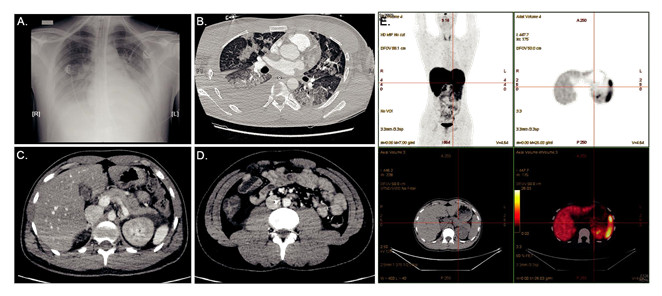
A 26-year-old Cambodian man was admitted to the emergency department unit during the Covid-19 outbreak. He presented with a 6-days history of nausea/abdominal pain, but without any history of polyuria/polydipsia/weight loss. The patient had no family history of diabetes and was contact case of Covid-19 positive individuals. He denied taking any medication. On admission, he suffered from confusional state and somnolence; BT=34°C (93.2°F); RR=34/min; BP=100/50 mmHg HR=75/min, Oxygen saturation=98%AA, BMI 20 kg/m2. Auscultation of the chest revealed fine crackles. Blood gases demonstrated severe metabolic acidosis (pH=6.9; HCO3-=3 mmol/L; acid lactic=2.5 mmol/L). Urine and blood sampling showed ketonuria at 0.8 g/L with major hyperglycaemia at 60.5 mmol/L (1100 mg/dL) confirming the diagnosis of diabetic ketoacidosis (DKA). An inflammatory state (leucocytes=16.109/L, CRP=40 mg/L Procalcitonin=4 ng/L), kidney function impairment (plasma creatinine=334 µmol/L), and a serum lipase at 2N were observed. Glycosylated hemoglobin HbA1c was found low at 6.2% with a normal Hb=13 g/dL. A PCR-test for SARS-CoV-2 was negative. The patient was treated with aggressive intra-venous fluids, intensive intravenously insulin therapy and a probabilistic antibiotic therapy. He was transferred to the intensive care unit (ICU), where he was intubated because of an acute respiratory distress syndrome with hypoxemia (pO2=62 mmHg). Full-body computed tomography (CT) scan reported severe pulmonary lesions peripherally distributed compatible with Covid-19, and right colitis in the abdomen. (Figure 1A-D). The investigation for another infection than Covid-19 evidence proved fruitless. Baseline hemocultures and cytobacteriological urine examination returned sterile. Iterative PCR for SARS-CoV-2 realized on nasopharyngeal swabs, during the first days after admission, remained negative. SARS-CoV-2 serum IgG antibody was also negative. Despite the large search for many viruses, in particular enterovirus (coxsackie, echovirus), herpes virus, HHV-6, mumps, influenza B, EBV, CMV, hepatitis A virus, no microbiologic agent was identified. Regarding the multi-organ failure, kidney function returned to normal with rehydration and closely monitoring of serum electrolytes and the diagnosis of acute tubular necrosis was retained. Regarding glucose homeostasis, the autoantibodies against glutamic acid decarboxylase (GAD), islet-associated antigen 2 (IA2) and insulin and zinc transporter 8 (ZnT8) were negative. Intravenous glucagon test showed severe impairment of insulin secretion with serum C-peptide<0.17 nmol/L after injection. Genotypic analysis revealed no human-leucocyte antigen (HLA) susceptibility for type 1 diabetes mellitus (T1D) or DF (DRB1*07:01;DQB1*02:02;DQA1*01:02;DRB1*02:01;DRB1*15:01;DQB1*05:02;DQA1*02:01;DPB1*03:01).
Abdominal CT-scan showed a normal volume pancreatic gland but peripancreatic fat infiltration (Figure 1C). 68Galium-PET/CT-DOTATOC performed one month later revealed no pancreatic insulitis. (Figure 1E). In accordance with the Japan Diabetes Society criteria [2], the patient was diagnosed with FD. The patient was transitioned to subcutaneous insulin infusion and a diabetic diet. He was discharged at day 20th, under multiple daily insulin injection therapy (13U basal and 26U prandial insulin).
Figure 1: (A) Chest X Ray showing bilateral interstitial pneumonia associated with right pleural effusion; (B) Pulmonary CT (parenchymal window) showing medium-abundance right pleural effusion and diffuse pulmonary involvement associating bilateral ground-glass opacities (GGO) with Crazy Paving aspect (combination of GGO, thickened polygonal septal lines and intralobular reticulations), and some consolidations (middle lobe); (C, D) Abdominal CT showing peripancreatic fluid next to the corporocaudal region without trophicity or pancreatic gland enhancement anomaly, and right colitis without signs of complication; (E) 68Galium-Positron Emission Tomography /CT-DOTATOC showing no pancreatic insulitis.
3. Conclusions
Fulminant diabetes is a very rare condition which is characterized by 1) a remarkably abrupt onset of the disease with acute ketosis or DKA within one week after the onset of hyperglycemic symptoms, 2) a low HbA1c value despite a high plasma glucose level, 3) an absence of insulin secretion capacity (low peptide-C levels after intravenous glucagon or meal load). It is considered a rapid and violent immune reaction targeted to viro-infected β-cells in genetically predisposed patients (HLADRB1*0405; DQB1*0401) that leads to a massive β-cell death [2]. The number of patients presumably amounts to 5,000-7,000 in Japan. In western countries, FD has been reported after anti-PD-1/PD-L1 inhibitors, alone or in association with anti-CTLA-4 antibodies treatments. To our knowledge, no case of FD during Covid-19 outbreak has been reported to date, though ketosis/DKA precipitated by Covid-19 have been observed [3]. In this case, no firm conclusion can be drawn on the triggering factor associated with this new-onset diabetes, and the negativity of Covid-19 sampling do not support this hypothesis. However, the Covid-19 diagnosis cannot be totally ruled out also, RT-PCR false-negative results have been reported (given the variation of viral RNA sequences) and given the social epidemic context. Furthermore, SARS-CoV-2 IgG testing was tested soon after diagnosis and other report from North West London, showing an increase in new-onset T1D in children during the COVID-19 pandemic, have showed similar results with only 9% of SARS-CoV-2 PCR positive and only 19% of IgG antibody positive [4]. Furthermore, CT lesions though not typical were evaluated as compatible by trained radiologists.
This case highlights the difficulty in proving viruses as causative agents in T1D, and more attention should be paid to the pancreatic beta cell as a site of infection.
We were not able to detect pancreatic insulitis even using sensitive functional imaging modalities such as 68Galium-PET/CT, which targets somatostatin receptor 2. This might be due to the small size of islets and their scattered location in the pancreas, the small and acute inflammatory lesion during the insulitis process. However, recent advances in noninvasive imaging techniques such as molecular studies applied to MRI, PET, and optical imaging indicate that there could be a role for diagnostic imaging in the evaluation of beta-cell number, mass, and function and lymphocyte infiltration/inflammatory activity in T1D. Patient HLA typing was unique and not associated to date with fulminant onset diabetes. Several data demonstrated increased ACE2 expression in alveolar AT2 cells, myocardium, kidney, and pancreas that may favor increased pancreatic cells binding of SARS-CoV-2 and hence islet damage. Other viruses such as enteroviruses have been implicated in the development of T1D in humans, suggesting that an initial infection might have significant, long-term consequences in some individuals. Whether fully lytic infection may arise in fulminant diabetes remains to be demonstrated.
Identifying new-onset diabetes during Covid-19 outbreak, seems crucial in clinical care, to better manage first-line intensive insulin therapy and obtain a better glycemic control in aim to decrease the risk of severe complications. Indeed, increased blood glucose on hospital admission for community acquired pneumonia predicts death in patients without pre-existing diabetes [5], highlighting the need for systematic blood glucose assessment in pulmonary infections.
To conclude, the emergence of new infectious diseases could lead to the development of new insulinopenic diabetes that may aggravate the prognosis of young patients.
References
- Rubino F, Amiel SA, Zimmet P, et al. New-Onset Diabetes in Covid-19. N Engl J Med 383 (2020): 789-790.
- Imagawa A, Hanafusa T, Awata T, et al. Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus: New diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J Diabetes Investig 3 (2012): 536-539.
- Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract 164 (2020): 108166.
- Unsworth R, Wallace S, Oliver NS, et al. New-Onset Type 1 Diabetes in Children During COVID-19: Multicenter Regional Findings in the U.K. Diabetes Care 43 (2020): 170-171.
- Lepper PM, Ott S, Nüesch E, et al. Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ 344 (2012): 3397.