Flow Cytometry-Based Assay to Identify A Competent Embryo in FET Cycles
Article Information
Berkay Akcay3, Neslihan Meric5, I Orcun Olcay3, Yagmur Ergun1, Kubra Boynukalin3, Aydin Arici1,2, Mustafa Bahceci3, Murat Basar1,2,4
1Department of Obstetrics, Gynecology, and Reproductive Sciences, Yale School of Medicine, New Haven, CT, USA
2Yale Fertility Center, 200 West Campus Drive, Orange, CT, 06477 USA
3Bahceci Health Group, Umut IVF Laboratory, Istanbul, Turkey
4Biruni University School of Medicine, Dept. of Histology & Embryology, Istanbul, Turkey
5Kütahya Health Sciences University, Engineering, and Natural Sciences, Kutahya, Turkey
*Corresponding author: Murat Basar, Department of Obstetrics, Gynecology, and Reproductive Sciences, Yale School of Medicine, New Haven, CT, USA.
Received: 02 June 2023; Accepted: 13 June 2023; Published: 19 June 2023
Citation: Berkay Akcay, Neslihan Meric, I Orcun Olcay, Yagmur Ergun, Kubra Boynukalin, Aydin Arici, Mustafa Bahceci, Murat Basar. Flow Cytometry-Based Assay to Identify A Competent Embryo in FET Cycles. Archives of Clinical and Biomedical Research. 7 (2023): 418-423.
Share at FacebookAbstract
Objectives: We aimed to investigate a non-invasive selection method to predict competent embryos before frozen-thawed embryo transfer.
Methods: Fifty-three random infertile patients undergoing a frozenthawed embryo transfer cycle at Bahceci Health Group Umut IVF Laboratory, Istanbul, were included in the study. Fifty-three patients’ embryos were thawed and cultured before transfer (100-120 min). Spent culture media was evaluated through flow cytometry for the PI (+) EV percentage.
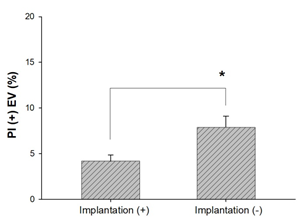
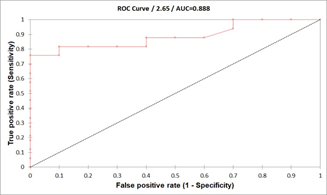
Results: Spent culture media with a lower percentage of PI (+) EVs were significantly higher implantation rates compared to higher PI+ EVs percentages (respectively; 4.18 ± 0.33, 7.9 ± 0.62; p<0.0001). Plotting data of confirmed competent embryos versus data from implantation failure yielded a 4.71% PI (+) EV count cut-off, corresponding to a maximum specificity and sensitivity. The AUC (area under the curve) was 0.888 (95% CI: 0.801–0.975).
Conclusion: Our result demonstrates that a PI (+) EV presence threshold in spent culture media can predict a healthy pregnancy outcome for frozen embryo transfer.
Keywords
ART; Extracellular vesicle; Frozen embryo transfer; Implantation; Spent culture media; Vitrification
ART articles; Extracellular vesicle articles; Frozen embryo transfer articles; Implantation articles; Spent culture media articles; Vitrification articles
Article Details
Introduction
Transferring more than one embryo increases the chance of establishing multiple gestations. Multiple gestations are known to raise the risk of adverse pregnancy outcomes. Preterm birth, low birth weight, fetal and infant death, and long-term disorders such as cerebral palsy can be counted as risks. Promoting an Elective Single Embryo Transfer (eSET) policy result in recognizing the risks associated with multiple pregnancies [1, 2]. Most laboratories rate the cleavage stage embryo based on the degree of fragmentation, the existence and number of nuclei, and the size, number, and symmetry of blastomeres per embryo. However, embryo grading procedures differ among fertility clinics [3-5]. Besides, the size of the blastocoel, the number of cells in the Inner Cell Mass (ICM), and the cohesion of the Trophectoderm (TE) cells are all considered when evaluating blastocysts [6]. Morphological evaluation has few predictive values in identifying the most viable embryos since morphology and developmental competency are not strongly correlated. Moreover, the timing of observations needs to be standardized to reduce subjectivity [7]. Currently, the two most common tools used widely for selecting the best embryo to transfer are embryo morphology assessment and preimplantation genetic testing for aneuploidies (PGT-A) [8]. Besides, closed embryo culture systems with time-lapse imaging generate enormous imaging data for analysis [9]. The number of studies was increasing to assess the most competent blastocyst to implant by developing new technologies, including using cell-free DNA from blastocoel fluid and spent media [10, 11]. EVs are nanoparticles surrounded by a lipid bilayer of varying size and content that cells use to communicate with each other and for many different purposes. According to their mechanisms and sources, they are classified into 3 groups: (exosomes, microvesicles, and apoptotic bodies [12]. It has been shown in various studies that the formations can be secreted throughout the entire preimplantation embryo development period, quickly crosses the zona pellucida, and assist the embryo in communicating with its environment [13-15]. Although many researchers have reported that some of these molecules can be used as a potential "non-invasive embryo selection biomarker," more studies are needed on the possible mechanisms of action of molecules and the standardization and effectiveness of tests developed using these biomarkers for clinical use [16]. In this study, we aimed to investigate implantation rates of frozen-thawed transfers, which can be predicted using this non-invasive selection method for the best competent embryo. We used flow cytometry to detect the propidium iodide (PI) positive (+) EV percentage, which indicates excessive dead material in the spent culture media. Our results demonstrate that the percentage of PI (+) EVs in the spent culture media (SCM) has a high prediction potential to assess competent blastocyst in frozen-thawed embryo transfer cycles.
Materials and Methods
Patients
Fifty-three random infertile patients undergoing a frozen-thawed embryo transfer cycle at Bahceci Health Group Umut IVF Laboratory, Istanbul, were included in the study. This study was approved by the Ethics Committee of the Acibadem Mehmet Ali Aydnlar University (No. 2019-8/5), and each patient signed written informed consent.
Blastocyst Vitrification Procedure
Embryo freezing was performed at room temperature (20-24°C). Laser pulse shrinkage of Day 5 (D5) and Day 6 (D6) expanded blastocysts was done using Mukaida's method [17]. Using an OCTAX laser (MTG, Germany), a single laser pulse opened the zona pellucida at the cellular junction of the trophectoderm cells located far away from the inner cell mass. According to the manufacturer's instructions, vitrification was performed after the blastocyst completed shrinkage with Irvine Vitrification Freeze-Kit (Irvine Scientific, Cat No. 90133- DSOC, USA). 50 µl drop of Equilibrium Solution (ES) was dispensed on an inverted lid of a petri dish. The specimen was transferred with a minimal volume of medium from the culture dish to the drop of ES, and embryos were equilibrated in the ES drop slowly by free-fall for 6-10 minutes. During the equilibration time in ES, a 50 µl drop of Vitrification solution (VS) was set. After equilibration in ES was completed, the embryos were transferred into the drop of VS for 30 seconds. The embryos were loaded on a straw and immersed in liquid nitrogen.
Blastocyst Warming Procedure
Warming was performed with the Vitrification Thaw Kit (Vit Kit®-Thaw, Irvine Scientific, USA) according to the manufacturer's instructions. The Cryotop (Kitazato, Japan) contents were immersed in 1 mL warmed Thawing Solution (TS) drop at 37 °C for 1 min. Then the blastocysts were transferred to a 50 μL Dilution Solution (DS) drop for 4 min, and finally, blastocysts were washed in two drops of Washing Solution (WS) for 4 min each.
Collection of Spent Culture Media (SCM)
In this study, the culture medium was collected after the embryo culture, and before the transfer was terminated (100-120 minutes after thaw). Before culture medium collection, the working area (Laminar Flow Cabinet, IVF Tech, Denmark) and microscopes were wiped with a DNA Away (Thermo Fisher Scientific, Waltham, MA, USA) surface cleaner to avoid possible contamination. Since the SCM was under oil, utmost care was taken to avoid contamination. SCM was collected using 290 µm diameter pipettes (EZ-Strip, #7-72-2290/1, CooperSurgical Fertility & Genomic Solutions, Målov, Denmark). As negative controls, the same amount of embryo culture medium was collected from the same dishes without being used for embryo culture.
PI (+) Extracellular Vesicle Detection
The necrotic activity of EVs obtained from spent culture media was examined through flow cytometry analysis by staining Propidium iodide (PI). As previously described [18], for PI staining, 100 µl of 4% paraformaldehyde was added to the embryo culture media (25 µl) and incubated at RT for 15 minutes. 150 µl of filtered PBS and 1 µl of PI dye were added to them and incubated for 30 minutes at RT in the dark. After the incubation period, the samples were run in the flow cytometer. The flow cytometer instrument was set to read 5000 extracellular vesicles in 180 seconds. Gates were taken according to the unstained sample.
Statistical Analysis
Statistical analyses were made with SigmaPlot Windows version 11.0 software using an independent t-test (p-value < .05) and binary logistic regression with an entering and Backward Stepwise Likelihood Ratio model (p < .05).
Results
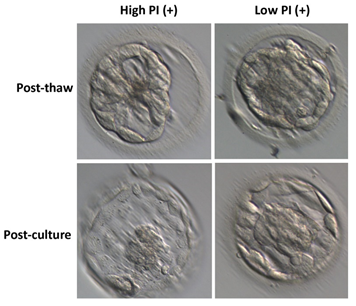
In this study, we aimed to identify competent embryos for transfer in vitro fertilization (IVF) procedures by analyzing the number of phosphatidylserine-positive extracellular vesicles (PI (+) EVs) present in the culture medium of frozen-thawed embryos. Only good-quality embryos, graded according to Gardner's criteria (3AA and higher), were included in the study. No difference between the high and low PI (+) EV groups was found in thaw survival rate and blastocyst quality.
Identification of competent embryos by determining the number of PI (+) EVs in the frozen-thawed culture medium
The transfer of 53 embryos resulted in 33 implantations, and all patients received a single embryo transfer. We observed that the culture media from transferred embryos that resulted in a pregnancy contained a lower percentage of PI (+) EVs than those that resulted in implantation failure. Additionally, when comparing culture media from embryos with a lower percentage of PI (+) EVs to those with a higher percentage within the "clinical pregnancy" group, a significant difference was found (4.18 ± 0.33 vs. 7.9 ± 0.62, respectively; p<0.0001). These findings support using PI (+) EV percentage as a marker for identifying competent embryos.
A cut-off level for identifying the competent embryos.
We performed receiver operating characteristic (ROC) analysis using different data sets to establish a cut-off level for identifying competent embryos. ROC analysis of confirmed competent embryos versus data from implantation failure yielded a cut-off of 4.71% PI (+) EVs, corresponding to maximum sensitivity and specificity. The area under the curve (AUC) was 0.888 (95% CI: 0.801-0.975). Similarly, plotting data from presumed competent embryos (i.e., those with the lowest PI (+) EV count among embryos from the same mother) against data from implantation failure resulted in a cut-off level of 964, with an AUC of 0.899 (95% CI: 0.837-0.960), a sensitivity of 0.875, and a specificity of 0.857.
Based on these data, transferring the embryo with the lowest PI (+) EV count is recommended, as it provides close to a 90% chance of successful implantation, assuming maternal receptivity. This approach could improve IVF success rates and reduce the number of embryos required for transfer, thereby increasing the safety and efficacy of IVF procedures.
Discussion
The eventual aim of in vitro fertilization (IVF) is a healthy birth from a single transferred embryo. Several methods for genetically assessing preimplantation embryos have been developed over the last thirty years. These have been used to avoid the transmission of a single gene disorder or, more controversially, to aid in selecting viable embryos for uterine transfer by distinguishing chromosomally normal embryos with high developmental potential from their compromised aneuploid siblings [19]. To determine the genetic status of an embryo, all previous and current PGT methods relied on sampling genetic material from embryos via biopsy of blastomeres or trophectoderm cells [20-23]. On the other hand, the micromanipulation techniques required for biopsy are invasive and not without risk [24]. It is hardly surprising that the recent discovery of DNA in embryo culture media and even in the fluid filling the blastocoel cavity of blastocysts has generated anticipation about the possibility of non-invasive PGT (niPGT), given the difficulties inherent with invasive embryo biopsy. The recent discovery of DNA in embryo culture media and even in the fluid filling the blastocoel cavity of blastocysts has generated anticipation about the possibility of non-invasive PGT (niPGT), given the difficulties inherent with invasive embryo biopsy [25, 26]. A spent Culture Medium (SCM) has recently been proposed as an alternative source of embryonic DNA. A growing amount of research has demonstrated the detection of cell-free DNA in SCM and emphasized the diagnostic potential of non-invasive SCM-based PGT for determining the genetic status of IVF-derived preimplantation human embryos [27]. Although the origin of cfDNA is still unknown, embryonic cell apoptosis may contribute to the presence of cfDNA in the spent culture media [28, 29]. In tumor growth, vascular function, immunology, and regenerative medicine, extracellular vesicles (EVs) are a significant class of membrane-bound structures extensively studied for their roles in intercellular communication. As discussed by Baxter et al., much of the current knowledge on the functions of EVs pertains to those derived from viable cells (e.g., exosomes and microvesicles) or apoptotic cells (e.g., apoptotic bodies). In contrast, EV generation from dying cells under non-apoptotic conditions remains poorly characterized [30]. Even though many studies investigated the roles of extracellular vesicles released from the cells in other physiological processes, EVs' precise mechanisms in embryo implantation have yet to be elucidated. It has been suggested that the three stages of embryo implantation are affected by the production of EVs by various tissues and cells, including the endometrium, decidua, embryo, seminal fluid, and oviduct [31-33]. To detect whether cells are alive, apoptotic, or necrotic by variations in plasma membrane integrity and permeability, propidium iodide (PI) and Annexin V are frequently used [34, 35]. The membrane's permeability determines whether PI may enter a cell; since the plasma membrane is intact, PI cannot stain living or early apoptotic cells. The integrity of the plasma and nuclear membranes deteriorates in late apoptotic and necrotic cells, allowing PI to flow through the membranes, crosslink into nucleic acids, and exhibit red fluorescence [36, 37]. Our study assessed the Propidium Iodide (PI) positivity percentage as an indicator sign for waste products released from the embryo to the culture media. When we examined the frozen embryo transfer (FET) groups, the PI+EV percentage was significantly higher than the fresh group. More interestingly, the clinical pregnancy rate was significantly lower when the PI (+) EV percentage was above 4.71% in the spent culture media of the transferred blastocyst. Many studies highlight that extracellular vesicle secreted from different origins had different effects on reproduction. In 2019, Kim et al. stated that outgrowth embryo derived EVs function as bioactive molecules and regulate mouse embryonic developmental competence in vitro and implantation potential in utero [31]. Similarly, in 2017, Qu et al. investigated porcine embryos derived from EVs and the results on the influence of their growth, viability, and pregnancy rates [38]. Furthermore, Giacomini et al. demonstrated that both the components of the endometrium, the epithelial and the stromal cells, could uptake embryo derived EVs [39]. In the study, co-cultures with monolayers of primary human endometrial cells, EVs dyed with Vybrant DiO fluorescent dye, efficient uptake of embryo derived EVs was shown, suggesting that only these vesicles may be internalized by endometrial cells using a particular mode of identification. Our result demonstrates that a PI (+) EV presence threshold in spent culture media can predict a healthy pregnancy outcome for frozen embryo transfer. Consistent with the previous studies, since extracellular vesicle has critical roles in the embryo-endometrium crosstalk, the viability of extracellular vesicles can be measured by flow cytometric analysis as a non-invasive method to detect the most competent embryo for successful implantation. Although numerous technical limitations remain to be addressed to maximize embryonic EV isolation, it now appears more likely than ever to begin to unravel the secrets that these vesicles may be holding. These findings, together with the knowledge at our disposal from the IVF lab, urge us to investigate further the potential of EVs to shed light on some of the mysteries surrounding reproductive medicine and their utility as indicators of embryonic competence.
Disclosure of interests
The authors report no competing interests to declare.
References
- Ombelet W, et al. Multiple gestation and infertility treatment: registration, reflection and reaction--the Belgian project. Hum Reprod Update 11 (2005): 3-14.
- In vitro fertilization and multiple pregnancies: an evidence-based analysis. Ont Health Technol Assess Ser 6 (2006): 1-63.
- Scott L, et al. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: prospective and applied data for increased pregnancy rates. Human Reproduction 22 (2006): 230-240.
- Racowsky C, et al. Standardization of grading embryo morphology. Journal of Assisted Reproduction and Genetics 27 (2010): 437-439.
- Hardarson T, et al. Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: Indications for aneuploidy and multinucleation. Human Reproduction 16 (2001): 313-318.
- Gardner DK, et al. Single blastocyst transfer: a prospective randomized trial. Fertility and Sterility 81 (2004): 551-555.
- Balaban B, et al. Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reproductive BioMedicine Online 22 (2011): 632-646.
- Babayev E. Choosing the best embryo with the help of artificial intelligence. Fertility and Sterility 114 (2020):
- Racowsky C, P Kovacs, WP Martins. A critical appraisal of time-lapse imaging for embryo selection: where are we and where do we need to go? Journal of Assisted Reproduction and Genetics 32 (2015): 1025-1030.
- Shitara A, et al. Cell-free DNA in spent culture medium effectively reflects the chromosomal status of embryos following culturing beyond implantation compared to trophectoderm biopsy. PLoS One 16 (2021): e0246438.
- Kuznyetsov V, et al. Minimally Invasive Cell-Free Human Embryo Aneuploidy Testing (miPGT-A) Utilizing Combined Spent Embryo Culture Medium and Blastocoel Fluid -Towards Development of a Clinical Assay. Sci Rep 10 (2020): 7244.
- Hawke DC, AJ Watson, DH Betts. Extracellular vesicles, microRNA and the preimplantation embryo: non-invasive clues of embryo well-being. Reproductive BioMedicine Online 42 (2021): 39-54.
- Battaglia R, et al. Identification of extracellular vesicles and characterization of miRNA expression profiles in human blastocoel fluid. Scientific Reports 9 (2019): 84.
- Vyas P, H Balakier, CL Librach. Ultrastructural identification of CD9 positive extracellular vesicles released from human embryos and transported through the zona pellucida. Syst Biol Reprod Med 65(2019): 273-280.
- Tesfaye D, N Menjivar, S Gebremedhn. Current knowledge and the future potential of extracellular vesicles in mammalian reproduction. Reprod Fertil Dev 34 (2021): 174-189.
- Capalbo A, et al. MicroRNAs in spent blastocyst culture medium are derived from trophectoderm cells and can be explored for human embryo reproductive competence assessment. Fertil Steril 105 (2016): 225-35.e1-3.
- T Mukaida, C Oka, T Goto, et al. Artificial shrinkage of blastocoeles using either a micro-needle or a laser pulse prior to the cooling steps of vitrification improves survival rate and pregnancy outcome of vitrified human blastocysts, Human Reproduction 21 (2006): 3246-3252.
- Pallinger E, et al. A simple and rapid flow cytometry-based assay to identify a competent embryo prior to embryo transfer. Sci Rep 7 (2017): 39927.
- Leaver M, D Wells. Non-invasive preimplantation genetic testing (niPGT): the next revolution in reproductive genetics? Hum Reprod Update 26 (2020): 16-42.
- Dokras A, et al. Trophectoderm biopsy in human blastocysts. Human Reproduction 5 (1990): 821-825.
- Handyside AH, et al. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature 344 (1990): 768-770.
- Verlinsky Y, et al. Analysis of the first polar body: preconception genetic diagnosis. Human Reproduction 5 (1990): 826-829.
- Kokkali G, et al. Birth of a healthy infant following trophectoderm biopsy from blastocysts for PGD of β-thalassaemia major: Case report. Human Reproduction 20 (2005): 1855-1859.
- Scott RT Jr , et al. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertility and Sterility 100 (2013): 624-630.
- Palini S, et al. Genomic DNA in human blastocoele fluid. Reproductive BioMedicine Online 26 (2013): 603-610.
- Stigliani S, et al. Mitochondrial DNA content in embryo culture medium is significantly associated with human embryo fragmentation. Human Reproduction 28 (2013): 2652-2660.
- Brouillet S, et al. Is cell-free DNA in spent embryo culture medium an alternative to embryo biopsy for preimplantation genetic testing? A systematic review. Reproductive BioMedicine Online 40 (2020): 779-796.
- Hardy K. Cell death in the mammalian blastocyst. Molecular Human Reproduction 3 (1997): 919-925.
- Hardy, K., Apoptosis in the human embryo. Reviews of Reproduction 4 (1999): 125-134.
- Baxter AA, et al. Analysis of extracellular vesicles generated from monocytes under conditions of lytic cell death. Scientific Reports 9 (2019): 7538.
- Kim J, et al. Embryotrophic effects of extracellular vesicles derived from outgrowth embryos in pre- and peri-implantation embryonic development in mice. Molecular Reproduction and Development 86 (2019): 187-196.
- Kurian NK, D Modi. Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy. Journal of Assisted Reproduction and Genetics 36 (2019): 189-198.
- Jankovicová J, et al. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. International Journal of Molecular Sciences 21 (2020): 7568.
- Vermes I, et al. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184 (1995): 39-51.
- Vermes I, C Haanen, C Reutelingsperger. Flow cytometry of apoptotic cell death. J Immunol Methods 243 (2000): 167-90.
- Darzynkiewicz Z, et al. Features of apoptotic cells measured by flow cytometry. Cytometry 13 (1992): 795-808.
- Faleiro L, Y Lazebnik. Caspases disrupt the nuclear-cytoplasmic barrier. J Cell Biol 151 (2000): 951-959.
- Qu P, et al. Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS One 12 (2017): e0174535.
- Giacomini E, et al. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Scientific Reports 7 (2017): 5210.