Evaluation of Effectiveness of Antibiotic Combination Therapy in Multi Drug Resistant Escherichia Coli in Vitro and in Vivo
Article Information
Nandita Banik1*, SM Shamsuzzaman2
1Microbiologist (WHO), Institute Of Epidemiology Disease Control and research, Dhaka, Bangladesh
2Professor, Department of Microbiology, Dhaka Medical College and Hospital, Dhaka, Bangladesh
*Corresponding author: Nandita Banik, Microbiologist (WHO), Institute Of Epidemiology Disease Control and research, Dhaka, Bangladesh
Received: 28 August 2021; Accepted: 08 September 2021; Published: 30 September 2021
Citation: Nandita Banik, SM Shamsuzzaman. Evaluation of Effectiveness of Antibiotic Combination Therapy in Multi Drug Resistant Escherichia Coli in Vitro and in Vivo. Fortune Journal of Health Sciences 4 (2021): 470-478.
Share at FacebookAbstract
The antimicrobial resistance to commonly used antibiotics including broad spectrum antibiotics like carbapenem and colistin and aminoglycosides are increasing among Escherichia coli which has resulted in emergence of MDR strains as well as limiting therapeutic options to treat infection by them. The use of combination therapy has been found to be an effective strategy to overcome this situation. The aim of the present study was to see the efficacy of combination of imipenem-colistin, imipenem-amikacin and colistin-amikacin both in vitro and in vivo in MDR Escherichia coli resistant to all of them. Total 65 Escherichia coli were included in this study isolated from urine, wound swab and blood samples of infected patients. Escherichia coli were identified by biochemical test followed by PCR. Antibiotic susceptibility pattern was performed by disc-diffusion method. MIC of imipenem, colistin and amikacin were done by agar dilution method. Antibiotic combinations (imipenem+colistin, imipenem+amikacin and colistin+amikacin) effectiveness in treating resistant Escherichia coli were evaluated in vitro by agar dilution method and in vivo by using a mouse model. Among 65 Escherichia coli 11 were MDR among which 2 were resistant to imipenem& colistin; 4 were resistant to imipenem & amikacin; 4 were resistant to amikacin & colistin; one was resistant to all the three drugs. Combination of two antibiotics of these three in vitro against resistant Escherichia coli showed synergistic effect when 1/4 or 1/8 MIC for each antimicrobial were used. The best in vivo efficacy appeared with imipenem-colistin and imipenemamikacin combination which showed 100% blood culture negative results after 72 hours of antibiotic combination therapy.
Keywords
Antibiotic combination; MDR; FIC; E.coli
Antibiotic combination articles, MDR articles, FIC articles, E.coli articles Antibiotic combination articles Antibiotic combination Research articles Antibiotic combination review articles Antibiotic combination PubMed articles Antibiotic combination PubMed Central articles Antibiotic combination 2023 articles Antibiotic combination 2024 articles Antibiotic combination Scopus articles Antibiotic combination impact factor journals Antibiotic combination Scopus journals Antibiotic combination PubMed journals Antibiotic combination medical journals Antibiotic combination free journals Antibiotic combination best journals Antibiotic combination top journals Antibiotic combination free medical journals Antibiotic combination famous journals Antibiotic combination Google Scholar indexed journals MDR articles MDR Research articles MDR review articles MDR PubMed articles MDR PubMed Central articles MDR 2023 articles MDR 2024 articles MDR Scopus articles MDR impact factor journals MDR Scopus journals MDR PubMed journals MDR medical journals MDR free journals MDR best journals MDR top journals MDR free medical journals MDR famous journals MDR Google Scholar indexed journals FIC articles FIC Research articles FIC review articles FIC PubMed articles FIC PubMed Central articles FIC 2023 articles FIC 2024 articles FIC Scopus articles FIC impact factor journals FIC Scopus journals FIC PubMed journals FIC medical journals FIC free journals FIC best journals FIC top journals FIC free medical journals FIC famous journals FIC Google Scholar indexed journals E.coli articles E.coli Research articles E.coli review articles E.coli PubMed articles E.coli PubMed Central articles E.coli 2023 articles E.coli 2024 articles E.coli Scopus articles E.coli impact factor journals E.coli Scopus journals E.coli PubMed journals E.coli medical journals E.coli free journals E.coli best journals E.coli top journals E.coli free medical journals E.coli famous journals E.coli Google Scholar indexed journals Escherichia coli articles Escherichia coli Research articles Escherichia coli review articles Escherichia coli PubMed articles Escherichia coli PubMed Central articles Escherichia coli 2023 articles Escherichia coli 2024 articles Escherichia coli Scopus articles Escherichia coli impact factor journals Escherichia coli Scopus journals Escherichia coli PubMed journals Escherichia coli medical journals Escherichia coli free journals Escherichia coli best journals Escherichia coli top journals Escherichia coli free medical journals Escherichia coli famous journals Escherichia coli Google Scholar indexed journals fluroquinolones articles fluroquinolones Research articles fluroquinolones review articles fluroquinolones PubMed articles fluroquinolones PubMed Central articles fluroquinolones 2023 articles fluroquinolones 2024 articles fluroquinolones Scopus articles fluroquinolones impact factor journals fluroquinolones Scopus journals fluroquinolones PubMed journals fluroquinolones medical journals fluroquinolones free journals fluroquinolones best journals fluroquinolones top journals fluroquinolones free medical journals fluroquinolones famous journals fluroquinolones Google Scholar indexed journals cephalosporins articles cephalosporins Research articles cephalosporins review articles cephalosporins PubMed articles cephalosporins PubMed Central articles cephalosporins 2023 articles cephalosporins 2024 articles cephalosporins Scopus articles cephalosporins impact factor journals cephalosporins Scopus journals cephalosporins PubMed journals cephalosporins medical journals cephalosporins free journals cephalosporins best journals cephalosporins top journals cephalosporins free medical journals cephalosporins famous journals cephalosporins Google Scholar indexed journals antimicrobials articles antimicrobials Research articles antimicrobials review articles antimicrobials PubMed articles antimicrobials PubMed Central articles antimicrobials 2023 articles antimicrobials 2024 articles antimicrobials Scopus articles antimicrobials impact factor journals antimicrobials Scopus journals antimicrobials PubMed journals antimicrobials medical journals antimicrobials free journals antimicrobials best journals antimicrobials top journals antimicrobials free medical journals antimicrobials famous journals antimicrobials Google Scholar indexed journals
Article Details
1. Introduction
Escherichia coli is one of the most common human pathogen. They are the leading cause of urinary tract infections, wound infections, diarrhoea and other illnesses [15]. The diseases caused by Escherichia coli are severe and require antibiotic therapy for treatment [22]. Bacterial resistance to commonly prescribed antimicrobials are raising both in developing as well as in developed countries [20]. Escherichia coli has been found highly resistant to commonly used antibiotics like penicillin, cephalosporins, fluroquinolones, Trimethoprim Sulfamethoxazole and other ß-lactam drugs [8]. The prevalence of carbapnenem-resistant Escherichia coli in human patients has increased in Bangladesh [3]. Carbapenemase-producing bacteria are usually multidrug resistant [16]. Polymyxin drugs, such as polymyxin B and colistin, remain active against carbapenemase producing pathogens and are considered to be last-resort antimicrobials in treating carbapenem resistant infections [24]. The recent increase in the use of colistin in clinical practice accompanied by its unbridled use in agriculture have contributed to the rapid dissemination of resistance [13]. This problem of wide scale resistance of bacteria against antibiotics is severe and need to be checked by employing effective measures. As a preventive measure the combined use of two or more antibiotics could be employed for killing multiple drug resistant bacteria. Treatment with more than one drug simultaneously can lower the survival rate of bacteria against resistance stemming from the occurrence of fortuitous mutations [22].
2. Materials and Methods
2.1 Isolation and identification of organisms:
Total 350 urine, blood and wound swab samples were included from the patients admitted in Dhaka Medical College Hospital after taking informed written consent. Escherichia coli were isolated and identified by observing pink colonies in Mac Conkey’s agar media, motility, indole positive, urease and citrate negative reactions [5] and confirmed by detecting uidA gene by PCR [4].
2.2 Antimicrobial susceptibility test
Susceptibility of isolates to 10 antimicrobials (ciprofloxacin, ceftriaxone, cefepime, amoxicillin/clavullenic acid, piperacillin/tazobactam, aztreonam, cefoxitin, amikacin, colistin, imipenem) were done by modified Kirby–Bauer disc diffusion method [2] and zones of inhibition were interpreted according to CLSI guidelines [6]. Escherichia coli ATCC 29212 was used as control strain to assess the performance of the method.
2.3 Determination of MIC
Minimum inhibitory concentration (MIC) of amikacin, imipenem and colistin were determined using agar dilution method [1, 9]. Eighty mg base of commercially available colistin injection vial was added to 10 ml distilled water to make a concentration of 8 mg/ml to prepare colistin stock solution. For each plate 50 ml Muller-Hinton agar media was prepared. 50 ml sterile Muller-Hinton agar was impregnated with 12.5 µl, 25 µl, 50 µl, 100 µl, 200 µl, 400 µl, 800 µl, 1600 µl of colistin stock solution to achieve concentration of 2µg/ml, 4µg/ml, 8µg/ml, 16µg/ml, 32µg/ml, 64µg/ml, 128µg/ml, 256µg/ml per plate respectively. Imipenem stock solution was prepared by adding 100 ml distilled water in 500mg base of commercially available imipenem injection vial to get 5mg/ml. 50 ml sterile Muller-Hinton agar was impregnated with 20 µl, 40 µl, 80 µl, 160 µl, 320 µl, 640 µl, 1280 µl, and 2560 µl of imepenem stock solution to achieve concentration of 2µg/ml, 4µg/ml, 8µg/ml, 16µg/ml, 32µg/ml, 64µg/ml, 128µg/ml, 256µg/ml per plate respectively. Commercially available amikacin injection vial was used as amikacin stock solution and the concentration was 250 mg/ml. 50 ml sterile Muller-Hinton agar was impregnated with 3.2 µl, 6.4 µl, 12.8 µl, 25.6 µl, 51.2 µl, 102.4 µl, 204.8 µl, 409.6 µl, 819.2 µl, 1638.4 µl, 3276.8 µl, 6553.6 µl, 13107.2 µl and 26214.4 µl of amikacin stock solution to achieve concentration of 16µg/ml, 32µg/ml, 64µg/ml, 128µg/ml, 256µg/ml, 512 µg/ml, 1024 µg/ml and 2048 µg/ml, 4096 µg/ml, 8192 µg/ml, 16384 µg/ml, 32768 µg/ml, 65536 µg/ml and 131072 µg/ml per plate respectively. To prepare bacterial inoculum, the turbidity of bacterial suspension in normal saline was compared with 0.5 McFarland turbidity standard and as 0.5 McFarland turbidity standard contain cfu/ml, 10 times dilution (one ml test inoculums compared to turbidity standard added when with 9 ml of normal saline) of test inoculums was done to achieve cfu/ml. To obtain cfu/spot on agar surface one µl of 10 times diluted inoculums were placed on Muller-Hinton agar plate. All the inoculated plates were incubated aerobically at 37ºC overnight. The lowest concentration of antibiotic impregnated Muller-Hinton agar media showing no visible growth was considered as MIC of that drug for that strain. Escherichia coli ATCC strain 25922 was used as control organism.
2.4 Antibiotic combinations in vitro
Combinations of imipenem-colistin, imipenem-amikacin and amikacin-colistin against MDR species including resistance to the drugs used in combination were undertaken to see synergistic, additive, indifferent or antagonistic effects by agar dilution method. For each sample 4 plates were prepared with 50ml Muller-Hinton agar media in each plate. The first plate of combination contained the MIC of each antibiotic for that sample. The 2nd plate contained two fold lower dilution than the MIC of both antibiotics for that sample. The 3rd plate contained four fold lower dilutions than the MIC of both antibiotics for that sample. The 4th plate contained eight fold lower dilutions than the MIC of both antibiotics for that sample. The Muller-Hinton agar plate was impregnated with respective amount of antibiotic stock solution according to above description. Then inoculum was prepared as mentioned above and all the plates were inoculated with 1 µl of inoculum followed by incubation at 37º overnight. In antibiotic combination Synergy was considered by agar dilution method when there was a fourfold or greater reduction in the MICs of both antibiotics. A reduction of less than four fold in the MICs of both antibiotics was considered additive. Indifference was found when neither drug exhibited a decreasing MIC, and an increase in the MIC was considered antagonism [9]. The fractional inhibitory concentration index (FICI) was also determined to evaluate the effects of antimicrobial combination as follow: synergistic (FICI≤0.5), partial synergistic (0.5< FICI <1), additive (FICI=1), indifferent (1< FICI ≤ 4), antagonistic (FICI >4), and calculated using the following equation [14].
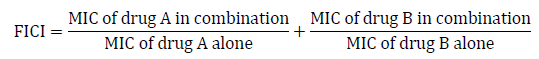
All the tests were performed in triplicate.
2.5 In vivo study
Forty mice (swis albino) were used for this purpose. The experiments were performed in immunocompetent female mouse weighting 15-20 grams. The mouse were purchased from icddr,b breeding house Dhaka, Bangladesh. Animals were maintained under adequate temperature (22-24º C) and humidity. The mice received a standard diet obtained from icddrb and sterile water. Mice were divided into 8 groups (A, B, C, D, E, F,G, H) with 5 mouse in each group. Group A, B, C, D, E, F, G were infected by intra-peritoneal injection of 250 µl of approximately cfu/ml bacterial inoculums using a 100 unit insulin syringe in the lower right abdomen [21]. Group H was not inoculated with bacterial inoculums. Group H was regarded as negative control group. Bacterial inoculums were obtained through a 24 hours subculture of a MDR (colistin, imipenem and amikacin resistant) Ecsherichia coli in MacConkey agar media at 37ºC. Group A, B, C, D, E, F received antimicrobial treatment intraperitoneally after 4 hours of infection at 12 hours interval for 3 days. Group A, B and C were treated individually only with amikacin (15mg/kg), imipenem (15mg/kg) and colistin (3.4mg/kg) respectively. Group D received amikacin plus imipenem (15mg/kg + 15mg/kg), Group E received amikacin plus colistin (3.4mg/kg + 15mg/kg) and Group F received imepenem plus colistin (3.4mg/kg + 15mg/kg) combination. Group G did not receive antimicrobial treatment. Group G was regarded as positive control. In order to confirm that these drugs were not toxic to the animal, another group of five uninfected mouse (Group H) were given each antibiotic for 72 hours (uninfected treat group/negative control). The animals were observed for 72 hours and the survival mouse were recorded every 12 hours. Blood samples were taken as detailed below. All the blood samples were processed for microbiological studies.
The infected animals were observed for 72 hours of treatment and the cumulative survival rates were recorded every 12 hours.
3. Microbiological study
After 72 hours of antibiotic treatment, blood samples were collected from mouse by cardiac puncture aseptically. At first, upper part of the chest was shaved by razor, then washed with alcohol pad followed by povidon iodine. After palpating the cardiac pulsation with the finger pulp, the area was washed with povidon iodine, then 100 unit insulin syringe needle was introduced through the skin in the heart of the mouse blindly. For blood culture 1.5ml of each mouse’s blood was collected and then incubated in sterile conical flask with 5 ml of TSB and incubated for 24 hours at 37ºC. Subculture was done in Blood agar and MacConkey agar media and incubated for 24 hours at 37ºC .Then the incubated plates were observed for positive or negative growth [10].
4. Results
Out of 350 samples 65 were Escherichia coli among which 11 (N1 to N11) were multidrug resistant. Among the MDR E. coli 90.91%, 81.82% and 45.45% were resistant to amikacin, imipenem and colistin respectively. The MIC value for imipenem, amikacin and colistin ranged from 16µg/ml to ≥128 µg/ml, 1,024 µg/ml to ≥ 65,536 µg/ml and 4 µg/ml to ≥ 256 µg/ml respectively (Table-1).
Table: 1 MIC of colistin, imipenem and amikacin in MDR Escherichia coli (N=11)
Combination of imipenem and amikacin against four MDR isolates which were resistant to both drugs showed reduction of their MIC value by four fold and eight fold after combination which were synergistic effect and this finding was strengthened by FICI≤0.5 (Table-3). Two MDR species found resistant to imipenem and colistin and MIC value of these drugs reduced by four fold after combination against these two species and FICI were 0.5, which proved synergistic effect (Table-2).
Table-3: Results of combination of imipenem with colistin in MDR E. coli resistant to both drugs.
Four MDR isolates were resistant to amikacin and colistin and combination of these two showed reduction of MIC by four fold in 3 isolates with FICI value 0.5 which was synergistic effect and by two fold in one isolates with FICI value 1 which was additive effect (Table-4).
Table-4: Result of combination of amikacin with colistin in MDR E. coli resistant to both drugs.
Combination of imipenem with amikacin and imipenem with colistin found more effective than colistin with amikacin combination as well as single durg therapy (table-5).
Table 5: Results of antibiotic therapy on the clearance of Escherichia coli from the blood of mice.
CL= Colistin IMP= Imipenem AK= Amikacin.
4. Discussion
Multidrug-resistant gram-negative bacteria are a major public health threat. However, intense efforts to limit their spread, the number of multidrug resistant gram-negative bacteria continues to increase globally [16]. Combined use of antimicrobial could be a better choice [18] in such cases. In the present study, combination of imipenem with colistin against MDR E. coli revealed synergistic effect in vitro as well as hundread percent blood culture negative result appeared in vivo using this combination. This finding is consistent with the previously reported combination therapy against MDR gram negative bacteria [23]. Colistin is a cationic peptide which disrupts bacterial cell membrane which may potentiate action of other antimicrobials in combination. Combination of imipenem with amikacin also showed synergism in all tested MDR isolates in vitro and hundread percent blood culture negative result appeared in vivo. Carbapenems were previously reported effective in combination with aminoglycosides against MDR gram negative bacteria [11, 12]. Imipenem is cell wall inhibitor and amikacin is protein synthesis blocker. So, this combination work well when used in combination. In this study Colistin and amikacin combination showed synergistic effect in 3 of the 4 test isolates and one showed additive effect. Synergism was also reported regarding this combination by other studies [25]. But in vivo experiment in this study showed 40% blood culture negative result. But this combination was reported as promising therapeutic option to treat infection by carbapenem resistant E. coli and Pseudomonas aeuroginosa [25, 19].
In the present study, mice were observed periodically for survival for 72 hours after intervention. In the present study the best in vivo result appeared in the group treated with imipenem-colistin and imipenem-amikacin combination. Hundread percent blood culture negative results were seen for these two combination in vivo. Previous in vivo studies reported these combination to be effective in infection by gram negative bacteria [23, 7].
5. Conclusion
The present study observed that combination therapy could be a good option for MDR Escherichia coli. Combination of imipenem with colistin, imipenem with amikacin and colistin with amikacin showed synergistic effect in vitro. Combination of colistin with amikacin found less effective (40% bacterial clearance) than other two combination (100% bacterial clearance) in vivo.
References
- Andrews JM. Determination of minimum inhibitory concentration. J Antimicrob Chemother 48 (2001): 5-16.
- Bauer AW, Kirby WMM, Sheris JC, Truck M. Antibiotic susceptibility testing by a standerized single disc method. Am J Clin Pathol 145 (1996): 225-230.
- Begum N, Shamsuzzaman SM. 2016. Emergence of carbapenemase producing urinary isolates at a tertiary care hospital in Dhaka, Bangladesh. Tzu Chi Medical Journal 28 (2016): 94-98.
- Bej AK, DiCesare JL, Atlas RM. Detection of Escherichia coli and Shigella spp. in water by using the polymerase chain reaction and gene probes for uid. Appl Environ Microbiol 57 (1991):1013-1017.
- Cheesbrough M. Antimicrobial susceptibility testing, p132-143. In: Cheesbrough M, (ed), District laboratory practice in tropical countries, Cambridge university press, India 2 (2009).
- Clinical and Laboratory Standard Institute (CLSI). 2018. Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standard Institute, Wayne, PA (2018).
- Dinc G, Demiraslan H, Elmali F, Ahmed SS, Alp E, Doganay M. Antimicrobial efficacy of doripenem and its combinations with sulbactam, amikacin, colistin, tigecycline in experimental sepsis of carbapenem-resistant Acinetobacter baumannii. New Microbiol 38 (2015): 67–73.
- Elsayed TI, Ismail HA, Elgamal SA, Gad HA. 2017. The Occurrence of Multidrug Resistant E. Coli which Produce ESBL and Cause Urinary Tract Infections. J Appl Microbiol Biochem 1 (2017): 8.
- Gombert ME, Aulicino TM. Synergism of imipenem and amikacin in combination with other antibiotics against Nocardia asteroides. Antimicrob Agents Chemother 24 (1983): 810–811.
- Hernandez MJR, Pachon J, Pichard C, Cuberos L, Martinez JI, Curiel AG, Caballero FJ, Moreno I, Mejias MEJ. Imipenem, doxycycline and amikacin in monotherapy and in combination in Acinetobacter baumannii experimental pneumonia. J Antimicrob Chemother 45 (2000): 493-501.
- Khaleq MAA, Abd AH, Dhahi MA. Efficacy of Combination of meropenem with gentamicin, and amikacin against resistant E. coli isolated from patients with UTIs: in vitro study. Iraqi J Pharm Sci 20 (2011): 66-74.
- Kadar B, Kocsis B, Toth A, Damjanova I, Szasz M, Kristof K, Nagy K, Szabo D. Synergistic antibiotic combinations for colistin-resistant Klebsiella pneumoniae. Acta Microbiol Immunol Hung 60 (2013): 201–209.
- MacNair CR, Stokes JM, Carfrae LA, Comyn AA, Coombes BK, Mulvey MR, Brown ED. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat Commun 9 (2018): 1-8.
- Marques MB, Brookings ES, Moser SA, Sonke PB, Waites KB. Comparative In Vitro Antimicrobial Susceptibilities of Nosocomial Isolates of Acinetobacter baumannii and Synergistic Activities of Nine Antimicrobial Combinations. J Antimicrob Chemother 41 (1997): 881-885.
- Melzer M, Peterson I. 2007. Mortality following bacteremic infection caused by extended spectrum beta-lactamase (ESBL) producing Escherichia coli compared to non- ESBL producing E.coli. J infect Dis 55 (2007): 254-259.
- Mlynarcik P, Roderova M, Kolar M. Primer evaluation for PCR and its application for detection of carbapenemases in Enterobacteriaceae. Jundishapur J Microbiol 9 (2016): e293142.
- Queiroz GM, Silva LM, Pietro RCLR, Salgado HRN. Microbial multi-resistance and available therapeutic options. Rev Bras Clin Med 10 (2012): 132-138.
- Schmid A, Wolfensberger A, Nemeth J, Schreiber PW, Sax H, Kuster SP. Monotherapy versus combination therapy for multidrug-resistant Gram-negative infections: Systematic Review and Meta-Analysis Sci Rep 9 (2019): 1–11.
- Tascini C, Ferranti S, Messina F, Menichetti F. In vitro and in vivo synergistic activity of colistin, rifampin, and amikacin against a multiresistant Pseudomonas aeruginosa isolate. Clin Microbiol Infect 6 (2000): 690–1.
- Thiraviam M, Yadesa D, Adugna T. 2014. Antibiotic resistant pattern of urinary tract infection causing Escherichia coli isolated from diabetic mellitus and non-diabetic mellitus patients with special reference to Rifampicin resistance. Int J Curr Microbiol App Sci 3 (2014): 668-674.
- Toledo PVM, Tuon FF, Bail L, Manente F, Arruda P, Arranha- Junior. Experimental model for treatment of extended spectrum beta lactamase producing Klebsiella pneumoniae. Arq Bras Cir Dig 27 (2014): 168-171.
- Yadav A, Yadav K, Ghosh A. The efficacy trial of some antibiotic combinations against multi-drug resistant Escherichia coli. Res. Environ Life Sci 9 (2016): 1109-1112.
- Yang H, Chen G, Hu L, Liu Y, Cheng J, Ye Y, Li J. 2016. Enhanced efficacy of imipenem-colistin combination therapy against multiple-drug-resistant Enterobacter cloacae: in vitro activity and a Galleria mellonella model. J Microbiol Immun Infect 51 (2016): 70-75.
- Yu Y, Walsh TR, Yang RS, Zheng M, Wei MC, Tyrrel JM, Wang Y, Liao XP, Sun J, Liu YH. Novel partners with colistin to increase its in vivo therapeutic effectiveness and prevent the occurrence of colistin resistance in NDM and MCR-co-producing Escherichia coli in a murine infection model. J Antimicrob Chemother 74 (2018): 87-98.
- Zhou YF, Tao MT, Feng Y, Yang RS, Liao XP, Liu YH, Sun J. Increased activity of colistin in combination with amikacin against Escherichia coli co-producing NDM-5 and MCR-1. J Antimicrob Chemother 72 (2017): 1723–30.
