Evaluation of the Consistency of the LI-RADS 2017 as a Non-Invasive Diagnostic Tool for the Detection of Hepatocellular Carcinoma in At-Risk Patients with a Focal Hepatic Lesion
Article Information
Rastgooyan Hemmat Allaha, Nazanin Sadraeia, Alishavandi Raanab, Bijan Bijanc, Sadabadi Yoonesd
a Radiology Department, Shiraz University of Medical Sciences, Shiraz, Iran
b Medical Researcher at Shiraz University of Medical Sciences, Shiraz, Iran
c University of California Davis, Sacramento, California
d Department of Pediatric Dentistry, Faculty of Dentistry, Shahed University, Tehran, Iran
*Corresponding Author: Nazanin Sadraei, Radiology Department, Shiraz University of Medical Sciences, Shiraz, Iran
Received: 17 March 2023; Accepted: 24 March 2023; Published: 22 May 2023
Citation: Nazanin Sadraei, Rastgooyan Hemmat Allah, Alishavandi Raana, Bijan Bijan, Sadabadi Yoones. Evaluation Of The Consistency Of The LI-RADS 2017 As A Non-Invasive Diagnostic Tool For The Detection Of Hepatocellular Carcinoma In At-Risk Patients With A Focal Hepatic Lesion. Journal of Radiology and Clinical Imaging. 6 (2023): 127-137.
Share at FacebookAbstract
Background: Hepatocellular carcinoma (HCC) is the most common type of primary hepatic malignancy. Liver cirrhosis and chronic HBV infection are the most common risk factors for HCC. [1] Imaging-based HCC diagnosis is considered an acceptable alternative for tissue biopsy in patients with chronic liver disease. Liver Imaging Reporting and Data System (LI-RADS) is a scoring system for reporting and interpreting focal hepatic lesions concerning their subcategories. LR-1 is used for definitely benign lesions, LR-2 for possible benign lesions, LR-3 for equivocal lesions, LR-4 for possible HCC, and LR-5 for definite HCC. LR-TIV is considered a malignant lesion within venous invasion.
Materials and methods: This retrospective study which has been implemented in Namazi university hospital tertiary care, reviews dynamic liver CTs of 29 patients with focal liver lesions and further evaluates the subsequent correlation with the final pathologic diagnosis of HCC. The period is taken from March 2016 to January 2019. The total number of 3000 cases containing pathological reports under HCC diagnosis during the specified 34 months with regards to this study were extracted from the Namazee hospital data center. A liver lesion biopsy was performed on 350 cases. HCC was proved histologically in 100 cases. CT images were available in 45 cases. Eventually, 29 cases were eligible to be evaluated by LI-RADS v2017. However, 16 patients were excluded due to various reasons such as lack of dynamic liver CT-scan protocol, age groups of less than 18, and history of previous intervention on liver lesions. It should be noted that a dynamic liver CT scan with acceptable quality according to LIRADS recommendations was carried out on all the 29 selected cases. Two board-certified radiologists experienced in cross-sectional liver imaging reviewed the case studies and randomly categorized them based on LIRADS v2017. The SPSS22 software was used for statistical analysis, followed by the Chi-square goodness of fit test and T-test. P-values larger than 0.05 were assumed as statistically significant.
Results: 29 patients, in which LIRADS applied to the 17 cases (58.62%) with major risk factors and 12 others with no RF for comparison. Among major imaging features of LI-RADS 2017, 100% of HCC patients showed a washout appearance and all of these lesions were measured above 20mm. Threshold growth could be assessed only in one patient. APHE was confirmed in 93.1%. The least encountered major imaging feature was the presence of enhancing capsule (27%). The most common ancillary features favoring malignancy were discrete nodules on ultrasound (79%),
LI-RADS articles; HCC articles; HBV articles; Cirrhosis articles; The major feature articles
Hepatocellular carcinoma articles Hepatocellular carcinoma Research articles Hepatocellular carcinoma review articles Hepatocellular carcinoma PubMed articles Hepatocellular carcinoma PubMed Central articles Hepatocellular carcinoma 2023 articles Hepatocellular carcinoma 2024 articles Hepatocellular carcinoma Scopus articles Hepatocellular carcinoma impact factor journals Hepatocellular carcinoma Scopus journals Hepatocellular carcinoma PubMed journals Hepatocellular carcinoma medical journals Hepatocellular carcinoma free journals Hepatocellular carcinoma best journals Hepatocellular carcinoma top journals Hepatocellular carcinoma free medical journals Hepatocellular carcinoma famous journals Hepatocellular carcinoma Google Scholar indexed journals Liver Imaging Reporting and Data System articles Liver Imaging Reporting and Data System Research articles Liver Imaging Reporting and Data System review articles Liver Imaging Reporting and Data System PubMed articles Liver Imaging Reporting and Data System PubMed Central articles Liver Imaging Reporting and Data System 2023 articles Liver Imaging Reporting and Data System 2024 articles Liver Imaging Reporting and Data System Scopus articles Liver Imaging Reporting and Data System impact factor journals Liver Imaging Reporting and Data System Scopus journals Liver Imaging Reporting and Data System PubMed journals Liver Imaging Reporting and Data System medical journals Liver Imaging Reporting and Data System free journals Liver Imaging Reporting and Data System best journals Liver Imaging Reporting and Data System top journals Liver Imaging Reporting and Data System free medical journals Liver Imaging Reporting and Data System famous journals Liver Imaging Reporting and Data System Google Scholar indexed journals CT scan articles CT scan Research articles CT scan review articles CT scan PubMed articles CT scan PubMed Central articles CT scan 2023 articles CT scan 2024 articles CT scan Scopus articles CT scan impact factor journals CT scan Scopus journals CT scan PubMed journals CT scan medical journals CT scan free journals CT scan best journals CT scan top journals CT scan free medical journals CT scan famous journals CT scan Google Scholar indexed journals LR-TIV articles LR-TIV Research articles LR-TIV review articles LR-TIV PubMed articles LR-TIV PubMed Central articles LR-TIV 2023 articles LR-TIV 2024 articles LR-TIV Scopus articles LR-TIV impact factor journals LR-TIV Scopus journals LR-TIV PubMed journals LR-TIV medical journals LR-TIV free journals LR-TIV best journals LR-TIV top journals LR-TIV free medical journals LR-TIV famous journals LR-TIV Google Scholar indexed journals Threshold growth articles Threshold growth Research articles Threshold growth review articles Threshold growth PubMed articles Threshold growth PubMed Central articles Threshold growth 2023 articles Threshold growth 2024 articles Threshold growth Scopus articles Threshold growth impact factor journals Threshold growth Scopus journals Threshold growth PubMed journals Threshold growth medical journals Threshold growth free journals Threshold growth best journals Threshold growth top journals Threshold growth free medical journals Threshold growth famous journals Threshold growth Google Scholar indexed journals Washout appearance articles Washout appearance Research articles Washout appearance review articles Washout appearance PubMed articles Washout appearance PubMed Central articles Washout appearance 2023 articles Washout appearance 2024 articles Washout appearance Scopus articles Washout appearance impact factor journals Washout appearance Scopus journals Washout appearance PubMed journals Washout appearance medical journals Washout appearance free journals Washout appearance best journals Washout appearance top journals Washout appearance free medical journals Washout appearance famous journals Washout appearance Google Scholar indexed journals arterial phase hyper-enhancement articles arterial phase hyper-enhancement Research articles arterial phase hyper-enhancement review articles arterial phase hyper-enhancement PubMed articles arterial phase hyper-enhancement PubMed Central articles arterial phase hyper-enhancement 2023 articles arterial phase hyper-enhancement 2024 articles arterial phase hyper-enhancement Scopus articles arterial phase hyper-enhancement impact factor journals arterial phase hyper-enhancement Scopus journals arterial phase hyper-enhancement PubMed journals arterial phase hyper-enhancement medical journals arterial phase hyper-enhancement free journals arterial phase hyper-enhancement best journals arterial phase hyper-enhancement top journals arterial phase hyper-enhancement free medical journals arterial phase hyper-enhancement famous journals arterial phase hyper-enhancement Google Scholar indexed journals SPSS22 software articles SPSS22 software Research articles SPSS22 software review articles SPSS22 software PubMed articles SPSS22 software PubMed Central articles SPSS22 software 2023 articles SPSS22 software 2024 articles SPSS22 software Scopus articles SPSS22 software impact factor journals SPSS22 software Scopus journals SPSS22 software PubMed journals SPSS22 software medical journals SPSS22 software free journals SPSS22 software best journals SPSS22 software top journals SPSS22 software free medical journals SPSS22 software famous journals SPSS22 software Google Scholar indexed journals HBV infection articles HBV infection Research articles HBV infection review articles HBV infection PubMed articles HBV infection PubMed Central articles HBV infection 2023 articles HBV infection 2024 articles HBV infection Scopus articles HBV infection impact factor journals HBV infection Scopus journals HBV infection PubMed journals HBV infection medical journals HBV infection free journals HBV infection best journals HBV infection top journals HBV infection free medical journals HBV infection famous journals HBV infection Google Scholar indexed journals PACS articles PACS Research articles PACS review articles PACS PubMed articles PACS PubMed Central articles PACS 2023 articles PACS 2024 articles PACS Scopus articles PACS impact factor journals PACS Scopus journals PACS PubMed journals PACS medical journals PACS free journals PACS best journals PACS top journals PACS free medical journals PACS famous journals PACS Google Scholar indexed journals
Article Details
1. Introduction
1.1 Hepatocellular carcinoma (HCC)
HCC is considered the most common type of primary hepatic malignancy comprising roughly 70 to 85 percent of overall hepatic malignancies. It is presumed as the second most common cause of cancer mortality worldwide [1-4]. More than 80 to 90 percent of HCCs occur in cirrhotic patients. The risk of HCC development depends on the underlying diseases e.g liver cirrhosis and chronic HBV infection which are the most common risk factors for developing HCC [5,6]. HCC is often diagnosed noninvasively by imaging without biopsy. Therefore, radiologists have an important role in the definite diagnosis of HCC [6], however, if image findings are not conclusive, biopsy or short-interval follow-up will be suggested [7]. Considering a screening program regarding the diagnosis of HCC is essential due to its morbidity and mortality significance. The so-called screening program of HCC is formed per every distinct risk population, which should carry various characteristics such as high diagnostic accuracy, low morbidity and mortality, well-defined recalling strategies, and available effective therapies after the diagnosis [8]. when classic criteria are present, Image-based HCC diagnosis is considered an alternative for tissue biopsy in patients with chronic liver disease [9]. Early-stage diagnosis of HCC allows for effective management by performing locoregional therapy (LRT) or resection and may also lead to a complete cure by orthotopic liver transplantation (OLT) [10].
1.2 LI-RADS (Liver Imaging-Reporting and Data System)
The American College of Radiology (ACR) was formed in 2008 as a committee of radiologists to design a comprehensive system for reporting and interpreting the findings of CT and MRI examinations of the liver in high-risk patients in the development of HCC. The first version of the LI-RADS was proposed in 2011 and was recently updated in 2017 [11]. LI-RADS is a scoring system for reporting and interpretation of hepatic lesion characteristics in computed tomography (CT) and magnetic resonance (MR) studies in high-risk patients for HCC development [12]. LI-1 is used for definitely benign lesions, LI-2 for probably benign lesions, LI-3 for equivocal lesions, LI-4 for probably HCC, and LI-5 for definite HCC [13,14]. LR-TIV means Malignant with venous invasion [15].
1.3 LI-RADS version 2017
According to the newest version of LI-RADS v2017, if a lesion is probably or malignant though not HCC specific, LR-M is applied and the treatment option may be determined based on the clinical context [16]. The Ancillary features (AFs) are divided into three subcategories, those that are in favor of malignancy, those which are HCC-specific, and those favoring benignity [17]. The eligible population for the LI-RADS application should have the following characteristics, adults older than 18 years of age, cirrhotic patients, patients with chronic hepatitis B, patients with current or prior HCC with or without cirrhosis, adult liver transplantation candidates and liver transplant recipients [15].
Patients with cirrhosis due to vascular causes, including Budd–Chiari syndrome, hereditary hemorrhagic telangiectasia, nodular regenerative hyperplasia, or cardiac cirrhosis are excluded. LI-RADS is not used in patients below 18 years of age since its validity has not been proven in pediatric populations [18]. Cirrhotic patients due to congenital hepatic fibrosis are also not eligible for LI-RADS application [15].
If the diagnosis could be not narrowed down due to various reasons such as image degradation or deletion, the LR-NC category is used to prevent the LI-RADS category from being overcrowded. For LR-NC lesions, repeating all sequence imaging in less than three months is considered acceptable. If no imaging modality could facilitate a diagnosis, a confirmatory biopsy would be suggested. LR-TIV –enhancing soft tissue with portal vein invasion– in prior versions, an unequivocal Tissue Invading Vein (TIV) was categorized as an LR-5V lesion, however, other malignancies such as cholangiocarcinomas and combination tumors e.g. hepatocholangiocarcinoma also may show venous invasion. TIV may be more clearly visible than the corresponding parenchymal mass and the mass may be undetected until recognition of the TIV prompts a closer investigation of the parenchyma. The LR-M category is more commonly associated with intrahepatic cholangiocarcinoma however may also be visual in combination tumors and metastases; nonetheless, metastases are very rare in cirrhotic livers [15].
1.4 Major Features
Five major criteria are used to determine the LI-RADS scoring system including, arterial phase hyper-enhancement, capsule appearance, threshold growth, washout appearance, and size. Multiple ancillary features can be applied to upgrade the LI-RADS category [15, 19].
1.5 Non-rim Arterial phase hyperenhancement (APHE)
APHE seems to be due to the formation of multiple unpaired arteries caused by neovascularization [15]. No lesion can be categorized in LI-RADS 5 category (Definite HCC) if APHE is absent. The optimal late arterial phase is essential for the unequivocal discrimination of nodule enhancement versus that of the liver background. Furthermore, it has higher priority than the underlying liver parenchyma during the hepatic arterial phase [20]. One of the impediments in the categorization of LI_RADS is imaging with infiltrative mass lesions accompanied by underexpression of angiogenic signaling pathways i.e. poorly differentiated HCC, which may not show the arterial phase hyperenhancement [15].
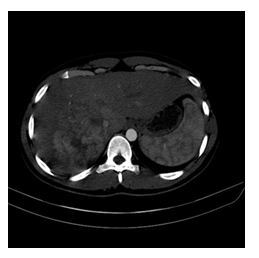
Figure 1: Non-rim Arterial phase hyperenhancement (APHE): Arterial hyperenhancement, is enhancement unequivocally more than underlying liver parenchyma during the hepatic arterial phase.
1.6 Enhanced capsule appearance
Enhanced capsule appearance, which demonstrates smoothed peripheral rim hyperenhancement in portal venous, delayed, or transitional phase imaging [21].
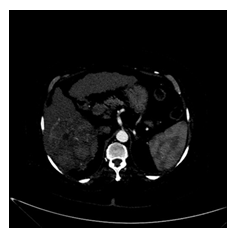
Figure 2: Enhanced capsule appearance: the peripheral rim of smooth hyper enhancement unequivocally thicker background nodule.
1.7 Threshold growth
Threshold growth in LI-RADS is defined as an increase in the lesion diameter by at least five millimeters that show either a more than 50 percent increase for less than 6 months or a more than 100 percent increase for more than 6 months. A newly discovered lesion with a diameter larger than 10 mm, which was found by comparing the last 24 months’ recorded exams, is considered an indicator of threshold growth [21].
1.8 Washout appearance
Washout appearance is identified as a visually assessed temporal decrease in the enhancement of the whole or some parts of the lesion in comparison to liver tissue from the earlier to later phase causing extracellular phase hypo enhancement [21].
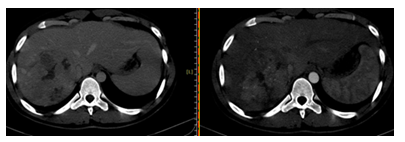
Figure 3: Washout appearance: decrease in the enhancement of the lesion compared to composite liver tissue from earlier to later Phase
1.9 Size
The largest outer-outer edge dimension of a lesion should be measured including the capsule. The possibility of HCC is increased significantly when the lesion size is larger than two centimeters [15].
1.10 Ancillary findings
In LI-RADS v2017, the use of ancillary imaging features is optional. Ancillary features can be used to upgrade or downgrade a lesion category by no more than one level, however, should not be used to upgrade a lesion to category LR-5, because no ancillary feature has sufficient specificity for the diagnosis of HCC. Often, if a lesion demonstrates one or more ancillary features in favor of malignancy, the observer can upgrade its category by one level, up to level LR-4. Moreover, if a lesion shows one or more features favoring benignity, the observer may downgrade the category by one level. If there are opposite ancillary features—features in favor of malignancy and those favoring benignity simultaneously —then the category cannot be changed [15].

Figure 4: Step 1 of the LI-RADS v2017 CT and MR imaging diagnostic algorithm: apply the algorithm. AASLD = American Association for the Study of Liver Diseases, OPTN = Organ Procurement and Transplantation Network. Reprinted from 2017 Version of LI-RADS for CT and MR Imaging: An Update.)
2. Materials and Methods
2.1 Study Area
In this retrospective cross-sectional study, we assessed the consistency of LI-RADS (Liver Imaging-Reporting and Data System) version 2017 in non-invasive diagnosis of HCC with pathologic reports, by using Triphasic (dynamic) liver CT scan in patients with the focal liver lesion(s) & with a definitive histologic diagnosis of HCC, in a time between March 2016 to January 2019 in Shiraz Namazee Hospital.
The pathologic data center of Shiraz Nemazee hospital was searched for reports, which were concordant with HCC diagnosis during these 34 months. 100 cases of histologically proven HCC were found and 29 cases with available dynamic liver CT-scan were eligible for the LI-RADS evaluation process.
2.2 Sampling methods
The study population consisted of adult patients above 18 years of age with pathologic reports of HCC in Namazee Hospital. Their respective dynamic liver CT scans were available furthermore; in 17 cases risk factors of HCC (cirrhosis, HBV infection) were identified, and also in 12 cases neither cirrhosis nor HBV infection was identified. with regards to LI-RADS scoring system version 17, Patients with cirrhosis due to vascular causes, such as Budd–Chiari syndrome, hereditary hemorrhagic telangiectasia, nodular regenerative hyperplasia, or cardiac cirrhosis were excluded from the study. Additionally, patients below 18 years of age were also excluded since the validity of the LI-RADS category has not been proven in pediatric populations. Lastly, cirrhotic patients due to congenital hepatic fibrosis were also excluded from the study. Patients who had a focal liver lesion(s), which were pathologically HCC, however, their respective CT scans were not available or were not acquired in a standard Triphasic fashion were also excluded from this study. The dynamic liver CT scans of the selected study group were evaluated according to LI-RADS scoring system version 2017. The evaluation process was carried out by two radiology specialists, experienced in abdominal CT-scan interpretation and reporting, subsequently, the LI-RADS category was applied for each patient separately. Demographic data i.e. name, age, sex, and further the presence of HBV were obtained and attached to CT scan images. CT scan qualities are interpreted as suitable or suboptimal for this study.
2.3 Data and statistical analysis
Five major criteria of the LI-RADS scoring system including size, arterial phase hyper-enhancement (APHE), washout appearance, capsule appearance, and threshold growth were assessed in dynamic liver CT scans accordingly. Several ancillary findings assessable and evaluable with CT scan were also evaluated. Ancillary features of discrete nodules identified in ultrasound US, subthreshold growth, corona enhancement, fat sparing in a solid mass, nodule within nodule appearance, mosaic architecture, blood product, and fat within mass were also evaluated.
Ancillary features such as size stability for more than two years, size reduction, parallel blood pool, and undistorted vessels in favor of benignity were also assessed. The SPSS22 software was utilized in the interpretation of data and subsequently, Chi-square and T-tests were applied to compare the qualitative and quantitative characteristics of the statistical data. Multi-variate and univariate regression tests were applied to investigate the impacts of different variables on the LI-RADS scoring procedure.
3. Results
Table 1 depicts the overall frequency of different variables in this study
Table 1: Frequency of different variables
Table 1: (Continued) Frequency of different variables
|
us discrete nodule |
corona enhancement |
non-enhancing capsule |
nodule in nodule |
mosaic architecture |
fat in mass |
LI-RADS |
|
23/25 |
Sep-29 |
Aug-29 |
May-29 |
17/29 |
Feb-29 |
1 |
|
-79.30% |
-31% |
-27.60% |
-17.20% |
-58.60% |
-6.90% |
-3.40% |
|
YES |
YES |
YES |
YES |
YES |
YES |
LR-4 |
|
Feb-25 |
20/29 |
21/29 |
24/29 |
Dec-29 |
27/29 |
20 |
|
-6.90% |
-69% |
-72.40% |
-82.80% |
-41.40% |
-93.10% |
-69% |
|
NO |
NO |
NO |
NO |
NO |
NO |
LR-5 |
|
8 |
||||||
|
27.6%) LR-TIV |
The age distribution of patients is given in table 2.
|
N |
Minimum |
Maximum |
Mean |
Std. Deviation |
|
|
age |
29 |
22 |
85 |
58.59 |
17.235 |
|
Valid N |
29 |
Table 2: Age distribution of patients
Table 3 depicts the frequency of major HCC risk factors (cirrhosis and/or HBV.
|
No HBV/no cirrhosis |
|||||
|
Frequency |
Percent |
Valid Percent |
Cumulative Percent |
||
|
Valid |
HBV &/or Cirrhosis |
17 |
58.6 |
58.6 |
58.6 |
|
No HBV/no cirrhosis |
12 |
41.4 |
41.4 |
100 |
|
|
Total |
29 |
100 |
100 |
||
Table 3: Presence of major HCC risk factors
In table 4 Lesion size distribution is shown.
|
lesions size |
|||||
|
N |
Minimum |
Maximum |
Mean |
Std. Deviation |
|
|
size |
29 |
22 |
190 |
86.4828 |
47.06502 |
|
Valid N |
29 |
||||
Table 4: Lesions size distribution
In table 5 frequency of the LI-RADS category is shown.
|
LI-RADS category |
|||||
|
Frequency |
Percent |
Valid Percent |
Cumulative Percent |
||
|
Valid |
LR-1 |
0 |
0 |
0 |
0 |
|
LR-2 |
0 |
0 |
0 |
0 |
|
|
LR-3 |
0 |
0 |
0 |
0 |
|
|
LR-4 |
1 |
3.4 |
3.4 |
3.4 |
|
|
LR-5 |
20 |
69 |
69 |
72.4 |
|
|
LRM |
0 |
0 |
0 |
0 |
|
|
LR-TIV |
8 |
27.6 |
27.6 |
100 |
|
|
Total |
29 |
100 |
100 |
||
Table 5: LI-RADS category
Figure 5 depicts the frequency of major imaging features.
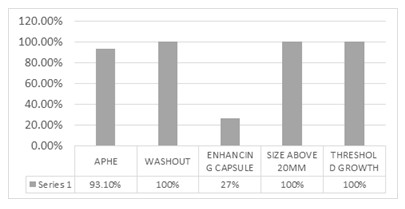
Figure 5: Major imaging features
In figure 6 the frequency of ancillary features favoring malignancy is observed.
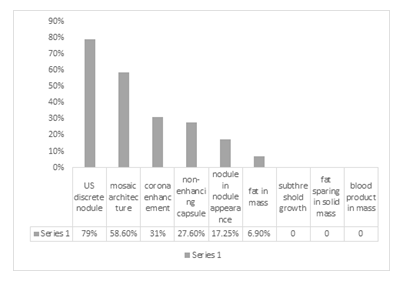
Figure 6: Ancillary features favoring malignancy
In figure 7 frequency of ancillary features favoring benignity is shown.
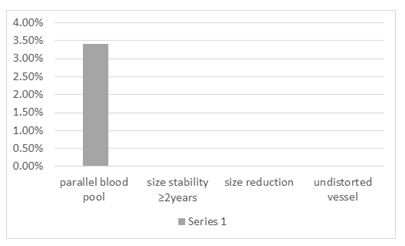
Figure 7: Ancillary features favoring benignity
In figure 8 frequency of Major features in cirrhotic vs non-cirrhotic patients is shown.
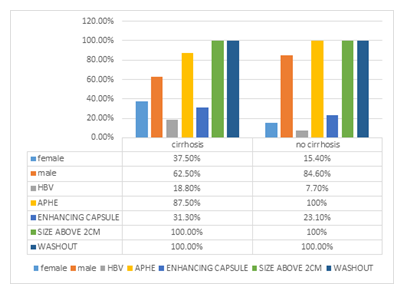
Figure 8: Major features in cirrhotic vs non-cirrhotic patients
In figure 9 frequency of Major features in HBV-positive vs HBV- negative patients are shown.
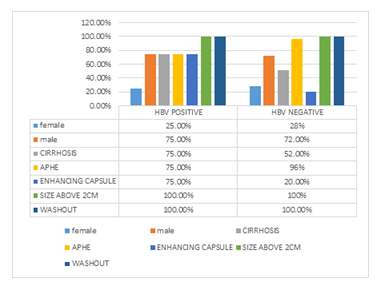
Figure 9: Major features in HBV positive vs HBV negative patient
In figure 10 frequency of Major features in a patient with vs patients without major HCC risk factors is shown.
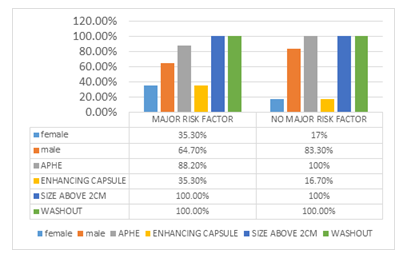
Figure 10: Major features in a patient with vs patients without major HCC risk factors
In figure 11 frequency of Major features in males vs females are shown.
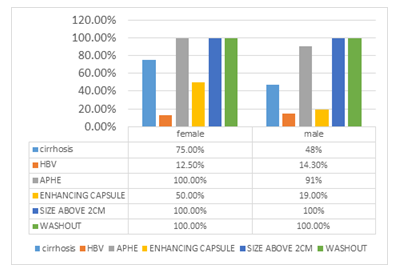
Figure 11: Major features in male vs female
In figure 12 Mean patients' age and lesion size in males vs females and also in a patient with vs patients without major HCC risk factors is shown.
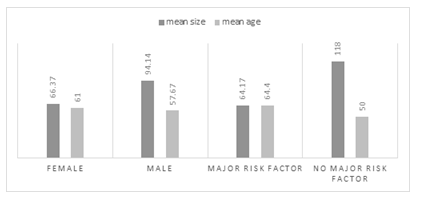
Figure12: Major features in male vs female and patient with vs without major HCC risk factors
In table 6 age and lesion size distribution in cirrhotic & non-cirrhotic patients are shown.
|
CIRRHOSIS |
N |
Minimum |
Maximum |
Mean |
Std. Deviation |
t |
Sig. |
|
|
(2-tailed) |
||||||||
|
yes |
age |
16 |
45 |
85 |
63.81 |
11.554 |
1.893 |
0.069 |
|
size |
16 |
22 |
150 |
63.9375 |
38.47851 |
3.341 |
0.002 |
|
|
Valid N |
16 |
|||||||
|
no |
age |
13 |
22 |
76 |
52.15 |
21.094 |
||
|
size |
13 |
39 |
190 |
114.2308 |
42.49736 |
|||
|
Valid N |
13 |
|||||||
Table 6: Age &lesions size distribution in cirrhotic & non-cirrhotic patients
In table 7 LI-RADS category in HBV positive vs HBV negative patients is shown.
|
HBV |
Frequency |
Percent |
Valid Percent |
Cumulative Percent |
Phi |
Approx. Sig |
||
|
yes |
Valid |
LR-5 |
1 |
25 |
25 |
25 |
0.424 |
0.022 |
|
LR-TIV |
3 |
75 |
75 |
100 |
||||
|
Total |
4 |
100 |
100 |
|||||
|
no |
Valid |
LR-4 |
1 |
4 |
4 |
4 |
||
|
LR-5 |
19 |
76 |
76 |
80 |
||||
|
LR-TIV |
5 |
20 |
20 |
100 |
||||
|
Total |
25 |
100 |
100 |
|||||
Table 7: LI-RADS category in HBV positive vs HBV negative patients
In table 8 presence of enhancing capsules in HBV-positive vs HBV-negative, patients are shown.
|
HBV |
Frequency |
Percent |
Valid Percent |
Cumulative Percent |
Phi |
Approx. Sig |
||
|
yes |
Valid |
yes |
3 |
75 |
75 |
75 |
0.424 |
0.0022 |
|
no |
1 |
25 |
25 |
100 |
||||
|
Total |
4 |
100 |
100 |
|||||
|
no |
Valid |
yes |
5 |
20 |
20 |
20 |
||
|
no |
20 |
80 |
80 |
100 |
||||
|
Total |
25 |
100 |
100 |
|||||
Table 8: Enhancing capsule in HBV positive vs HBV negative patients
In table 9 Mean patient’s age and lesion size in a patient with vs without major HCC risk factors are shown.
|
No HBV or cirrhosis |
N |
Minimum |
Maximum |
Mean |
Std. Deviation |
t |
Sig. (2-tailed) |
|
|
no |
age |
17 |
45 |
85 |
64.41 |
11.457 |
2.332 |
0.027 |
|
size |
17 |
22 |
150 |
64.1765 |
37.26968 |
3.643 |
0.001 |
|
|
Valid N |
17 |
|||||||
|
yes |
age |
12 |
22 |
76 |
50.33 |
20.939 |
||
|
size |
12 |
39 |
190 |
118.0833 |
41.94901 |
|||
|
Valid N |
12 |
|||||||
Table 9: Mean patient’s age and lesion size in a patient with vs patients without major HCC risk factors
4. Discussion
In this cross-sectional retrospective study, we assessed the consistency of LI-RADS (Liver Imaging-Reporting and Data System) version 2017 in non-invasive diagnosis of HCC (hepatocellular carcinoma) with pathologic reports. A triphasic (dynamic) liver CT scan in patients with the focal liver lesion(s) accompanied by a definite pathological diagnosis of HCC was used in the time of March 2016 to January 2019 in Shiraz Nemazee Hospital. All 29 cases were known cases of HCC according to the available pathological reports which were carried out by an expert pathologist in the liver pathology report. The dynamic liver CT scans were extracted from the PACS system. The quality of all dynamic liver CT scans was recognized as suitable and hence considered acceptable for the evaluation of imaging characteristics.
Five major criteria of LI-RADS v.2017 were assessed in the dynamic liver CT scans, including size, arterial phase hyper-enhancement (APHE), washout appearance, capsule appearance, and threshold growth. Several ancillary findings that were assessable & evaluable via CT scan were also evaluated. (including US discrete nodule, subthreshold growth, corona enhancement, fat sparing in a solid mass, a nodule in nodule appearance, mosaic architecture, blood product in mass, fat in mass which favoring malignancy and size stability more than 2 years, size reduction, parallel blood pool, and the undistorted vessel which were in favor of benignity)
Finally, the obtained LI-RADS category was tabulated as LR-1 to 5, LR-M, or LR-TIV. Among the major imaging features of LI-RADS 2017, 100 percent of HCC patients showed a washout appearance, & all lesions were above 20 mm in size (cut off of LI-RADS for HCC size). Threshold growth was assessable in only one patient (due to lack of available prior dynamic liver CT scan for comparison), which was achieved to threshold growth. Moreover, APHE was perceived to be the most common image finding (93.1%). On the other hand, the least common major imaging feature of LI-RADS 2017 in our study was the presence of enhancing capsule (27%). The data for evaluation of threshold growth was available in only one patient (only one patient out of the twenty-nine cases had a prior dynamic liver CT scan for comparison) which was positive for achieving threshold growth (more than 100% diameter increase more than six months) In the evaluation of ancillary features that favor malignancy, the most common findings were US discrete nodule (79%), mosaic architecture (58.6%), corona enhancement (31%), non-enhancing capsule (27.6%), nodule within nodule appearance (17.2%) and fat in mass (6.9%) respectively. no feature showed subthreshold growth, fat sparing in a solid mass, or blood product in mass. Diversely the evaluation of ancillary features favoring benignity resulted in only one case with a parallel blood pool and none of the cases showed ancillary features of size stability of more than two years, size reduction, and undistorted vessel.
None of the mentioned ancillary features were used in LI-RADS scoring levels that favored malignancy or benignity. According to LI-RADS 2017, any change in the categories is not permitted hence all patients with an exception of one was categorized in LR-5 or LR-TIV. The only LR-4 lesion had no ancillary feature favoring benignity to decrease its LI-RADS category.
Out of the 29 cases, none of them were assigned scores of LI-1, LR-2, or LR-3. Only one patient (3.4%) was categorized as LR-4. 20 (69%) patients were categorized as LR-5. No LR-M lesions were detected. eight patients (27.6%) were categorized as LR-TIV. it was found that the higher the LR-RADS category is the higher the chance of HCC. Applying the high LR-RADS category (LR-4, LR-5, LR-TIV) to the dynamic liver CT scan is significantly consistent with the non-invasive diagnosis of HCC. Conversely, no significant relationship has been detected among cirrhosis with the presence of non-rim APHE, enhancing capsule, a discrete nodule in the US, corona enhancement, non-enhancing capsule, a nodule in nodule appearance, mosaic architecture, fat in mass, parallel blood pool, LI-RADS category, reported HCC type, sex, HBV infection, and age.
An inverse relationship was discovered between cirrhosis and lesion size; i.e. the average size of the liver lesion(s) in non-cirrhotic HCC patients was more than that of the liver lesion(s) in cirrhotic HCC patients.
No significant relation has been detected between HBV infections and sex, age, lesion(s) size cirrhosis, presence of non-rim APHE, a discrete nodule in the US, corona enhancement, non-enhancing capsule, nodule within nodule appearance, mosaic architecture, and fat in mass, parallel blood pool or HCC type category.
Significant relation between HBV and the presence of enhancing capsule was found; i.e. in HBV-positive HCC patients, the possibility of enhancing capsule presence in the liver lesions within the dynamic liver CT scan is higher compared to HBV-negative HCC patients. There was a significant relation between HBV and LI-RADS category; i.e. in HBV-positive HCC patients, the possibility of LR-TIV presence is higher compared to HBV-negative HCC patients.
No significant relation has been detected between major HCC risk factors (HBV or cirrhosis) and gender, presence of APHE, enhancing capsule, a discrete nodule in the US, corona enhancement, presence of non-enhancing capsule, nodule within nodule appearance, mosaic architecture, fat in mass, parallel blood pool, LI-RADS category, or HCC type category.
A significant relation was detected among major HCC risk factors (HBV or cirrhosis); i.e. the average size of the liver lesion(s) in HCC patients with major HCC risk factors is less than that of the liver lesion(s) compared to HCC patients without major HCC risk factors. A significant relation was found between major HCC risk factors (HBV or cirrhosis) and patients' age; i.e. HCC patients with major HCC risk factors are older and have higher average age compared to HCC patients without major HCC risk factors.
No significant relation has been detected between sex and presence of APHE, enhancing capsule, a discrete nodule in reported US, corona enhancement, non-enhancing capsule, nodule within nodule appearance, mosaic architecture, fat in mass, parallel blood pool, LI-RADS category, HCC type category, patient age, lesion(s) size, cirrhosis, HBV infection major HCC risk factors (cirrhosis and HBV infection).
Limitations
One limitation of this study was the relatively small sample, because of the lack of available data concerning HCC patients’ archived dynamic liver CT scans due to the degradation of some of the data in the PACS system.
5. Conclusions
We found that the use of the LI-RADS category & applying the high LR-RADS category to a liver lesion (LR-4, LR-5, LR-TIV) in dynamic liver CT scan can have significant consistency with the non-invasive diagnosis of HCC. Significant relation between cirrhosis with lesion size was found; i.e. the average size of the liver lesion(s) in non-cirrhotic HCC patients is more than the average size of the liver lesion(s) in cirrhotic HCC patients. There is a significant relation between HBV and the presence of enhancing capsule; i.e. in HBV-positive HCC patients, the chance of the presence of enhancing capsule in liver lesions in dynamic liver CT scan is higher compared to HBV-negative HCC patients. Significant relation has also been detected amongst HBV and LI-RADS categories; i.e. in HBV-positive HCC patients, the chance of the presence of LR-TIV is higher compared to HBV-negative HCC patients. We found that there is a significant relation between major HCC risk factors (HBV or cirrhosis); i.e. the average size of the liver lesion(s) in HCC patients with major HCC risk factors is less than that of the liver lesion(s) comparing to HCC patients without major HCC risk factors. this could be due to the earlier diagnosis of clinical presentation & consequent earlier diagnostic workup.
There is a significant relation between major HCC risk factors (HBV or cirrhosis) and patient’s age; i.e. HCC patients with major HCC risk factors are older and have higher average age compared to HCC patients without major HCC risk factors. The most common ancillary features that are expected to manifest in favor of malignancy seem to be US discrete nodule (79%), mosaic architecture (58.6%) and corona enhancement (31%), and non-enhancing capsule (27.6%) respectively. Further nodules within nodule appearance (17.2%) and fat in mass (6.9%) were seen to be less common. It is worth mentioning that none of the patients demonstrated subthreshold growth, fat sparing in a solid mass, or blood product in mass.
In the evaluation of ancillary features favoring benignity, only one patient showed a parallel blood pool conversely none of the cases have shown ancillary features of size stability for more than two years, size reduction, and undistorted vessel. It can be assumed that the absence of ancillary features that favor benignity could bring up suspicions concerning malignancy.
Acknowledgment
The present article was extracted from the thesis written by Hemmat Allah Rastgooyan and was financially supported by the Shiraz University of Medical Science, Shiraz, Iran, grants No #19116.
References
- You M-W, Yun S. Differentiating between hepatocellular carcinoma and intrahepatic cholangiocarcinoma using contrast-enhanced MRI features: a systematic review and meta-analysis. Clinical radiology 19 (2019): 30.
- Liu W, Qin J, Guo R, et al. Accuracy of the diagnostic evaluation of hepatocellular carcinoma with LI-RADS. Acta Radiologica 59(2018): 140-6.
- Bae JS, Kim JH, Yu MH, et al. Diagnostic accuracy of gadoxetic acid-enhanced MR for small hypervascular hepatocellular carcinoma and the concordance rate of Liver Imaging Reporting and Data System (LI-RADS). PloS one 12(2017): e0178495.
- Tang A, Hallouch O, Chernyak V, et al. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdominal Radiology 43(2018): 13-25.
- Basha MAA, AlAzzazy MZ, Ahmed AF, et al. Does a combined CT and MRI protocol enhance the diagnostic efficacy of LI-RADS in the categorization of hepatic observations? A prospective comparative study. European radiology 28(2018): 2592-603.
- Tang A, Fowler KJ, Chernyak V, et al. LI-RADS and transplantation for hepatocellular carcinoma. Abdominal radiology (New York) 43(2018): 193-202.
- Petruzzi N, Mitchell D, Guglielmo F, et al. Hepatocellular carcinoma likelihood on MRI exams: evaluation of a standardized categorization system. Academic radiology 20(2013): 694-8.
- Ayuso C, Rimola J, Vilana R, et al. Corrigendum to "Diagnosis and staging of hepatocellular carcinoma (HCC): Current guidelines." Eur. J. Radiol 101 (2018): 72-81.
- Davenport MS, Khalatbari S, Liu PS, et al. Repeatability of diagnostic features and scoring systems for hepatocellular carcinoma by using MR imaging. Radiology 272(2014): 132-42.
- Ludwig DR, Fraum TJ, Cannella R, et al. Hepatocellular carcinoma (HCC) versus non-HCC: accuracy and reliability of Liver Imaging Reporting and Data System v2018. Abdominal radiology (New York) 44 (2019).
- Abd Alkhalik Basha M, Abd El Aziz El Sammak D, El Sammak AA. Diagnostic efficacy of the Liver Imaging-Reporting and Data System (LI-RADS) with CT imaging in categorising small nodules (10-20 mm) detected in the cirrhotic liver at screening ultrasound. Clin Radiol 72(2017): e1-.e11.
- Granata V, Fusco R, Avallone A, et al. Critical analysis of the major and ancillary imaging features of LI-RADS on 127 proven HCCs evaluated with functional and morphological MRI: Lights and shadows. Oncotarget 8(2017): 51224-37.
- Jha RC, Mitchell DG, Weinreb JC, et al. LI-RADS categorization of benign and likely benign findings in patients at risk of hepatocellular carcinoma: a pictorial atlas. AJR American journal of roentgenology 203(2014): W48-69.
- Schima W, Heiken J. LI-RADS v2017 for liver nodules: how we read and report. Cancer imaging: the official publication of the International Cancer Imaging Society 18(2018):14.
- Elsayes KM, Hooker JC, Agrons MM, et al. 2017 Version of LI-RADS for CT and MR Imaging: An Update. Radiographics : a review publication of the Radiological Society of North America, Inc 37(2017): 1994-2017.
- Kim YY, An C, Kim S, et al. Diagnostic accuracy of prospective application of the Liver Imaging Reporting and Data System (LI-RADS) in gadoxetate-enhanced MRI. European radiology 28(2018): 2038-46.
- Chernyak V, Tang A, Flusberg M, et al. LI-RADS((R)) ancillary features on CT and MRI. Abdominal radiology (New York) 43(2018):82-100.
- Chernyak V, Santillan CS, Papadatos D, et al. LI-RADS((R)) algorithm: CT and MRI. Abdominal radiology (New York) 43(2018):111-26.
- Hicks RM, Yee J, Ohliger MA, et al. Comparison of diffusion-weighted imaging and T2-weighted single shot fast spin-echo: Implications for LI-RADS characterization of hepatocellular carcinoma. Magnetic resonance imaging 34(2016): 915-21.
- Hope TA, Fowler KJ, Sirlin CB, et al. Hepatobiliary agents and their role in LI-RADS. Abdominal imaging 40(2015):613-25.
- Narsinh KH, Cui J, Papadatos D, et al. Hepatocarcinogenesis and LI-RADS. Abdominal radiology (New York) 43(2018):158-68.
